Leiomiosarcoma of the Subcutaneous Tissue the Difficulties of Clinical Diagnosis: Description of a Case
A B S T R A C T
Aims: Leiomyosarcomas are infrequent malignant tumors of smooth muscle, mainly derived from blood vessels or viscera. Superficial leiomyosarcomas are rare soft tissue sarcomas resulting from the dermis or subcutaneous tissue. They show a preference for proximal ends and tend to be slow-growing. They clearly show different histological and prognostic characteristics depending on whether they are cutaneous or subcutaneous.
Case Report: We reported the case of subcutaneous leiomyosarcoma resulting in the medial subinguinal region of the right thigh in a 58-year-old female. Leiomyosarcoma is an entity whose clinical presentation may appear non-specific, making diagnosis difficult. We discussed the case with a brief review of the literature and the difficulties of a first approach clinical diagnosis.
Conclusion: In conclusion, a long period of patient follow-up is recommended to capture a subsequent malignant progression of the disease.
Keywords
Immunohistochemistry, subcutaneous leiomyosarcoma, smooth muscle differentiation, soft tissue tumors
Background
Subcutaneous leiomyosarcomas (LMS) are rare aggressive mesenchymal neoplasms. They represent 4-6.5% of soft tissue sarcomas [1]. Cutaneous leiomyosarcomas originate for the most part from the cells of the smooth muscle erector of the hair. They represent 2-3% of superficial sarcoma, having no preference for a particular sex. They are located in the limbs and in particular in the piliferous areas, generally small in size (less than 2 cm), even if the subcutaneous tumors grow reaching larger sizes. The symptoms are pain and changes in the epidermis [1, 2]. Since clinical signs are not strongly indicative of malignancy and vary from person to person, a definitive diagnosis may be delayed until the next stage of the disease [1].
Subcutaneous SML is thought to result from the smooth muscle wall of the small and medium blood vessels of the subcutaneous. They usually occur in patients between 50 and 70 years of age, with prevalence in women ranging from 2: 1 to 3: 1. Although they can occur anywhere on the body, they are most frequently found in the lower extremities. The thigh has been reported as a frequently affected site. The etiology of these tumors is relatively unknown, although antecedent traumatic injuries, ionizing radiation, chemicals, sunlight and lupus vulgaris have been associated. Pol R.A. et al. (2012) also describe a case that developed on a smallpox scar [3]. Massi D. et al. 2010 reported a patient with a pleomorphic dermal SML with minimal extension in subcutaneous fat who developed metastases (colon, lung, laterocervical lymph nodes) 15 years after the initial excision [4]. Other researchers have defined cutaneous SMLs as tumors that arise in the dermis and that only secondarily or subsequently invade the subcutaneous tissue.
The tumors were classified as purely dermal when the lesion was confined within the expanded and papillary reticular dermis, without extension in the subcutaneous fat, or cutaneous with minimal extension to the subcutaneous tissue when most of the tumor occupies the expanded dermis but with a minimum extension detected in the underlying fat. There is evidence that the depth of the tumor can significantly influence the clinical outcome. The dissemination of the gelatinous matrix at the time of surgery may contribute to the phenomenon of local recurrence. True subcutaneous SMLs are known for more frequent local recurrence (50-70% of cases) and distant metastasis in about 30-40% of cases.
The LMS are classified according to the grading system described by Coindre et al., where the points are assigned to the level of differentiation (1 = very similar to normal tissue; 2 = certain histogenetic classification; 3 = undifferentiated), of Mitotic Index for 2 mm2 (1 = 0-9; 2 = 10-19; 3 = 20 or more) and necrosis (0 = none; 1 = less than 50%; 2 = more than 50%) [5, 6]. Cancers with a total score of 2 or 3 are classified as Grade I, those with a score of 4 or 5 as Grade II and those with a score of 6-8 as Grade III. Leiomyosarcomas present a macroscopic fasciculated appearance of whitish-gray or pink colour; tumors originating in the dermis often appear poorly defined due to the intricate intertwining of tumor fascicles, surrounding collagen and hair erector muscle. On the contrary, subcutaneous tumors tend to compress the surrounding tissue generating a capsule.
As we have said, they occur in at least three distinct clinical manifestations: cutaneous SML, which usually has a benign course due to their small size and superficial position; those derived from large vessels such as the inferior vena cava; and those resulting from deep soft tissues. Subcutaneous LMS is considered to be derived from the muscle fibers of the vessels. Although cutaneous SML is usually treated with surgical excision, it has high local recurrence rates ranging from 30 to 50%. Examples of distant metastasis have been published in the literature. Subcutaneous LMS has a recurrence rate higher than cutaneous LMS (50-70%) with distant metastasis. Radiotherapy or chemotherapy has been used in four cases without success [7, 8]. Subcutaneous LMS is a well-circumscribed mass with a collagen pseudocapsule. It initially exists at the dermohypodermal junction presumably linked to the hypodermic plexus. Atypical smooth muscle cells are randomly distributed and typically comprise a vascular model related to the tissue of origin. The vascular origin can explain the propensity of the subcutaneous SML to relapse and to metastasize.
The histopathology diagnosis of LMS requires an aspect of dense cellularity, nuclear atypia, and pleomorphism with at least one mitotic figure per field of 5-10. The superficial LMS present variable coloring for vimentin, actin, desmin, HHF35, and cytokeratins induced by the various phenotypes. The actin stain is 100% and 66% desmin in cutaneous SML. The metastasis and mortality rate for subcutaneous SML is 33% and inadequate excision puts you at risk of metastasis and possibly fatal disease due to recurrent lesions that tend to be larger and involve deeper structures. Oliver et al. (1991) reported that the risk of local recurrence is related to the adequacy of the excision, while the metastatic diffusion is linked to the depth, the histological degree of the SML and the DNA content [9]. Jensen et al. (1996) described their only patient with local recurrences due to incomplete margins after the initial surgery [10]. However, if the microscopic margins are negative, then relapses and survival are not affected by increasing the margin of excision of the soft tissues.
Therefore, this rare neoplasm of smooth muscle must be diagnosed at the time of the first surgery. A large local excision is recommended and it would seem that narrow margins with a tumor-free plan should be sufficient; however, all patients must be followed carefully. A wrong preoperative diagnosis is common as it is often confused with other formations. The typical histological picture is dominated by the proliferation of "spindle cells", characterized by an elongated nucleus with abundant cytoplasm, and by positivity for actin and myoglobin. The core is usually central, with rounded edges or with a characteristic cigar shape. Perinuclear vacuoles may be noted in some cells. In more undifferentiated tumors the nucleus is larger, hyperchromatic and often loses its central position. Multinuclear giant cells are common. The appearance of the cytoplasm varies according to the degree of differentiation: the less differentiated cells have numerous well-oriented myofibrils, identifiable with Masson's trichrome staining as longitudinal lines to the cell, red in colour and parallel to each other; in the less differentiated cells the longitudinal striations are less numerous and with a less rigorous orientation. Glycogen is present in the cytoplasm.
As for the ultrastructural characteristics, the differentiated LMS have nuclei with deep grooves and numerous and thin well-oriented myofilaments. Large pinocytic vesicles and intercellular connections are identified; the basal lamina affects the entire cell membrane. The presence of these characteristics is a diagnosis of smooth muscle cells. Less differentiated tumors show loss of myofilaments, the wrinkled endoplasmic reticulum, and free ribosomes take on greater prominence. The basal lamina also can be incomplete. In SMLs of subcutaneous tissue, small in size, necrosis and bleeding are generally rare. Giant cells may be present, but they are never numerous, mitotic figures are frequent, but these tumors are moderately differentiated. The identification of muscle antigens by immunohistochemistry, such as desmin, can help in the diagnosis of LMS. Subcutaneous leiomyosarcomas generally have a favorable prognosis. Local recurrences are frequent (50%). The treatment of choice is an extensive resection of the neoplasm. We report here the case of a patient with a giant subcutaneous leiomyosarcoma of the right thigh FNCLCC (Fèdèration Nationale des Centers de Lutte Contre le Cancer) with score 2, differentiation 1, mitosis 1, necrosis 0.
Case Report
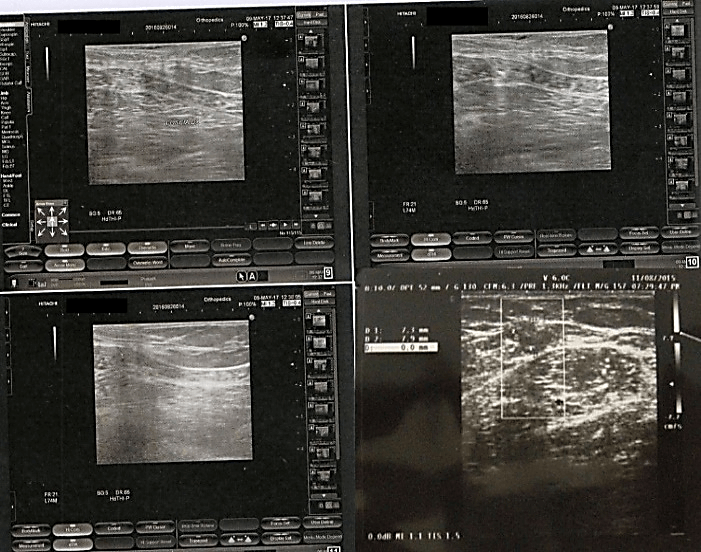
58-year-old female patient with asthma syndrome operated ten years before uterine myomectomy, has an asymmetrical, bulky tumor on the medial face of the right thigh, in the sub-groin area that had grown in about 10 years. The patient had 2 children and did not have a family history of cancer and currently in apparent well-being. He was not addicted to tobacco or alcohol. Physical examination at the time of hospitalization revealed a tumor of about 8 x 3 cm in size. The lesion that had grown in recent weeks had also become painful. The overlying skin had appeared reddish with vascular congestion, but without ulceration. It was not accompanied by regional lymphadenopathy. The ultrasound examination is shown in (Figure 1) and description. A lipoma was diagnosed and therefore without performing further investigations the patient underwent exeresis under local anesthesia.
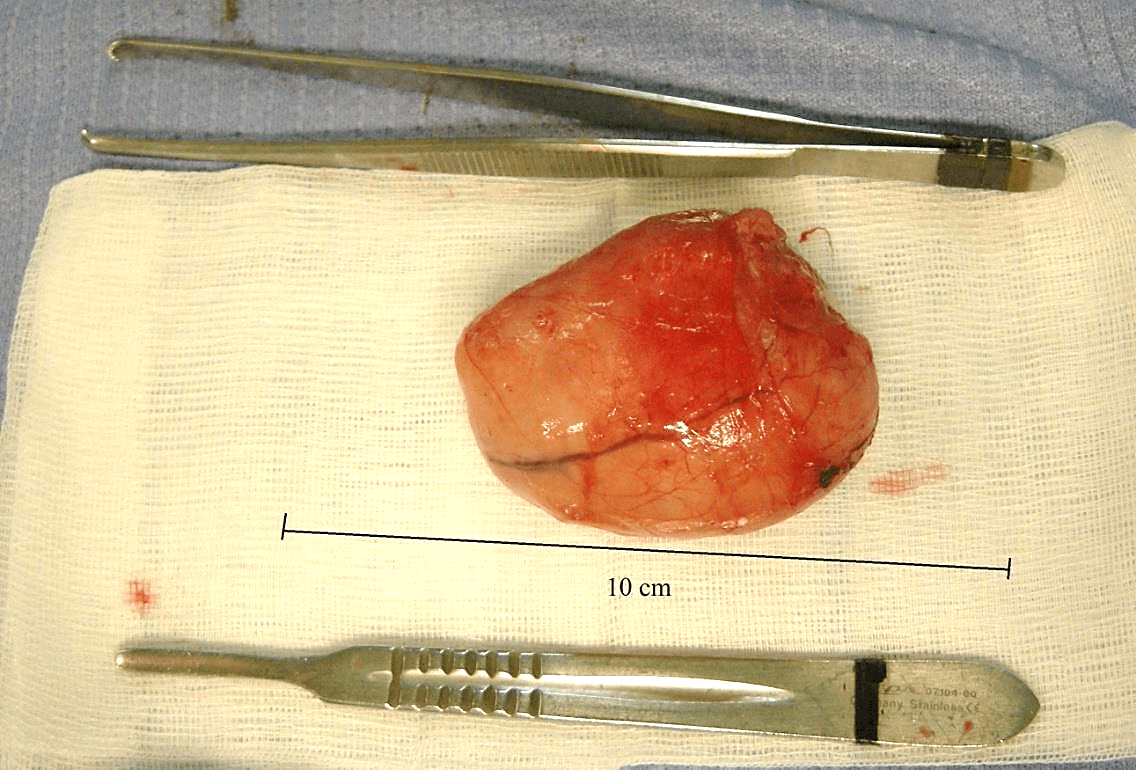
The resection exceeded the macroscopic margins of the tumor by 2 cm. The section margin of the tumor, which measured 8 x 3.1 cm, was yellowish-white in color, without central necrosis and hemorrhage on its cutting surface (Figure 2), with a hard-elastic consistency.
Figure 1: Ultrasound: Presence in the subcutaneous seat along the medial face of the proximal III of the right thigh, with an iso-hypo-echogenic oval solid nodular formation of about 80 x 31 mm with regular margins, of elastic soft consistency, mobile on the surface and deep, moderately vascularized, to be referred to lipoma at first instance.
Figure 2: Macroscopic aspect of the lesion.
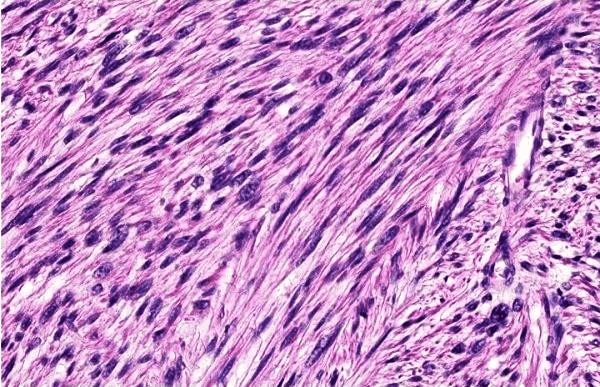
Routine histological examination with hematoxylin-eosin revealed that the tumor consisted of fusiform cells arranged in sheets and bundles bundled with cigar cores (Figure 3). Giant tumor cells have occasionally been found. Mitotic figures were scarce, around 1 per field at high magnification. All margins were free. The histological study was performed with S100 - / ++ /; Desmin - / ++ /; SMA - / ++ /. In immunohistochemistry (IHC), cancer cells tested positive for smooth muscle actin (SMA) (+), Desmina (+) and Ki-67 (monoclonal antibody, which reacts with a nuclear antigen expressed in all phases of the cell cycle except G0) (proliferative index = 20%), but negative for S-100 (-) (Figure 4 ab), a mitotic index between 5 and 9 MF x 10 HPF and detected a moderately differentiated leiomyosarcoma. According to the FNCLCC classification: score 2, differentiation 1, mitosis 1, necrosis 0.
Figure 3: Histological sample of the excised specimen (hematoxylin-eosin stain 40x).
Figure 4: A) Tumor cells positive for SMA (40x). B) Tumor cells showing cytoplasmic positivity for SMA (40x).
Following the Histopathological diagnosis and the preoperative diagnostic error, the patient underwent reoperation for the enlargement of the excision with 5 cm of surgical margins, with ligation and section of the Safena Internal vein at the origin, which on this occasion determined the removal of the perilymphoglandular subcutaneous adipose tissue, two lymph nodes, and the deep muscle fascia, which were found to be free of neoplasia. The patient underwent an oncological follow-up for 5 years without a demonstration of recurrence or distant metastasis. At the last follow-up visit, the clinical and laboratory examination (X-rays, Nuclear Magnetic Resonance, and Bone Scanning) did not reveal any sign of recurrence or metastasis of the tumor. The patient is currently able to carry out her daily activities.
Discussion
Subcutaneous leiomyosarcomas are often well-circumscribed nodular lesions associated with clinically normal overlying skin and a hemispherical skin elevation ranging from 0.6 to 5 cm in diameter [2, 8, 11]. Dermal leiomyosarcomas, on the other hand, usually appear as small compact nodules less than 2 cm in size, but their subcutaneous counterparts are larger with an average tumor size of 4 cm at presentation [4, 10, 12]. The median duration from start to presentation is 12 months [4]. The clinical presentation is not specific and leiomyosarcomas can be misdiagnosed for clinical reasons [6, 13]. Since some tumors are not firmly attached to surrounding structures, they can be considered harmless and excoriate or ulcerated as if they were benign [2, 9]. Awareness of the misleading characteristics of this tumor is, therefore, necessary to avoid delays in diagnosis and treatment.
In our clinical case, the consistency of the neoplasm, upon intraoperative macroscopic examination, made us doubt the preoperative diagnosis of lipoma, despite the presence of a capsule (Figure 2). According to what was reported by Jena S. et al. (2014) surgical excision is the primary treatment for subcutaneous SML [2]. Until now, a large local excision with 3-5 cm of surgical margins has been recommended, but recent work has shown the success of treatment with a narrow margin of excision as well as with the micrographic excision of Mohs. Inadequate excision or local excision without adequate margin leads, however, to recurrence and increases the risk for metastatic disease and potentially lethal disease.
In another case reported by Angeloni M. et al. (2008) an excisional biopsy of the lesion performed previously on the patient did not clarify the true nature of the lesion since the described diagnosis was "unspecified soft tissue sarcoma" [1]. According to a Scandinavian study of 225 patients with non-visceral soft tissue leiomyosarcoma local treatment is considered adequate when the surgical margin is wide or when it has been completed with adjuvant radiation therapy [13, 14]. An intralesional surgical margin with or without adjuvant radiotherapy and a marginal halo surgical margin (independent of tumor depth) is considered an inadequate local treatment. Postoperative radiation therapy is recommended after marginal or intralesional surgery, generally at a dose of 50 Gy in 2-Gy fractions.
In the study of Svarvar et al. 97 of 225 patients (43%) were reported to have undergone first surgery before extensive excision, of which 9.6% of 97 patients had had intralesional margin treatment and 32% had had margin excision surgical, only 59% of all patients had a large surgical margin. Demonstrating that inadequate surgical treatment does not correlate with the appearance of metastases but with local recurrence. The size of the neoplasm has proven to be a significant prognostic factor for metastasis, local recurrence, and death. Hence the need for a correct preoperative diagnosis and a correct surgical approach. It is well known, that an adequate surgical margin is the most important factor for local control. Clinical findings can lead to confusion with other skin cell tumors. A differential histological diagnosis must be made against atypical fibroxanthoma (whose cells are usually negative for Actina smooth muscle), dermatofibrosarcoma protuberans (CD34 positive cells), angiosarcoma and squamous cell carcinoma (cytokeratin positive cells) or melanoma (cells marked positive for S-100 protein) (Table 1) [6, 15].
Table 1: Selected immunohistochemical stains a san aid to the diagnosis of spindle cell tumors of the skin.
|
Variable |
Atypical fibroxanthoma |
Dermatofibroma protuberans |
Angiosarcoma |
Spindle cell SCC |
Leiomyosarcoma |
Our Case |
|
S100 |
- |
- |
- |
- |
R |
- |
|
Desmin |
- |
- |
- |
- |
+ |
+ |
|
CD34 |
V |
+ |
V |
- |
R |
- |
|
Factor VIII |
- |
- |
+ |
- |
- |
- |
|
CK |
- |
- |
- |
+ |
V |
- |
|
EMA |
- |
- |
- |
V |
- |
- |
|
SMA |
V |
V |
V |
R |
+ |
+ |
SCC: squamous cell carcinoma; CK: cytokeratin; EMA: epithelial membrane antigen; SMA: smooth muscle actin; R: rarely positive; V: variable; -: negative; +: positive.
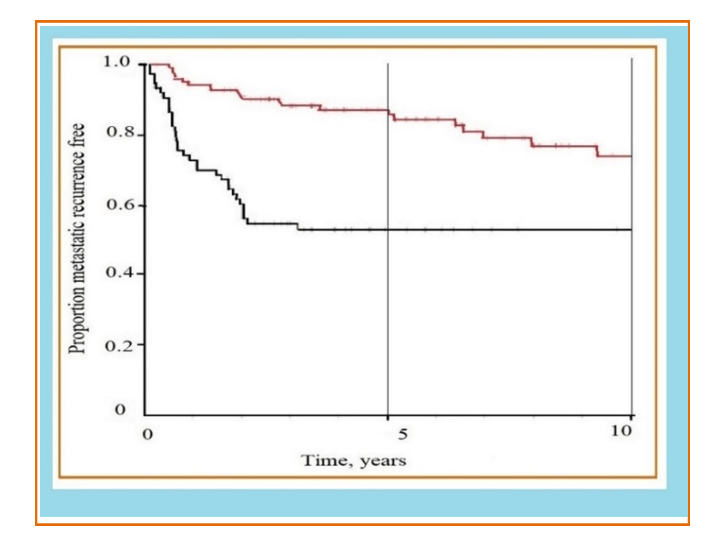
Figure 5: Survival without metastatic recurrence is illustrated by tumor size. The red line indicates small tumors (≤ 5cm), and the black line illustrates large tumors (>5cm). At 0 years, 5 years and 10 years, the number of patients at risk was 201, 82 and 33, respectively. (from Svarvar C. et al. modified, 2006).
Ciurea M.E. et al. (2014) reported a case in which an LMS had been clinically diagnosed as dermatofibrosarcoma [12]. Pasquali P. et al. (2015) reports the case of an 84-year-old patient in whom the soft tissue ultrasound shows features compatible with a hematoma of the forearm and a second case in the left lumbar area interpreted as a skin metastasis of another tumor [13]. Clinically, these lesions are also interpreted as pyogenic granuloma, basal cell or epidermoid carcinoma, fibroids, nodular lesions or those suggestive of metastasis as in the case reported by Pasquali et al. The histological study allows diagnostic affiliation by requiring a second intervention with a greater risk of metastasis and local recurrence (Figure 5). Angeloni et al. report in their work that Miyajima et al. related tumor size to advanced staging and found that they were the only factors that independently correlated with reduced survival [16-19].
Conclusions
Leiomyosarcoma is a rare entity whose clinical presentation may appear non-specific, making diagnosis difficult. Clinical diagnosis is difficult and can only be determined after a histopathological examination [8]. Differential diagnosis must be made with any solitary nodule developed on the skin or subcutaneous: lipoma, dermatofibroma, dermatofibrosarcoma, neurofibroma, etc. In most cases, histological differentiation with specific immunohistochemical markers is necessary.
In conclusion, this review indicates that when dealing with LMS involving the skin it is appropriate to correctly recognize and indicate the depth of the subcutaneous extension, as even a minimal subcutaneous involvement, or more deeply with extension in the fat, can be associated with metastasis remotely. Due to the potential for local recurrence and/or metastasis, careful and prolonged clinical follow-up is strongly recommended in these cases. Although the diagnosis is delayed and the size of the soft tissue leiomyosarcomas is significant and can adversely affect the final result, effective recovery can be expected. Physicians should, therefore, be aware of the misleading characteristics of this tumor to avoid delays in diagnosis and treatment.
Article Info
Article Type
Case ReportPublication history
Received: Tue 07, Apr 2020Accepted: Wed 22, Apr 2020
Published: Wed 29, Apr 2020
Copyright
© 2023 Alessandro Crisci. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSCR.2020.02.06
Author Info
Alessandro Crisci Michela Crisci Raffaele D’Adamo
Corresponding Author
Alessandro CrisciDepartment of Medicine, Surgery and Dentistry “Salernitan Medical School”, University of Salerno, Fisciano (SA), Italy
Figures & Tables
Table 1: Selected immunohistochemical stains a san aid to the diagnosis of spindle cell tumors of the skin.
|
Variable |
Atypical fibroxanthoma |
Dermatofibroma protuberans |
Angiosarcoma |
Spindle cell SCC |
Leiomyosarcoma |
Our Case |
|
S100 |
- |
- |
- |
- |
R |
- |
|
Desmin |
- |
- |
- |
- |
+ |
+ |
|
CD34 |
V |
+ |
V |
- |
R |
- |
|
Factor VIII |
- |
- |
+ |
- |
- |
- |
|
CK |
- |
- |
- |
+ |
V |
- |
|
EMA |
- |
- |
- |
V |
- |
- |
|
SMA |
V |
V |
V |
R |
+ |
+ |
SCC: squamous cell carcinoma; CK: cytokeratin; EMA: epithelial membrane antigen; SMA: smooth muscle actin; R: rarely positive; V: variable; -: negative; +: positive.

References
- Jena S, Bhattacharya S, Roy S (2014) Giant Subcutaneous Leiomyosarcoma of Anterior Abdominal Wall. Case Rep Surg 2014: 308916. [Crossref]
- Oliver GF, Reiman HM, Gonchoroff NJ, Muller SA, Umbert IJ (1991) Cutaneous and subcutaneous leiomyosarcoma: a clinicopathological review of 14 cases with reference to antidesmin staining and nuclear DNA patterns studies by flow cytometry. Br J Dermatol 124: 252-257. [Crossref]
- Andrè MC, Antunes JV, Reis MD, Filipe PL, Almeida LM (2011) Cutaneous leiomyosarcoma on the trunk. An Bras Dermatol 86: 999-1002. [Crossref]
- Salemis NS (2013) Recurrent subcutaneous trunk leiomyosarcoma: management and review of the literature. J Nat Sci Biol Med 4: 238-242. [Crossref]
- Pijpe J, Broers GH, Plaat BE, Hundeiker M, Otto F et al. (2002) The relation between histological, tumor-biological and clinical parameters in deep and superficialleiomyosarcoma and leiomyoma. Sarcoma 6: 105-110. [Crossref]
- Lee KC, Kim MS, Choi H, Na CH, Shin BS (2013) Rapid Growing Superficial Cutaneous Leiomyosarcoma of the Face. Ann Dermatol 25: 237-241. [Crossref]
- Liao WC, Lin JT, Ma H, Chen WYK, Yeh FL (2006) Primary cutaneous leiomyosarcoma. J Plastic Recondtrut Anesthetic Sur.
- Ciurea ME, Georgescu CV, Radu CC, Georgescu CC, Stoica LE (2014) Cutaneous leiomyosarcoma-Case Report, J Med Life 7: 270-273. [Crossref]
- Pasquali P, Freites-Martinez A, Hernandez C, Valero Lugo J, Fortuño Á (2015) Cutaneous and subcutaneous leiomyosarcoma: report of two cases. Dermatol Online J 21. [Crossref]
- Jensen ML, Jensen OM, Michalski W, Nielsen OS, Keller J (1996) Intradermal and subcutaneous leiomyosarcoma: a clinicopathological and immunohistochemical study of 41 cases. J Cutan Pathol 23: 458-463. [Crossref]
- Pol RA, Dannenberg H, Robertus JL, van Ginkel RJ (2012) Cutaneous leiomyosarcoma arising in a smallpox scar. World J Surg Oncol 10: 148. [Crossref]
- Massi D, Franchi A, Alos L, Cook M, Di Palma S et al. (2010) Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology 56: 251-262. [Crossref]
- Svarvar C, Böhling T, Berlin O, Gustafson P, Follerås G et al. (2007) Clinical Course of Nonvisceral Soft Tissue Leiomyosarcoma in 225 Patients from the Scandinavian Sarcoma Group. Cancer 109: 282-291. [Crossref]
- Geraci G, Pisello F, Sciumè C, Li Volsi F, Platia L et al. (2007) Unusual acute onset of pedunculated extragastric leiomyosarcoma. Case report. G Chir 28: 265-269. [Crossref]
- Akshay Bali, Ranjit Kangle, Maitrayee Roy, Bhagyashree Hungund (2013) Primary cutaneous leiomyosarcoma: a rare malignant neoplasm. Indian Dermatol Online J 4: 188-190. [Crossref]
- Angeloni M, Muratori F, Magarelli N, Chalidis BE, Ricci R et al. (2008) Exophytic growth of a neglected giant subcutaneous leiomyosarcoma of the lower extremity. A case report. Int Semin Surg Oncol 5: 11. [Crossref]
- Enchev E, Dimitrov E, Minkov G, Nikolov St, Ovcharov I (2017) Leiomyosarcoma in A Patient with Accessory Kidney - Clinical Problems, Imaging and Treatment. J Sur 182.
- Weiler L, Poulalhon N, Slama A, Guillaud-Bataille M, Thomas L (2016) Isolated cutaneous leiomyosarcoma revealing a novel germline mutation of fumarate hydratase gene. Br J Dermatol 175: 1104-1106. [Crossref]
- Kalkonde MY, Venkatarajan S, Dang L, Yang DJ, Madjidi A et al. (2010) A case of desmoplastic leiomyosarcoma: a rare variant of cutaneous leiomyosarcoma. Dermatol Online J 16: 4. [Crossref]
