Lack of Pathological Response of Rectal Cancer to Neoadjuvant Chemoradiotherapy is Associated with Poorer Long-Term Oncological Outcomes
A B S T R A C T
Purpose: Tumor regression scores are used to evaluate local response to preoperative treatment. Complete pathological response (tumor regression score=0) is associated with excellent prognosis. In this study we evaluated the prevalence and impact of poor-to-no pathological response to neoadjuvant treatment (tumor regression score=3), based on a recently revised grading system on long term oncologic outcomes among rectal cancer patients.
Methods: This retrospective study included rectal cancer patients who received. neoadjuvant chemoradiotherapy and surgical resection at our medical center. Pathological specimens were ren evaluated and graded (grades 0-3) based on the revised tumor regression scores. Disease free survival and cancer specific survival rates were documented and matched with the patients’ tumor regression scores.
Results: Initially 78 patients were included. 6 patients were later excluded from the long-term follow up since they developed disease progression during neoadjuvant treatment. Among the other 72 patients, 38 (52.8%) were classified as tumor regression score=3 (no response) and 34 (47.2%) tumor regression score=0-2 (any level of response). Conversion from laparoscopy to open surgery was higher in the tumor regression score=3 group (21% vs. 2.9%, p=0.02). Follow-up ranged from 5 months to 12 years. Nine (12.5%) patients experienced disease recurrence, 8 with tumor regression score=3. Most recurrences were metastases to liver and lungs. Disease free survival was lower in tumor regression score=3 patients as compared to tumor regression score=0-2.
Conclusions: Non-responders to neoadjuvant therapy are at higher risk for disease recurrence, conversion to open surgery and disease-related mortality. Systemic recurrence of tumor regression score=3 tumors suggests an aggressive biology of these tumors. Further studies are needed to characterize tumors that are less likely to respond to radiotherapy and to personalize preoperative treatment decisions.
Keywords
Tumor regression score, neoadjuvant, response, rectal cancer
Introduction
Colorectal cancer is the third most common malignant neoplasm and the fourth cause of malignancy-related mortality worldwide, with one-third of the tumors located in the rectum [1]. Currently, the treatment of choice for locally advanced rectal cancer (stage II to III) is preoperative chemo-radiotherapy (CRT) followed by resection of the rectum and total mesorectal excision [2, 3]. This multimodal therapy has been recommended by the National Comprehensive Cancer Network (NCCN) since 2006 [4, 5] and became the standard of care for patients with locally advanced rectal cancer (LARC), after it was shown to reduce local recurrence and improve survival when combined with adjuvant chemotherapy [4-7]. Tumor response to neo-adjuvant CRT varies among patients and ranges from complete response, to partial response, to virtually no response or even tumor progression [8]. The Modified Ryan Scheme for Tumor Regression Score (Table 1) was recommended for routine use by the College of American Pathologists [9]. Tumor Regression Score (TRS) is a well-accepted measure of the local response of malignant rectal tumors to preoperative CRT. TRS is determined by the pathologist on a scale of 0-3 according to a well-defined set of histopathological features associated with radiation induced tissue changes [10]. TRS 0 indicates pathological complete response (pCR), TRS 1-2 indicates different grades of partial response and TRS 3 indicates poor or no response. Correlation between the level of tumor regression and oncological outcome is quite evident [11-14]. PCR is evident in up to 20% of patients and was shown to be associated with long-term oncologic benefits, including excellent DFS and OS rates [17-19]. Emerging data suggest that patients with partial/intermediate response (varying tumor infiltration identified in the post-radiotherapy surgical specimen) will also experience favorable long-term oncological outcomes [20-22]. Studies focusing on the non-responders' group (TRS 3) are less common. Many studies grouped partial responders and non-responders together comparing them to complete responders while recent data suggest that partial responders are more similar to complete responders than they are to non-responders in terms of long-term outcomes [14, 23-25]. The aim of this study was to evaluate the prevalence and impact of poor pathological response (TRS 3) based on the recently adopted TRS grading system by the college of the American pathologists on long-term oncologic outcomes (DFS and cancer specific survival (CSS) among rectal cancer patients treated with neoadjuvant CRT before surgical resection [9].
Table 1: Modified Ryan Scheme for Tumor Regression Score.
|
Description |
Tumor Regression Score |
|
No viable cancer cells (complete response) |
0 |
|
Single cells or rare small groups of cancer cells (near complete response) |
1 |
|
Residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells (partial response) |
2 |
|
Extensive residual cancer with no evident tumor regression (poor or no response) |
3 |
Methods
This retrospective cohort study was approved by the Institutional Review Board of Meir Medical Center and performed in accordance with the ethical standards of the Declaration of Helsinki. The medical records of all patients diagnosed with rectal adenocarcinoma who underwent preoperative CRT before resection surgery in our medical center, from July 2005 to February 2018 were reviewed. Rectal cancer was diagnosed via histological examination of a biopsy sample corroborating adenocarcinoma. Patients with distant metastasis discovered before or during surgery and patients with positive resection margins (either macroscopic or microscopic) were not included in the long-term evaluation due to the fact that metastatic disease and incomplete resection are powerful predictors of prognosis and could confound the effect of TRS on long term oncologic outcomes [27, 28]. Tumoral response to the neoadjuvant treatment was determined from specimens retrieved from the archives. Slides were reviewed and classified by the same pathologist based on the well-validated Tumor Regression Score (TRS) [9].Demographic and disease-related data (age, gender, clinical and pathological staging, date of surgery, surgical approach, neo-adjuvant regimen date and duration, surgery interval, administration of adjuvant chemotherapy and resection margins status (R0 - negative margins, R1 - microscopic involvement and R2- macroscopic residual tumor) were reviewed and extracted from electronic and paper medical files. Pre-operative clinical staging and post-operative pathological staging were determined according to the TNM staging system of the combined American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) updated in 2017 [29]. All patients received CRT at a median radiation dose of 50.4 Gy accompanied by fluoropyrimidine-based chemotherapy as a radio-sensitizing agent. Interval between completion of neoadjuvant CRT and surgery was 6-8 weeks after long course radiotherapy and 4-6 weeks after short course radiotherapy. Patients underwent anterior resection, low anterior resection or abdominoperineal resection, all of which included total mesorectal excision. Adjuvant chemotherapy was administered to a subset of patients according to pathological staging. The multimodality treatment administered to the patients, including indications, course, and components was in consonance with the NCCN Rectal Cancer clinical practice guidelines [4]. Data on long-term outcomes included recurrent disease, date and form of recurrence (local or systemic), and date and cause of death. Post-operative surgical follow-up consisted of routine physical examination, blood levels of carcinoembryonic antigen, computed tomography imaging and colonoscopy based on a local protocol.
I Data Analysis
The cohort was divided into two groups according to TRS: Responders included all patients with any level of response to neo-adjuvant CRT (TRS 0-2) and Non responders included patients with no response to neo-adjuvant CRT (TRS 3). Univariate comparison analysis was performed to ensure sufficient similarity between groups. The primary study outcome was disease recurrence. Secondary outcomes were conversion to open surgery, systemic recurrence and disease-related mortality (DRM). Long-term oncological outcomes were compared between the groups. DFS was defined as the time between surgery and a proven evidence for local or distant disease recurrence. Cancer specific survival was defined as the time between surgery and cancer related mortality. DFS and CSS were assessed and compared between the groups using Kaplan-Meier survival analysis. Statistical analysis was performed using Statistical Package for Social Sciences Software (SPSS, Version 25.0, IBM, Armonk, NY, USA). Data are described as numbers and percentage for nominal parameters and as mean and standard deviation for continuous variables. In the univariate comparison analysis, chi-square and Fisher's Exact test were used to analyze categorical variables, and student's T-test was used for continuous variables. For more than two group comparison, Bonferroni correction was used. Kaplan-Meier survival analysis was used to evaluate long-term, disease-free-survival and CSS according to TRS group. Differences in Kaplan-Meier analysis was determined using the Mantel-Cox Log Rank test. Statistical significance was set at P-value of 0.05 or less.
Results
Seventy-eight patients with rectal cancer who underwent preoperative CRT followed by surgical resection at our institution, from July 2005 to February 2018 were identified and included in the study. Six patients with a primary evaluation of a respectable, curable disease were treated with nCRT, but experienced disease progression to an extent that rendered their tumors incurable with surgical resection. as one patient had liver metastasis discovered during surgery. In 3 patients, local disease extended to involve the pelvic walls, making complete resection impossible and 2 patients had microscopically-involved surgical margins. Interestingly, all had TRS 3 tumors and experienced DRM. Tgus, the final study cohort included 72 patients.
I Patients, feasibility and safety
Of the 72 patients included in the study, 44 (61.1%) were males and 28 (38.8%) were females. Mean age was 63.1 ± 12.7 years (standard deviation). According to pre-operative evaluation, all patients had stage II or III rectal cancer (LARC) with the exception of one who had stage I and underwent neo-adjuvant CRT for sphincter preservation. A total of 65 (90.2%) patients underwent long course radiotherapy, and 7 (9.7%) short course radiotherapy. Surgical approaches were 24 (33.3%) open, 39 (54.1%) laparoscopic, and 9 (12.5%) began laparoscopically and converted to open procedures. All 72 patients had R0 resection margins. 31 (43.1%) patients received adjuvant chemotherapy. Follow-up ranged from 5 months to 12 years (mean 59.3 ± 42.5 months).
II Response According to Trs
Of 72 patients, 15 (20.8%) were classified as TRS 0, 6 (8.3%) as TRS 1, 13 (18.1%) as TRS 2 and 38 (52.8%) as TRS 3. There were 34 (47.2%) patients in the responders' group (TRS 0-2) and 38 (52.8%) in the non-responder’s group (TRS 3). Demographics, stage and treatment parameters were comparable between the two groups (Table 2). These parameters included age, gender, pre-operative staging, course of radiotherapy (long vs. short), whether adjuvant chemotherapy was administered and duration of follow-up. This similarity enabled the comparison of long-term outcomes.
III Conversion to Open Surgery
The groups did not differ in the number of patients who were scheduled to laparoscopic approach. However, conversion to open surgery was significantly higher in the TRS=3 group. (21% vs 2.9%, P=0.02).
IV Long-Term Outcomes
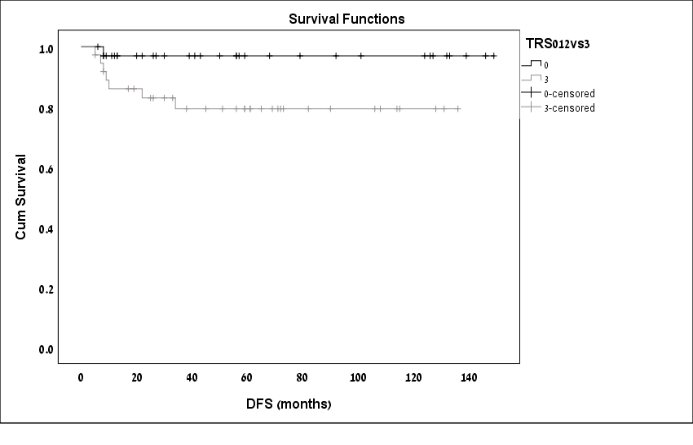
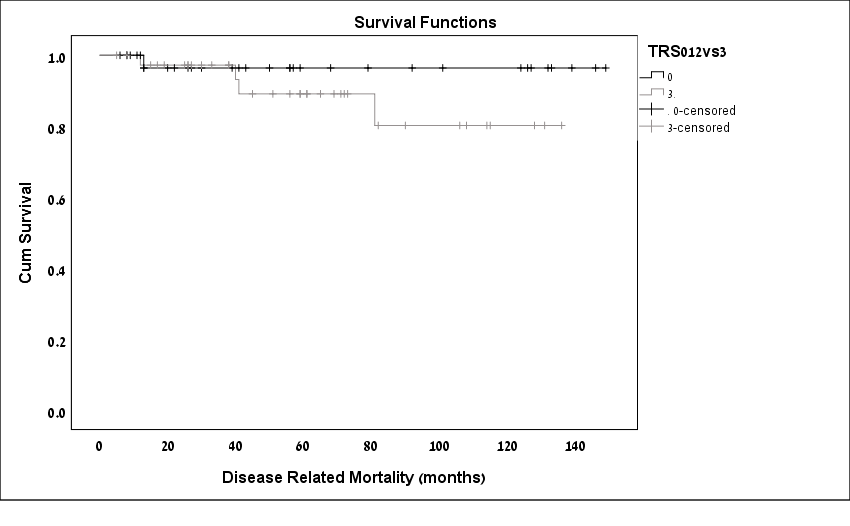
Comparison of long-term outcomes between the responders and the non-responders are displayed in Table 3. Non-responders developed disease recurrence in a higher rate [(21%) vs. (2.9%), n respectively; p=0.02). Kaplan-Meier DFS curves demonstrated significantly shorter DFS rates among the non-responders as compared to the responders (p=0.04; Figure 1). Most of the recurrences were in the form of distant metastasis to liver and lungs. Among the 9 patients with recurrent disease, 6 (66.6%) had systemic recurrence, 5 of whom were in the group patients of the non-responders (TRS 3). Disease related mortality Overall, 5 patients (6.94%) experienced DRM, all as a result of recurrent disease. Four were TRS 3 and 1 was TRS 1. Kaplan-Meier survival analysis was used to evaluate cancer-specific survival according to TRS. Kaplan-Meier DRM curves demonstrated poorer CCS rates among the non-responders; however, the difference between the groups was not statistically significant (P= 0.24; Figure 2).
Table 2: Univariate comparison between responders and non-responders.
|
Clinical and disease-related features |
Responders (TRS 0-2) |
Non-responders (TRS 3) |
P-value |
|
|
N = 34 (47.2%) |
N = 38 (52.8%) |
|||
|
Gender |
Male |
22 (64.7%) |
22 (57.9%) |
0.554 |
|
Female |
12 (35.3%) |
16 (42.1%) |
||
|
Mean age (years) |
63.08 ± 13.87 |
63.18 ± 11.44 |
0.668 |
|
|
Pre-operative staging |
Stage 1 |
1 (2.9%) |
0 (0%) |
0.894 |
|
Stage 2 |
16 (47.1%) |
19 (50%) |
||
|
Stage 3 |
17 (50%) |
19 (50%) |
||
|
Radiotherapy |
Short course |
3 (8.8%) |
4 (10.5%) |
0.808 |
|
Long course |
31 (91.2%) |
34 (89.5%) |
||
|
Surgical approach |
Lap (intent to treat) |
23 (67.7%) |
25 (65.8%) |
0.86 |
|
Open |
11 (32.3%) |
13 (34.2%) |
0.86 |
|
|
Lap converted to open |
1 (2.9%) |
8 (21%) |
0.02 |
|
|
Post-operative staging |
Stage 0 |
12 (35.3%) |
0 (0%) |
0.0001 |
|
Stage 1 |
9 (26.5%) |
11 (28.9%) |
0.815 |
|
|
Stage 2 |
8 (23.5%) |
17 (44.7%) |
0.059 |
|
|
Stage 3 |
5 (14.7%) |
10 (26.3%) |
0.226 |
|
|
Adjuvant chemotherapy |
Yes |
12 (35.3%) |
19 (50%) |
0.208 |
|
No |
22 (64.7%) |
19 (50%) |
||
|
Mean follow-up (months) |
|
59.97 ± 48.70 |
58.76 ± 36.76 |
0.914 |
Table 3: Comparison of long-term outcomes.
|
Long-term outcomes |
TRS 0-2 (responders) |
TRS 3 (non-responders) |
P-value |
|
|
N= 34 (47.2%) |
N= 38 (52.8%) |
|||
|
Recurrence |
1 (2.9%) |
8 (21%) |
0.03 |
|
|
Distant recurrence |
1 (2.9%) |
5 (13.15%) |
0.11 |
|
|
Prognosis |
|
|
|
|
|
Living without disease |
30 (88.2%) |
26 (68.4%) |
0.052 |
|
|
Living with disease |
0 (0%) |
4 (10.5%) |
||
|
Died without disease |
3 (8.8) |
4 (10.5%) |
||
|
Died of disease |
1 (2.9%) |
4 (10.5%) |
||
Figure 1 : Kaplan-Meier Disease Free Survival curves by patients TRS.
Figure 2 : Kaplan-Meier cancer specific survival curves by patients TRS.
Discussion
Following years of research and numerous large-scale studies, it is safe to say that neo-adjuvant CRT followed by radical surgical resection is a beneficial treatment strategy for patients with LARC [6,7]. The effects of this treatment are tumor downstaging, increased likelihood of curative resection, and reduced risk of local recurrence [3]. However, with routine clinical use of preoperative CRT, it was evident that tumor response to this treatment ranges from complete to absent response [8, 15, 26]. This led to the development of various scales designed to quantify the level of response to neoadjuvant therapy [14]. Many studies have shown that response to nCRT correlates with prognosis and specifically DFS [11-13]. When evidence regarding patients with pCR begun to accumulate, suggesting excellent DFS and OS rates, there was a substantial shift in focus towards predicting and improving the rate of pCR [16, 17, 19]. The extremely favorable prognosis of these patients led to the concept of "watch and wait" approach in which patients with pCR avoid radical surgery [30, 31]. Successful implementation of the ‘watch and wait’ approach is probably the best evidence for the beneficial effect of nCRT.
However, even though nCRT results in clinically significant tumor regression for most patients, a considerable portion of patients exhibit very poor or no response to treatment. The rate of non-responders in current literature is about 20-30% [14, 32]. However, 53% of the patients in the current study had poor to no response to nCRT. The higher non-response rate is probably related to the use of different tumor regression scales. All previous studies shared the same remarkable limitation, which was the absence of a uniform method to rat tumor response [14, 23]. Several systems to quantify tumor response have been advocated, complicating implementation of a universal, standard definition for tumor regression. The current study used the Modified Ryan Scheme for Tumor Regression Score, due to its high validity, good inter-observer reproducibility, and because it is the official pathologic tumor staging scale for resected specimens, recommended by the College of American Pathologists [9, 10, 33]. A retrospective study by Park et al.found that the 5-year RFS and OS rates progressively increased relative to poor response to complete response, and that TRS was the most important predictor of oncologic outcome after radical resection; more important even than clinical staging [33]. In the Modified Ryan Scheme, the TRS 3 group includes non-responders as well as poor responders, while in other regression scales this group includes non-responders only [14]. This may explain the high rate of non-responders in our study. Our results indicate that, when compared with patients who had any level of response to nCRT (TRS 0-2), rectal cancer patients with a poor response (TRS 3) are at significantly higher risk for disease recurrence and tend to develop distant metastases following surgical resection. With the exception of one patient from the responders’ group who died as a result of recurrent metastatic disease, the negative long-term outcomes (recurrence and DRM) occurred attributed to the non-responders (TRS 3). Reviewing the course of the TRS 1 patient with recurrent disease, we saw that he was diagnosed with disseminated metastatic disease within 6 months after surgery, suggesting undiagnosed micro-metastases that were not apparent during the initial staging or surgery. Also noteworthy is this patient did not receive adjuvant chemotherapy. Despite the particulars of this case, other studies have reported that in the rare cases of recurrence among patients with pCR, the pattern of failure was systemic [33, 34]. Conversion from laparoscopic to open surgery was another differentiating factor. While there was no difference between the groups in the initial surgical approach, non-responders had a significantly higher rate of conversion from laparoscopic to open procedure. With responsive tumors, neo-adjuvant CRT can result in a smaller, mobile tumor which is easier to resect then a large, bulky tumor. In light of this, it is clear why the non-responders arrived to surgery with larger, less mobile tumors, making laparoscopic resection extremely difficult and sometimes impossible; thus, requiring conversion to open surgery. While analyzing the patients who did not meet the study inclusion criteria for long term follow up, we discovered an interesting pattern. Six patients with a primary evaluation of a resectable, curable disease, were treated with nCRT, but experienced disease progression to an extent that rendered their tumors incurable with surgical resection. During 4-6 weeks of CRT and an additional 6-8-week interval period, the malignancy progressed, as one patient had liver metastasis discovered during surgery. In 3 patients, local disease extended to involve the pelvic walls, making complete resection impossible and 2 patients had microscopically-involved surgical margins. We mention these patients because they all had TRS 3 tumors and experienced DRM. The uniqueness of this study lies in its focus on the long-term oncological outcomes of the non-responder TRS 3 patients. Other studies reached the same conclusions regarding the poor prognosis and high recurrence rate among non-responder patients [33-36]. The major limitation of this study is its retrospective nature and the relatively small sample size, which was probably the reason for our inability to demonstrate statistically significant differences in distant recurrence and DRM rates between the groups. Despite these limitations, we were able to substantiate our initial hypothesis regarding the higher recurrence rate of TRS 3 tumors. Another limitation lies in the fact that we did not evaluate other possible prognostic factors (such as lympho-vascular invasion, perineural invasion, CRM, distance from anal verge etc.). However, since none of these factors or other clinical or pathological factors were proved to be predictive in terms of response to neoadjuvant treatment this limitation is less of importance. To conclude, the primary finding of this study is that rectal cancer patients with poor or no response to neoadjuvant treatment based on the Modified Ryan Scheme for Tumor Regression Score demonstrate increased risk for non-curative resection (R1, R2 resection), conversion to open surgery, and poor long term, oncologic outcomes (recurrence and DRM), as compared with patients with any better level of response (TRS 0-2). The systemic mode of recurrence of TRS 3 tumors found in this study and others, suggests that these tumors have an inherently aggressive, malignant biologic phenotype that makes them less responsive to CRT and also increases their propensity to metastasize [24, 33, 34].
The need to expand therapeutic options is gaining relevance in LARC as part of a general shift in modern medicine towards personalized medicine. The variability of tumor response to irradiation has increased the need to find a way to predict the tumor's potential response, however, currently this does not exist. Even though many researchers have undertaken this challenge, until now, none of the potential predictive markers are accurate enough to change clinical treatment algorithms [37]. Studies to discover tumor characteristics associated with poor response, that can be identified at the preliminary diagnostic biopsy are urgently needed. With these patients lies the potential for developing different treatment strategies, which may lead to upfront surgery or to exchanging radiotherapy with systemic chemotherapy.
Acknowledgment
The authors thank Nava Jelin for statistical analysis and Faye Schreiber for language editing.
Conflicts of interest
None
Funding
None
Article Info
Article Type
Research ArticlePublication history
Received: Tue 22, Oct 2019Accepted: Thu 14, Nov 2019
Published: Tue 26, Nov 2019
Copyright
© 2023 Shmuel Avital. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2019.5.18
Author Info
Debora Kidron Lauren Lahav Liron Berkovich Moshe Mishaeli Nasreen Haj Yahya Shmuel Avital
Corresponding Author
Shmuel AvitalDepartment of Surgery B, Meir Medical Center, Kfar Saba, Israel
Figures & Tables
Table 1: Modified Ryan Scheme for Tumor Regression Score.
|
Description |
Tumor Regression Score |
|
No viable cancer cells (complete response) |
0 |
|
Single cells or rare small groups of cancer cells (near complete response) |
1 |
|
Residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells (partial response) |
2 |
|
Extensive residual cancer with no evident tumor regression (poor or no response) |
3 |
Table 2: Univariate comparison between responders and non-responders.
|
Clinical and disease-related features |
Responders (TRS 0-2) |
Non-responders (TRS 3) |
P-value |
|
|
N = 34 (47.2%) |
N = 38 (52.8%) |
|||
|
Gender |
Male |
22 (64.7%) |
22 (57.9%) |
0.554 |
|
Female |
12 (35.3%) |
16 (42.1%) |
||
|
Mean age (years) |
63.08 ± 13.87 |
63.18 ± 11.44 |
0.668 |
|
|
Pre-operative staging |
Stage 1 |
1 (2.9%) |
0 (0%) |
0.894 |
|
Stage 2 |
16 (47.1%) |
19 (50%) |
||
|
Stage 3 |
17 (50%) |
19 (50%) |
||
|
Radiotherapy |
Short course |
3 (8.8%) |
4 (10.5%) |
0.808 |
|
Long course |
31 (91.2%) |
34 (89.5%) |
||
|
Surgical approach |
Lap (intent to treat) |
23 (67.7%) |
25 (65.8%) |
0.86 |
|
Open |
11 (32.3%) |
13 (34.2%) |
0.86 |
|
|
Lap converted to open |
1 (2.9%) |
8 (21%) |
0.02 |
|
|
Post-operative staging |
Stage 0 |
12 (35.3%) |
0 (0%) |
0.0001 |
|
Stage 1 |
9 (26.5%) |
11 (28.9%) |
0.815 |
|
|
Stage 2 |
8 (23.5%) |
17 (44.7%) |
0.059 |
|
|
Stage 3 |
5 (14.7%) |
10 (26.3%) |
0.226 |
|
|
Adjuvant chemotherapy |
Yes |
12 (35.3%) |
19 (50%) |
0.208 |
|
No |
22 (64.7%) |
19 (50%) |
||
|
Mean follow-up (months) |
|
59.97 ± 48.70 |
58.76 ± 36.76 |
0.914 |
Table 3: Comparison of long-term outcomes.
|
Long-term outcomes |
TRS 0-2 (responders) |
TRS 3 (non-responders) |
P-value |
|
|
N= 34 (47.2%) |
N= 38 (52.8%) |
|||
|
Recurrence |
1 (2.9%) |
8 (21%) |
0.03 |
|
|
Distant recurrence |
1 (2.9%) |
5 (13.15%) |
0.11 |
|
|
Prognosis |
|
|
|
|
|
Living without disease |
30 (88.2%) |
26 (68.4%) |
0.052 |
|
|
Living with disease |
0 (0%) |
4 (10.5%) |
||
|
Died without disease |
3 (8.8) |
4 (10.5%) |
||
|
Died of disease |
1 (2.9%) |
4 (10.5%) |
||
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90. [Crossref]
- Chari RS, Tyler DS, Anscher MS, Russell L, Clary BM et al. (1995) Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 221: 777-786. [Crossref]
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W et al. (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30: 1926-1933. [Crossref]
- Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ et al. (2018) Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16: 874-901. [Crossref]
- Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S et al. (2012) Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 13: 579-588. [Crossref]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup Wh et al. (2001). Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345: 638-646. [Crossref]
- Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M et al. (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27: 5124-5130. [Crossref]
- Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ et al. (2007) Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 109: 1750-1755. [Crossref]
- Tang HL, Berlin J, Branton P, Burgat LJ, Carter DK et al. (2016) Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. College of American Pathologists, Based on AJCC/UICC TNM, 7th edition.
- Ryan R, Gibbons D, Hyland JM, Treanor D, White A et al. (2005) Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 47: 141-146. [Crossref]
- Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M et al. (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23: 8688-8696. [Crossref]
- Wheeler JM, Dodds E, Warren BF, Cunningham C, George BD et al. (2004) Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: correlation with rectal cancer regression grade. Dis Colon Rectum 47: 2025-2031. [Crossref]
- Kuo LJ, Liu MC, Jian JJ, Horng CF, Cheng TI et al. (2007) Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol 14: 2766-2772. [Crossref]
- Kong JC, Guerra GR, Warrier SK, Lynch AC, Michael M et al. (2018) Prognostic value of tumour regression grade in locally advanced rectal cancer: a systematic review and meta-analysis. Colorectal Dis 20: 574-585. [Crossref]
- Ruo L, Tickoo S, Klimstra DS, Minsky BD, Saltz L et al. (2002) Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg 236: 75-81. [Crossref]
- Maas M, Nelemans PJ, Valentini V, Das P, Rödel C et al. (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11: 835-844. [Crossref]
- Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD et al. (2003) A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 46: 298-304. [Crossref]
- Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C et al. (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72: 99-107. [Crossref]
- Martin ST, Heneghan HM, Winter DC (2012) Systematic review and metaanalysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 99: 918-928. [Crossref]
- Geva R, Davidovics H, Soyfer S, Pelles-Avraham S, Klausner JM et al. (2017) Does residual microscopic disease after chemoradiotherapy for locally advanced rectal cancer translate into a good clinical outcome? Colorectal Dis 19: 237-242. [Crossref]
- Janjan NA, Crane C, Feig BW, Cleary K, Dubrow R et al. (2001) Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol 24: 107-112. [Crossref]
- Huebner M, Wolff BG, Smyrk TC, Aakre J, Larson DW (2012) Partial pathologic response and nodal status as most significant prognostic factors for advanced rectal cancer treated with preoperative chemoradiotherapy. World J Surg 36: 675-683. [Crossref]
- Abdul-Jalil KI, Sheehan KM, Kehoe J, Cummins R, O'Grady A et al. (2014) The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis 16: O16-O25. [Crossref]
- Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T et al. (2014) Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 32: 1554-1562. [Crossref]
- Lee YC, Hsieh CC, Chuang JP (2013) Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a metaanalysis. Dis Colon Rectum 56: 1093-1101. [Crossref]
- Hong YS, Kim DY, Lim SB, Choi HS, Jeong SYet al. (2011) Preoperative chemoradiation with irinotecan and capecitabine in patients with locally advanced resectable rectal cancer: longterm results of a Phase II study. Int J Radiat Oncol Biol Phys 79: 1171-1178. [Crossref]
- Brenner H, Kloor M, Pox CP (2014) Colorectal cancer. Lancet (London, England) 383:1490-1502. [Crossref]
- Compton CC (2002) Pathologic prognostic factors in the recurrence of rectal cancer. Lancet 383: 1490-1502. [Crossref]
- Gospodarowicz MK, Brierley JD, Wittekind C (2017) TNM Classification of Malignant Tumours. John Wiley & Sons.
- Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr et al. (2004) Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 240: 711-718. [Crossref]
- Kong JC, Guerra GR, Warrier SK, Ramsay RG, Heriot AG (2017) Outcome and Salvage Surgery Following “Watch and Wait” for Rectal Cancer after Neoadjuvant Therapy: A Systematic Review. Dis Colon Rectum 60: 335-345. [Crossref]
- Bujko K, Kolodziejczyk M, Nasierowska-Guttmejer A, Michalski W, Kepka L et al. (2010) Tumour regression grading in patients with residual rectal cancer after preoperative chemoradiation. Radiother Oncol 95: 298-302. [Crossref]
- Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA et al. (2012) Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 30: 1770-1776. [Crossref]
- Jalilian M, Davis S, Mohebbi M, Sugamaran B, Porter IW et al. (2016) Pathologic response to neoadjuvant treatment in locally advanced rectal cancer and impact on outcome. J Gastrointest Oncol 7: 603-608. [Crossref]
- Sim SH, Kang MH, Kim YJ, Lee KW, Kim DW et al. (2014) P21 and CD166 as predictive markers of poor response and outcome after fluorouracil-based chemoradiotherapy for the patients with rectal cancer. BMC Cancer. 14: 241. [Crossref]
- Gash KJ, Baser O, Kiran RP (2017) Factors associated with degree of tumour response to neo-adjuvant radiotherapy in rectal cancer and subsequent corresponding outcomes. Eur J Surg Oncol 43: 2052-2059. [Crossref]
- Dayde D, Tanaka I, Jain R, Tai MC, Taguchi A (2017) Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. Int J Mol Sci 18. [Crossref]
