Journals
KRAS Mutation as Predictor Factor in Locally Advanced Rectal Cancer
A B S T R A C T
Introduction: Standard treatment of locally advanced rectal cancer (LARC) includes neoadjuvant chemo-radiotherapy followed by total mesorectal excision (TME). The role of KRAS as a biomarker in rectal cancer remains equivocal. We evaluate the Tumor Regression Grade (TRG), Relapse-Free Survival (RFS) and Overall Survival (OS) according to the KRAS oncogene status in LARC.
Material and Method: We evaluated the KRAS status in 23 patients with LARC. Tumor DNA was obtained from pretreatment biopsy tissues.
Results: KRAS mutation was found in 30,4% of the patients. TRG (1-2) after CRT were 56,2% and 42,8%, for wild-type and mutant KRAS groups (p= NS). After a median follow-up of 31 months, there was no difference in RFS (47,7 vs 23,3 months) or OS (51,5 vs 30 months) between wild-type and mutant-type KRAS groups, respectively.
Conclusions: Although KRAS status seems to have slightly better prognosis in LARC, it does not reach significant results (probably due to insufficient sample) in TRG, RFS or OS.
Keywords
Rectal cancer, neoadjuvant treatment, KRAS
Introduction
Combined chemotherapy and radiation therapy (CRT) before total mesorectal excision (TME) has become the standard treatment for patients with locally advanced rectal cancer (LARC) [1, 2]. This has shown to obtain an excellent tumor response and long- term survival [3]. Effectively, the National Comprehensive Cancer Network Guidelines recommended preoperative pelvic chemo-radiation followed by TME for LARC [4]. However, some patients have poor tumor response and long-term oncologic outcomes. Patients with a pathologic response (pCR) have a better prognosis compared with non-pCR patients [5, 6]. In addition, a certain proportion of patients also tend to experience toxicity and treatment- related complications, including leucopenia, thrombocytopenia, radiation proctitis and poor wound healing [7].
In this setting, the identification of predictive biomarkers of poor response to neoadjuvant CRT could be used to select the optimal treatment for rectal cancer patients avoiding significant morbidity in patients who will not benefit from these treatment [8]. Several molecular markers like epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF) or tumor protein 53 and some of them have been already applied to the treatment regimen [9]. Between these biomarkers, the mutation of Kirsten RAS (KRAS) oncogene is one of the most commonly studied mutations in colorectal cancer, yet its role in rectal cancer remains controversial [8]. Activation of the KRAS proto-oncogene is an essential step in carcinogenesis and it appears in 30-40% in colorectal cancer [10]. KRAS is a molecular transducer and important component of the EGFR pathway and is now widely accept as a predictor of poor response to anti-EGFR monoclonal antibodies such a cetuximab and panitumumab in metastatic colorectal cancer and testing for KRAS mutation has been incorporated into treatment [11, 12].
Nevertheless, few trials have investigated the KRAS status and clinical outcomes in LARC patients treated with neoadjuvant CRT followed by Surgery (TME). In this setting, there have been few clinical analyses finding that the KRAS mutation did not predict the clinical efficacy of neoadjuvant CRT followed by TME [13]. Therefore, we aimed to examine the clinical association among KRAS oncogene status and the response to neoadjuvant CTRT in LARC.
Materials and methods
I Patients Eligibility
Twenty-third LARC patients treated at our Hospital between January 2013 and August 2018 who received preoperative CRT followed by TME, were retrospectively analyzed. Eligibility patients had 1. histological confirmed rectal adenocarcinoma, 2. clinical stage of cT3-4N0-2M0, and 3. radical resection of the rectal tumor. Exclusion criteria were: patients with a history of malignancy other than rectal cancer, anti-EGFR therapy with preoperative radiation, or distant metastasis at the time of diagnosis.
All patients underwent preoperative irradiation of 45Gy in 25 daily fractions to the whole pelvis al 1.8 Gy/day and 5,4 Gy in 3 fractions to the primary tumor and visible nodes. Concurrent chemotherapy consisted of Capecitabine (825mg/m2 twice daily during radiation time). All patients underwent TME, 6-8 weeks after the end of preoperative treatment. According to risk factors, adjuvant chemotherapy was administrated (XELOX, FOLFIRI or XEL). Clinical staging examination before CRT consisted of digital rectal examination, complete blood count, level of carcinoembryonic- antigen (CEA), video colonoscopy, chest and abdomen computed tomography and pelvic magnetic resonance imaging with endorectal ultrasonography (EUS). The pathologic tumor stage was categorized according to the tumor-node-metastasis classification of the AJCC Criteria, 7th edition.
Tumor DNA from all patient was obtained from pretreatment biopsy tissues. Standard polymerase chain reaction analysis was performed to detect specific mutations in KRAS using established primers. Tumor regression grade after CRT (TRG)was classified according to the Mandard grading system; good responders were defined as Mandard TRG1 and TRG2 and bad responders as Mandard TRG3, TRG4 or TRG5 [14]. We compared preclinical and post-CRT pathological stages and defined downstaging as ypStage ypT0-2N0M0.
II Statistical Analysis
The study was designed to identify whether KRAS status is associated with tumor response to preoperative CRT and patient survival. Overall survival (OS) was defined as the time from the start of CRT to death. Relapse-free survival (RFS) was defined as the time from the start of CRT to any type of recurrence. Kaplan-Meier method and the log-rank test were used to compare survival distributions. Correlations between mutation status and tumor regression were assessed by the Chi square test. Multivariate analysis using a logistic regression model was performed to determinate associations between categorical variables and tumor response after CRT. All statistical test were 2-sided, and p-values <0.05 were considered statistically significant.
Results
All patients received the prescribed CRT as planned. Of the 23 included patients, 18 were men (78%) and 5 were women, with a median of age of 63 (range:49-75) years. The clinicopathological characteristics of the patients are summearized in (Table 1).
Table 1: Clinical-pathological characteristics of locally advance rectal cancer patients
|
Characteristics |
No. of patients, % |
|
Gender Male Female |
18 (78,3) 5 (21,7) |
|
Age (years) Median (range) ≤60 >60 |
63 (49-75) 11 (47,8) 12 (52,2) |
|
Tumor location (cm to anal margin) High (11-15) Medium (6-10) Low (0-5) |
4 (17,4) 13 (56,5) 6 (26,1) |
|
Clinical tumor classification cT3 cT4 |
19 (82,6) 4 (17,4) |
|
Clinical nodal classification cN0 cN1 cN2 |
1 (4,3) 7 (30,4) 15 (65,3) |
|
Histologic Grade High Moderate Poor |
9 (39,1) 11(47,8) 3 (13,1) |
|
CEA level, ng/ml ≤5 >5 |
12 (52,2) 11 (47,8) |
|
Tumor regression grade (TRG) TRG 1-2 TRG 3-4-5 |
12 (52,2) 11 (47,8) |
Table 2: Comparison of Tumor Response with regard to KRAS Mutation Status
|
Wild-type (n=7) |
Mutant –Type (n=16) |
||
|
KRAS status |
No. of patients, % |
p-Value |
|
|
Regression tumor No Yes |
7 (43,7) 9 (56,3) |
4 (57,1) 3 (42,9) |
0,554 |
I Tumor regression and oncogene mutation
KRAS mutation status was found in 7 of 23 patients (30,4%). After CRT, 6(26%) of the 23 patients were downstaged. Tumor response with regard to KRAS oncogene status is shown in (Table 2). In total, 9 of the 16 patients (56,2%) with wild-type KRAS achieved significant tumor regression (TRG1-TRG2) after preoperative CRT, whereas only 3 of the 7 patients (42.8%) with mutant-KRAS had a detectable response. There were no statistically significant difference in tumor regression rates between the 2 groups (p=0.55). In the multivariate analysis, age gender, downstaging, KRAS status and CEA level significantly did not affect the tumor response after preoperative CRT.
Figure 1: There was no statistically significant difference in Relapse-free survival between the wild-type group and the mutant-type group (p=0,08)
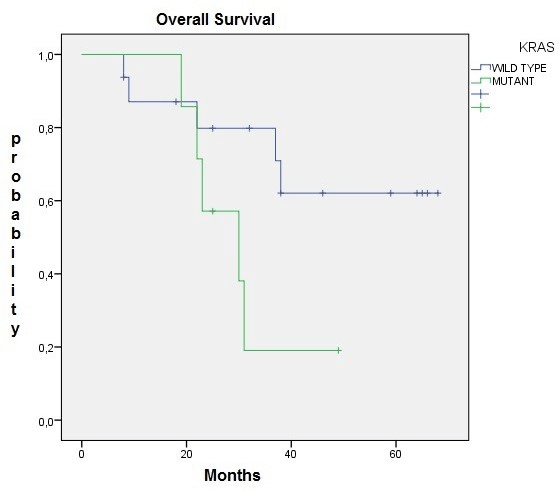
Figure 2: There was no statistically significant difference in Overall Survival between the wild-type group and the mutant-type group (p=0,07)
Discussion
The KRAS oncogene mutation is involved in the transition of adenoma to carcinoma in colorectal cancer, being found in about 30-35% [15]. Several trials examining tumors with KRAS mutation and anti-EGFR therapy have revealed the poor prognostic effect of KRAS mutations in metastatics colorectal cancer patients [16]. As pointed earlier, 5-FU or capecitabine- based chemotherapy concurrent radiotherapy is the recommended regimen before TME. There are currently no effective predictors of response to preoperative CRT in LARC. Clinical trials over the KRAS oncogene status and treatment outcomes in LARC are limited. Thus, we focused on the potential of KRAS oncogene status as a biological predictive marker for rectal cancer in LARC.
There are some clinical studies showing the good responders after preoperative CRT have better outcomes than poor responders in the control of local and distant failure. For this reason, tumor response can be an important prognostic factor in rectal cancer. In our analysis the downstaging rate was 26%, similar to others studies whereby downstaging rate of 30-40% [17]. Lee JW et al. found that downstaging was not associated with KRAS mutation status. In the current study, KRAS mutation was not found to be predictive of tumor response to neoadjuvant CRT in LARC [19]. Some studies have been reported that rectal cancer with KRAS mutation is likely to have poor response to neoadjuvant CRT compared with wild-type tumors [12, 18]. Nevertheless, in the meta-analysis by Clancy et al, there were no statistically differences in tumor response between the wild-type and mutant-type KRAS groups, irrespective of the chemotherapy regimen [13].
Several studies analyzed the association of RFS and OS between KRAS mutation groups (wild-type vs mutant). Luna –Perez et al. found KRAS mutations in rectal cancer to be associated with longer RFS and OS [20]. While these data were retrieved from tissue taken after preoperative CRT, we analized biopsies taken prior to neoadjuvant treatment. Lee JW et al. found that KRAS mutation status was not associated with postoperative prognosis in rectal cancer as well as this study that no difference is observed in RFS and OS between both groups [19]. Curiosly, in our study we found near significant differences in outcomes and responses regarding the KRAS status.
There are some limitations in my study. First, these cohorts of patients are those who due to their bad prognosis factors, requested KRAS mutation status in pre-treatment biopsy. They are not all LARC patients treated in our center. Thus, its prior poor prognosis might affect the final results. Secondly, this study should be understood in view of inherent biases of a retrospective study design, and we have evaluated small number of patient cohort. Thirdly, the follow-up period is far too short to draw definitive conclusions. In summary, we found that the KRAS mutation status does not influence the tumor response and survival in patients treated with neoadjuvant CRT followed by curative surgery. It would be interesting to carry out prospective trials with longer follow-up examining KRAS mutation in locally advanced rectal cancer for an increased understanding.
Conflicts of interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 30, Jul 2019Accepted: Tue 13, Aug 2019
Published: Sat 24, Aug 2019
Copyright
© 2023 Fernando Arias. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2019.03.03
Author Info
Flamarique Sonia Asin Gemma Campo M Fernando Arias Guerrero David Rodriguez M
Corresponding Author
Fernando AriasRadiation Oncology Service, Complejo Hospitalario de Navarra, Pamplona, Spain
Figures & Tables
Table 1: Clinical-pathological characteristics of locally advance rectal cancer patients
|
Characteristics |
No. of patients, % |
|
Gender Male Female |
18 (78,3) 5 (21,7) |
|
Age (years) Median (range) ≤60 >60 |
63 (49-75) 11 (47,8) 12 (52,2) |
|
Tumor location (cm to anal margin) High (11-15) Medium (6-10) Low (0-5) |
4 (17,4) 13 (56,5) 6 (26,1) |
|
Clinical tumor classification cT3 cT4 |
19 (82,6) 4 (17,4) |
|
Clinical nodal classification cN0 cN1 cN2 |
1 (4,3) 7 (30,4) 15 (65,3) |
|
Histologic Grade High Moderate Poor |
9 (39,1) 11(47,8) 3 (13,1) |
|
CEA level, ng/ml ≤5 >5 |
12 (52,2) 11 (47,8) |
|
Tumor regression grade (TRG) TRG 1-2 TRG 3-4-5 |
12 (52,2) 11 (47,8) |
Table 2: Comparison of Tumor Response with regard to KRAS Mutation Status
|
Wild-type (n=7) |
Mutant –Type (n=16) |
||
|
KRAS status |
No. of patients, % |
p-Value |
|
|
Regression tumor No Yes |
7 (43,7) 9 (56,3) |
4 (57,1) 3 (42,9) |
0,554 |

References
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355: 1114-1123. [Crossref]
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351: 1731-1740. [Crossref]
- Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M et al. (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABPR-03. J Clin Oncol 27: 5124-5130. [Crossref]
- Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ et al. (2018) Rectal cancer National, Version 2.2018 NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16: 874-901. [Crossref]
- Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA et al. (2012) Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 30: 1770-1776. [Crossref]
- Li J, Yuan J, Liu H, Yin J, Liu S et al. (2016) Lymph nodes regression grade is a predictive marker for rectal cancer after neoadjuvant therapy and radical surgery. Oncotarget 7: 16975-16984. [Crossref]
- Lin JZ, Peng JH, Qdaisat A, Lu ZH, Wu XJ et al. (2016) Preoperative chemoradiotherapy creates an opportunity to perform sphincter preserving resection for low-lying locally advanced rectal cancer based on an oncologic outcome study. Oncotarget 7: 57317-57326. [Crossref]
- Yokota T (2012) Are KRAS/BRAF mutations potent prognostic and/or predictive biomarkers in colorectal cancers? Anticancer Agents Med Chem 12: 163-171. [Crossref]
- Edden Y, Wexner SD, Berho M (2012) The use of molecular markers as a method to predict the response to neoadjuvant therapy for advanced stage rectal adenocarcinoma. Colorectal Dis 14: 555-561. [Crossref]
- Gaedcke J, Grade M, Jung K, Schirmer M, Jo P et al. (2010) KRAS and BRAF mutations in patients with rectal cancer treated with preoperative chemoradiotherapy. Radiother Oncol 94: 76-81. [Crossref]
- Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A et al. (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 96: 1166-1169. [Crossref]
- Chang DZ, Kumar V, Ma Y, Li K, Kopetz S (2009) Individualized therapies in colorectal cancer: KRAS as a marker for response to EGFR-targeted therapy. J Hematol Oncol 2: 18. [Crossref]
- Clancy C, Burke JP, Coffey JC (2013) KRAS mutation does not predict the efficacy of neo-adjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Surg Oncol 22: 105-11. [Crossref]
- Santos MD, Silva C, Rocha A, Matos E, Nogueira C et al. (2014) Prognostic Value of Mandard and Dworak Tumor Regression Grading in Rectal Cancer: Study of a Single Tertiary Center. ISRN Surg 2014: 310542. [Crossref]
- Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR et al. (2001) Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer 85: 692-696. [Crossref]
- Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D et al. (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359: 1757-1765. [Crossref]
- Rodel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M et al. (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23: 8688-8696. [Crossref]
- Bengala C, Bettelli S, Bertolini F, Sartori G, Fontana A et al. (2010) Prognostic role of EGFR gene copy number and KRAS mutation in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy. Br J Cancer 103: 1019-1024. [Crossref]
- Lee JW, Lee JH, Shim BY, Kim SH, Chung MJ et al. (2015) KRAS mutation status is not a predictor for tumor response and survival in rectal cancer patients who received preoperative radiotherapy with 5-fluoropyrimidine followed by curative surgery. Medicine (Baltimore) 94: e1284. [Crossref]
- Luna-Perez P, Segura J, Alvarado I, Labastida S, Santiago-Payan H et al. (2000) Specific c-K-ras gene mutations as a tumor-response marker in locally advanced rectal cancer treated with preoperative chemoradiotherapy. Ann Surg Oncol 7: 727-731. [Crossref]
