Kaposi’s Sarcoma in the Era of ART: A Single Institutional Retrospective Review
A B S T R A C T
Background: Kaposi’s Sarcoma (KS) is an angioproliferative tumor characterized by 4 sub-types: classic (CKS), endemic, immunosuppression related, and epidemic (AIDS-KS). AIDS-KS prevalence has decreased since the introduction of potent combination anti-retroviral therapy (ART). Even so, KS lesions develop in patients with undetectable viral loads and high CD4 counts. KS is variable across its subtypes, disease course, and clinical outcomes. Treatment must therefore be individualized.
Results: 130 patients with a diagnosis of KS were identified, of which 95 (73.1%) had AIDS-KS and 31 (23.8%) had CKS. There were 4 patients with immunosuppression therapy-related KS and no endemic cases. 18.9% of AIDS-KS patients had metastatic disease vs. 6.5% in the CKS group. At KS diagnosis, 50.5% of AIDS-KS patients had CD4 count >200 cells/mm3 and 33.7% had HIV viral load level <=20 copies/mL. Among the 53 patients who received chemotherapy, 45 were AIDS-KS patients (84.9%). The most commonly used chemotherapy was doxorubicin hydrochloride liposomal injection (78.4%) with an average of 10 cycles. Other chemotherapy utilized includes paclitaxel and interferon. 16 pts (12.3%) died, of which only two died of disseminated AIDS-KS.
Conclusions: Our retrospective study confirms that 75% of pts diagnosed with KS have AIDS-KS. Despite introduction of ART and the well-controlled nature HIV/AIDS, KS continues to occur. AIDS-KS patients are younger, more likely to have metastatic disease and more frequently treated with chemotherapy. Poorly controlled HIV confers a worse outcome in AIDS-KS. Further investigations are required to better understand the etiology of AIDS-KS in pts with undetectable HIV viral loads and immune recovery.
Keywords
Kaposi’s sarcoma, KS-associated herpesvirus, AIDS-related Kaposi’s Sarcoma, classical Kaposi’s Sarcoma
Introduction
KS is an angioproliferative tumor with four different subtypes described. The pathogenesis of KS hinges on complex multifactorial events including immunosuppression. Since the 1950’s, the important role of viral agents has been recognized [1]. In 1994, HHV8 or KS-associated herpesvirus (KSHV) sequences were found to be present in greater than 90% of KS tissues obtained from patients with Acquired Immunodeficiency Syndrome (AIDS). HHV8 has since been shown to be present in a majority of all subtypes of KS. KSHV can be transmitted via both sexual and non-sexual routes. The modes of transmission may vary in different parts of the world. In non-endemic areas, such as the United States and Western Europe, sexual transmission may be the primary mode of transmission. The global seroprevalence of KSHV is generally high in areas where non-Human Immunodeficiency Virus (non-HIV) associated forms of KS are common (i.e. African and Mediterranean regions), whereas in the United States and Western Europe, seroprevalence may be generally low (<10%) [2, 3]. In the San Francisco Men’s Health study, the prevalence of KSHV infection was reported to be high among men who have sex with men (MSM) (37.6%) [4]. In addition, among the men who were infected with both HIV and HHV8 at baseline, the 10-year probability of developing KS was 49.6%, thus supporting the observation that HHV8 has an etiologic role in KS and is sexually transmitted among men [4].
In the current view of KS pathogenesis, though HHV-8 infection is a common underlying etiology, further factors are usually required for development of KS (i.e. genetic, immunologic, and environmental). Both systemic and local immunodeficiency play a significant role in the pathogenesis of KS. The prevalence of KS is significantly higher in AIDS patients and post-transplant patients as compared to the general population [5]. In addition, certain conditions which dysregulate local immunologic homeostasis, such as lymphedema or podoconiosis, can often lead to predisposition for KS [6].
Since the initial introduction of potent combination anti-retroviral therapy (ART) in 1996, there has been a sharp decline in the incidence of AIDS-KS. Using the AIDS and cancer registries in the US, among 5131 patients with AIDS-KS, the standardized incidence ratio was found to be 22 100 and 3640 respectively between 1990-1995 and 1996-2002 (P < 0.0001) [7]. Even so, KS lesions develop in patients with undetectable HIV viral loads and high CD4 counts. The pathogenesis in such patients remains unclear.
KS is variable across its clinical course and various subtypes. Therefore, treatment is widely heterogenous and must be individualized. Patients with AIDS-KS should be administered ART and are most likely to achieve remission if they are ART-naïve, have limited disease, and achieve optimal HIV control. However, there remain a subset who require further therapies in addition to ART.
We conducted a retrospective review of the cohort of patients who have been diagnosed and treated for KS at the Lurie Comprehensive Cancer Center of Northwestern University between 2005 and 2017 to describe the patient demographics, clinical characteristics and treatments administered across a modern era with effective ART widely available.
Methods
Study approval was obtained from the Institutional Review Board at Northwestern University. A retrospective cohort was identified of KS patients evaluated and treated between 2005 and 2017 at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Patients were identified through the Enterprise Data Warehouse using ICD code for KS diagnosis. Inclusion criteria were patient age greater than 18 and documentation of HIV status in the electronic medical record. Patient data was reviewed for demographics, clinical and pathological features and treatment modalities. Descriptive and comparative statistics were used to assess for predictors of disease severity and treatment.
The following data were recorded by chart review of the patient’s electronic medical record. Patient demographics including age at KS diagnosis, age at HIV diagnosis, gender, and ethnicity were recorded. Clinical characteristics were recorded including stage of KS, HIV status, and HIV disease parameters (viral load, CD4 count, ART regimen, and presence of other AIDS-defining illnesses at time of KS diagnosis).
Finally, the treatment modalities were reviewed and described. If chemotherapy was prescribed, the type, dose, and number of cycles of chemotherapy was recorded. If patient is currently deceased, cause of death was recorded. All research was performed in accordance with relevant guidelines and regulations. A waiver of consent and HIPAA authorization was obtained.
Results
I Demographics
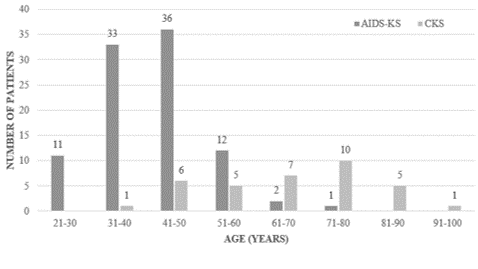
This retrospective study comprised of 130 patients who were diagnosed and treated for KS between 2005 and 2017, including 95 AIDS-KS, 31 CKS, and 4 immunosuppression therapy-related KS patients. Seven patients were excluded from the initial cohort as they did not have documentation of HIV sero-status. The mean age at time of diagnosis was 48.7 ± 15.6 (range 25 - 95) years. The mean age of diagnosis of HIV was 35.4 ± 8.8 (range 22-69) years. The mean number of years after HIV diagnosis at which time KS was diagnosed was 6.8 ± 7.9 years. Age distribution patterns indicate that CKS was more common among elderly patients of age 71-80 years (32.2%) and AIDS-KS was more common among patients of age 41-50 years (37.8%) (Figure 1).
Figure 1: Distribution of KS patients by age at diagnosis.
Of the 130-patient cohort, 11 were female and 120 were male patients. Among the female patients, all were HIV-negative. Among the 95 AIDS-KS patients, there were 47 cases (49.5%) in self-identified white patients, 26 cases (27.4%) in black or African American patients, two cases in self-identified Hispanic patients, and one case in a self-identified Asian patient. The rest either declined to list ethnicity or selected other. In the CKS patients, the majority of cases (22, 70.9%) were identified in white patients. There were three cases of CKS in black patients, and the rest were unlisted. Demographic characteristics of the study cohort is summarized in (Table 1).
II Clinical Characteristics
AIDS-KS patients were more likely to have metastatic disease at time of KS diagnosis. There were 23 cases (17.6%) of metastatic KS (KS involving the viscera and/or lymph nodes) identified. 21 (91.3%) of these were in AIDS-KS patients. Of the 107 cases of localized KS, 74 were in AIDS-KS patients and 33 were in HIV-negative patients.
Table 1: Demographics of KS patients.
|
AIDS-KS |
CKS |
Immunosuppression Therapy-Related KS |
||
|
N |
95 |
31 |
4 |
|
|
Gender |
||||
|
M |
95 (100%) |
23 (74.2%) |
1 (25%) |
|
|
F |
0 |
8 (25.8%) |
3 (75%) |
|
|
Age at diagnosis |
||||
|
21-40 |
44 (46.3%) |
1 (3.2%) |
0 |
|
|
41-60 |
48(50.5%) |
9(29%) |
2 (50%) |
|
|
>60 |
3 (3.2%) |
21(67.7%) |
2 (50%) |
|
|
Ethnicity |
||||
|
Caucasian |
47 (49.5%) |
22(70.9%) |
2 (50%) |
|
|
African American |
26 (27.4%) |
3 (9.7%) |
0 |
|
|
Hispanic |
2 (2.1%) |
0 |
0 |
|
|
Asian |
1 (1.1%) |
1 (3.2%) |
0 |
|
|
Other/ unknown |
19 (20%) |
5 (16.1%) |
2 (50%) |
|
Table 2: Clinical characteristics of AIDS-KS patients by CD4 count at time of KS diagnosis.
|
CD4 count < 200 cells/mm3 |
CD4 count > 200 cells/mm3 |
|
|
N |
39 |
44 |
|
Stage of disease |
||
|
Localized |
28 (71.8%) |
37 (84.1%) |
|
Metastatic |
11 (28.2%) |
7 (15.9%) |
|
HIV viral load |
||
|
Undetectable |
12 (30.8%) |
29 (65.9%) |
|
> 20 copies/mL |
27 (69.2%) |
15 (34.1%) |
|
HAART during KS diagnosis |
||
|
Yes |
25 (64.1%) |
29 (65.9%) |
|
No |
14 (35.9%) |
15 (34.1%) |
|
Presence of other AIDS defining illness |
||
|
Yes |
25 (64.1%) |
12 (27.3%) |
|
No |
14 (35.9%) |
32 (72.7%) |
Among the 95 AIDS-KS patients, 50 (52.6%) patients were on ART at time of KS diagnosis. 31 (32.6%) patients were not on ART at time of KS diagnosis, and ART status was unknown in 13 (13.7%) patients. 42 AIDS-KS patients (44.2%) had another AIDS-defining illness present at time of KS diagnosis, whereas 53 patients (55.8%) did not.
44 AIDS-KS (46.3%) patients had CD4 counts of greater than or equal to 200/mm3 at time of KS diagnosis. 39 AIDS-KS (41.1%) patients had CD4 counts less than 200/mm3, and CD4 counts were unknown in 12 (12.6%) patients. Additionally, 32 AIDS-KS patients (33.7%) had undetectable HIV viral loads at time of KS diagnosis. Notably, the HIV viral loads were unknown for 14 patients (14.7%) (Table 2).
III Treatment Characteristics
AIDS-KS patients were more likely to require chemotherapy. Among the study cohort of 130 patients, 52 patients (40 %) received chemotherapy, and of these, 45 were AIDS-KS patients. 41 patients (31.5%) only required ART and these were all AIDS-KS patients. 7 patients (5.4%) were treated surgically (two of these were AIDS-KS patients). Six patients (4.6%) received only radiation therapy (two of these were AIDS-KS patients). One patient received surgery, radiation and chemotherapy, and this patient ultimately required eight cycles of paclitaxel followed by alitretinoin gel for recurrence. 13 patients (10%) were treated with observation only (all were CKS patients). The 4 immunosuppression therapy-related KS patients were treated in addition with alteration of their immunosuppression regimen. Treatment regimens are summarized in (Table 3).
Table 3: Treatment Regimens Used for KS Patients.
|
|
AIDS-KS |
CKS |
Immunosuppression therapy-related KS |
|
Stage of disease |
|||
|
Localized |
74 (77.9%) |
29 (93.5%) |
4 (100%) |
|
Metastatic |
21 (22.1%) |
2 (6.5%) |
0 |
|
Treatment |
|||
|
HAART |
41 (43.2%) |
0 |
0 |
|
Observation |
0 |
13(41.9%) |
0 |
|
Chemotherapy |
45(47.4%) |
5(16.1%) |
2 (50%) |
|
Surgery |
2 (2.1%) |
5 (16.1) |
0 |
|
Radiation |
3 (3.2%) |
2 (6.5%) |
1 (25%) |
|
Surgery + Radiation |
0 |
2 (6.5%) |
0 |
|
Surgery + Radiation + Chemo |
0 |
1 (3.2%) |
0 |
|
Other/unknown |
4 (4.2%) |
2 (6.5%) |
1 (25%) |
Of the chemotherapy regimens prescribed, the most common was doxorubicin hydrochloride liposomal injection (40 patients, 78.4%) with an average of ten cycles. Other chemotherapy regimens used included etoposide (1 patient, 1.9%) and paclitaxel (4 patients, 7.8%). 16 patients (12.3%) died, of which only two died of disseminated AIDS-KS.
Discussion
HIV-associated malignancies were more common in the pre-ART era [8]. Non-Hodgkin Lymphoma and KS accounted for the most common malignancy-associated morbidity and mortality in HIV patients in the pre-ART era [9]. In previous retrospective observational studies held during the late ART period (at least 5 years after the widespread availability of potent ART), a decrease in the predominance of KS has been observed in women and black demographic groups [10]. However, despite declines in incidence of KS and non-Hodgkin lymphoma in th ART-era, they a continue to be among the most common cancers within HIV populations [11].
Our cohort of 130 KS patients was comparable to the sample size of other similar retrospective observational studies. As mentioned above, the majority of AIDS-KS patients in the post-ART era were white males, similar to other cohorts [10]. A large European study in the early 1990s showed that a low CD4 count was the only significant risk factor for development of KS in a population of HIV-positive patients who were co-infected with HHV-8 [12]. In addition, ART played a protective role on development of KS and mortality of KS patients. Multiple factors have been studied in development of KS such as degree of immunosuppression, temporal order of HHV-8 and HIV infection, HIV or HHV-8 viral load and homosexual behavior. Prevalence of HHV-8 infection and incidence of AIDS-KS is higher among HIV-positive MSM, which suggests that HHV-8 can be sexually transmitted [13]. However only 30-50% of men who are coinfected with HHV8 and HIV develop KS within 5-10 years, suggesting that multiple risk factors must be in play [14].
In our cohort, we noted that 46.3% of the AIDS-KS cohort developed KS with CD4 counts measuring greater than or equal to 200 cells/mm3. Additionally, 50.6% of the AIDS-KS cohort of patients were on ART at time of KS diagnosis. These findings may be related to the small sample size and/or be an indication of other risk factors which may be accounting for the development of KS in these patients. For the future, a study involving a larger number of AIDS-KS patients where we also collect information about HHV8 status and indication of sexual practices may show what we have seen in the larger cohort studies looking at risk factors among HIV-positive patients in developing AIDS-KS.
Our data supports that CKS is a disease of the elderly, with the mean age at diagnosis being between 70 and 80 years. Researchers in the United States have reported that the average age of CKS is 63 and the highest incidence is seen in the sixth through eighth decade of life [15]. CKS has been described to be a slow and indolent disease with average survival time reported of eight to thirteen years, and cases of spontaneous tumor regression has also been reported [15]. This was supported in our data in that 91.3% of the CKS patients had localized disease and 56.5% of the CKS patients were treated with observation only. Due to the indolent nature of CKS, the causes of mortality in CKS patients is usually other diseases of old age and/or secondary malignancies, as supported by our data above [15].
There are many treatment modalities for KS and standard of practice may differ among different centers. A retrospective phase II study in Germany showed that pegylated liposomal doxorubicin is a safe and effective drug for treatment of advanced AIDS-KS without adverse effects on the patient’s CD4 cell counts and/or HIV viral loads [16]. A small retrospective study of 20 CKS patients showed that pegylated liposomal doxorubicin showed an improvement in objective response and pain intensity in patients who had been pretreated for CKS, thereby concluding that it is a well-tolerated second line treatment option for CKS [17].
The most commonly used chemotherapy regimen in our cohort was liposomal doxorubicin injection followed by paclitaxel. Predictors for receiving chemotherapy included metastatic disease at diagnosis. Approximately half of the AIDS-KS patients who received chemotherapy were already on ART at time of KS diagnosis, suggesting that ART regimens only for the treatment of KS are more effective in ART-naïve patients.
Conclusion
To summarize, we studied three subtypes of KS in our cohort: AIDS-KS, CKS, and immunosuppression therapy-related KS. AIDS-KS patients comprised of 75% of all patients diagnosed with KS. Though many studies have shown the prevalence of AIDS-KS decrease in the post-ART era, we show that KS continues to develop in this patient population despite well-controlled HIV/AIDS. The pathogenesis of KS hinges on local and/or systemic immunosuppression and the invasion of host by KS-associated herpesvirus. We saw that our AIDS-KS patients continued to develop KS in the presence of well-controlled HIV, defined as CD4 count greater than or equal to 200 cells/mm3. AIDS-KS patients tend to be younger at diagnosis, are more likely to have metastatic disease, and more frequently require systemic chemotherapy. We did see that poorly controlled HIV portends a worse outcome in AIDS-KS, similar to observations reported by previous studies. Further investigations in the pathogenesis of KS are required to determine which HIV-positive individuals are more susceptible to developing KS in the presence of well-controlled HIV.
Author Contributions
MA and KF are responsible for the concept and design of the work, KS and MA are responsible for acquisition, analysis and interpretation of the data, CA is responsible for interpretation of the data and all authors reviewed the manuscript.
Competing Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 07, Jan 2020Accepted: Sat 01, Feb 2020
Published: Mon 10, Feb 2020
Copyright
© 2023 Mark Agulnik. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2020.01.01
Author Info
Chad Achenbach Kamya Sankar Kelly Foster Mark Agulnik
Corresponding Author
Mark AgulnikDepartment of Medicine at Northwestern University, Chicago, IL, USA
Figures & Tables
Table 1: Demographics of KS patients.
|
AIDS-KS |
CKS |
Immunosuppression Therapy-Related KS |
||
|
N |
95 |
31 |
4 |
|
|
Gender |
||||
|
M |
95 (100%) |
23 (74.2%) |
1 (25%) |
|
|
F |
0 |
8 (25.8%) |
3 (75%) |
|
|
Age at diagnosis |
||||
|
21-40 |
44 (46.3%) |
1 (3.2%) |
0 |
|
|
41-60 |
48(50.5%) |
9(29%) |
2 (50%) |
|
|
>60 |
3 (3.2%) |
21(67.7%) |
2 (50%) |
|
|
Ethnicity |
||||
|
Caucasian |
47 (49.5%) |
22(70.9%) |
2 (50%) |
|
|
African American |
26 (27.4%) |
3 (9.7%) |
0 |
|
|
Hispanic |
2 (2.1%) |
0 |
0 |
|
|
Asian |
1 (1.1%) |
1 (3.2%) |
0 |
|
|
Other/ unknown |
19 (20%) |
5 (16.1%) |
2 (50%) |
|
Table 2: Clinical characteristics of AIDS-KS patients by CD4 count at time of KS diagnosis.
|
CD4 count < 200 cells/mm3 |
CD4 count > 200 cells/mm3 |
|
|
N |
39 |
44 |
|
Stage of disease |
||
|
Localized |
28 (71.8%) |
37 (84.1%) |
|
Metastatic |
11 (28.2%) |
7 (15.9%) |
|
HIV viral load |
||
|
Undetectable |
12 (30.8%) |
29 (65.9%) |
|
> 20 copies/mL |
27 (69.2%) |
15 (34.1%) |
|
HAART during KS diagnosis |
||
|
Yes |
25 (64.1%) |
29 (65.9%) |
|
No |
14 (35.9%) |
15 (34.1%) |
|
Presence of other AIDS defining illness |
||
|
Yes |
25 (64.1%) |
12 (27.3%) |
|
No |
14 (35.9%) |
32 (72.7%) |
Table 3: Treatment Regimens Used for KS Patients.
|
|
AIDS-KS |
CKS |
Immunosuppression therapy-related KS |
|
Stage of disease |
|||
|
Localized |
74 (77.9%) |
29 (93.5%) |
4 (100%) |
|
Metastatic |
21 (22.1%) |
2 (6.5%) |
0 |
|
Treatment |
|||
|
HAART |
41 (43.2%) |
0 |
0 |
|
Observation |
0 |
13(41.9%) |
0 |
|
Chemotherapy |
45(47.4%) |
5(16.1%) |
2 (50%) |
|
Surgery |
2 (2.1%) |
5 (16.1) |
0 |
|
Radiation |
3 (3.2%) |
2 (6.5%) |
1 (25%) |
|
Surgery + Radiation |
0 |
2 (6.5%) |
0 |
|
Surgery + Radiation + Chemo |
0 |
1 (3.2%) |
0 |
|
Other/unknown |
4 (4.2%) |
2 (6.5%) |
1 (25%) |
References
- Oettle AG (1962) Geographical and racial differences in the frequency of Kaposi’s sarcoma as evidence of environmental or genetic causes. Acta Unio Int Contra Cancrum 18: 330-363.[Crossref]
- Dedicoat M, Newton R (2003) Review of the distribution of Kaposi's sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi's sarcoma. Br J Cancer 88: 1-3. [Crossref]
- Weiss RA, Whitby D, Talbot S, Kellam P, Boshoff C (1998) Human herpesvirus type 8 and Kaposi's sarcoma. J Natl Cancer Inst Monogr 23: 51-54. [Crossref]
- Martin JN, Ganem DE, Osmond DH, Page Shafer KA, Macrae D et al. (1998) Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med 338: 948-954. [Crossref]
- Schwartz RA (2004) Kaposi’s Sarcoma: an update. J Surg Oncol 87: 146-151. [Crossref]
- Ruocco V, Astarita C, Guerrera V, Lo Schiavo A, Moscariello CG et al. (1984) Kaposi’s Sarcoma on a lymphdematous immunocompromised limb. Int J Dermatol 23: 56-60. [Crossref]
- Engels EA, Pfeiffer RM, Goedert JJ , Virgo P, McNeel TS et al. (2006) Trends in cancer risk among people with AIDS in the United States. AIDS 20: 1645-1654. [Crossref]
- Patel P, Hanson DL, Sullivan PS , Novak RM, Moorman AC et al. (2008) Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 148: 728-736. [Crossref]
- Cornett PA, Volbrding PA (2010) Malignant diseases in human immunodeficiency virus infection. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Disease. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier 2010: 1765.
- Rihana N, Nanjappa S, Sullivan C, Velez AP, Tienchai N et al. (2018) Malignancy Trends in HIV-infected patients over the past 10 years in a single-center retrospective observational study in the United States. Cancer Control 25: 1073274818797955. [Crossref]
- Yanik EL, Tamburro K, Eron JJ, Damania B, Napravnik S et al. (2013) Recent cancer incidence trends in an observational clinical cohort of HIV-infected patients in the US, 2000-2011. Infect Agent Cancer 8: 18. [Crossref]
- Martro E, Esteve A, Schulz TF, Sheldon J, Gambús G et al. (2007) Risk factors for human Herpesvirus 8 infection and AIDS-associated Kaposi’s sarcoma among men who have sex with men in a European multicenter study. Int J Cancer 120: 1129-1135. [Crossref]
- Gambus G, Bourboulia, Esteve A, Lahoz R, Rodriguez C et al. (2011) Prevalence and distribution of HHV8 in different subpopulations, with and without HIV infection, in Spain. AIDS 15: 1167-1174. [Crossref]
- O’ Brien TR, Kedes D, Ganem D, Macrae DR, Rosenberg PS et al. (1999) Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi’s Sarcoma. J Infect Dis 180: 1010-1017. [Crossref]
- Safai B (1984) Kaposi’s sarcoma: a review of the classical and epidemic forms. Ann N Y Acad Sci 437: 373-382. [Crossref]
- Hengge UR, Esser S, Rudel HP, Goos M (2001) Long-term chemotherapy of HIV-associated Kaposi’s sarcoma with liposomal doxorubicin. Eur J Cancer 37: 878-883. [Crossref]
- Lorenzo G, Trollo RD, Montesarchio V, Palmieri G, Nappa P et al. (2008) Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi Sarcoma. Cancer 112: 1147-1152. [Crossref]
