Is Saline Injection a True Sham/Placebo Treatment in Randomized Controlled Trials? A Systematic Review
A B S T R A C T
Objective: To explore whether saline is a real sham/placebo agent, or it has potential therapeutic effects when used as control treatment in randomized controlled trials for the management of discogenic low back pain.
Methods: A comprehensive literature search was conducted investigating the effects of saline as a placebo in the treatment of chronic pain when administered into the intervertebral disc. Following stepwise filtering, selected articles were assessed for their levels of evidence, followed by a discussion of their contribution to the understanding of the role of saline in chronic pain management.
Results: Out of 95 articles that described the administration of intradiscal saline solution used as a placebo for chronic pain management, 8 articles met all of the inclusion criteria. Their levels of evidence ranged from 1a to 4 (Oxford Centre CEBM). Intradiscal administration of saline solution was found to have measurable therapeutic benefits. In some studies, the pain relief was similar to that provided by local anaesthetics and steroids.
Conclusion: Although the exact mechanism of the analgesic effects of saline is not clear, yet the use of intradiscal saline appears to have some analgesic benefits like local anaesthetics and steroids when used individually. Researchers should practice caution when designing RCTs using intradiscal saline injection as a sham/placebo treatment for the control arm or maybe, when possible, avoid the use of intradiscal saline injection as a sham treatment.
Keywords
Sham, saline, intradiscal injection, discogenic pain
Introduction
Randomized controlled trials (RCTs) are considered the gold standard methodology in clinical research. It is commonly used to evaluate the therapeutic effects of a new treatment or intervention [1]. In order to eliminate or minimize the placebo effect, research subjects need to be blinded to which treatment arm they were randomized to. By doing so, the quality and reliability of the research outcomes improve significantly [2]. To further minimize the potential bias, researchers expand blinding to include the study evaluation team and sometimes the investigator(s) administering the therapy and biostatisticians analysing the data to further increase the reliability and validity of the results. When evaluating the safety and efficacy of an interventional procedure, the only way to blind participants is to randomize subjects to receive either the study intervention or a sham treatment that is administered in a way that simulates the study intervention. For example, the sham treatment is performed using the same or at least a very similar technique to the active treatment with the exception of substituting the active agent/treatment with an inactive agent. Saline solution is used in many clinical trials as the inactive or sham treatment agent without clear data to support the notion that it is, in reality, an inactive agent. One cannot ignore or underestimate the importance of the placebo/sham treatment arm in RCT and how it significantly strengthens the level of evidence a specific clinical trial provides. Although the positive results in the control arm could be explained by the placebo effect, we should not ignore the unknown potential therapeutic effect of injecting saline or other inactive formulation at the treatment target.
In a well-designed RCT published in New England Journal of Medicine (NEJM), Friedly et al. compared epidural injections of glucocorticoids plus lidocaine or lidocaine alone for symptom control in patients with spinal canal stenosis. At 6 weeks, there was no statistically significant difference in Roland-Morris Disability Questionnaire (RMDQ) scores between the 2 groups, albeit both groups showed some improvement in their pain scores. On the Swiss Spinal Stenosis Questionnaire (SSSQ) satisfaction scale, 67% of patients who received glucocorticoids plus lidocaine reported being very or somewhat satisfied with their treatment, as compared with 54% of those who received only lidocaine (P=0.01) [3]. The authors did not provide an explanation for the long-term improvement in the lidocaine group. It is well-established, that lidocaine is a short-acting local anaesthetic; therefore, the prolonged pain relief and improved disability at 6 weeks must have a different mechanism than local anaesthesia. In another literature review conducted by Bar-Or et al., it was assumed that intra-articular saline injection that is used as placebo for knee osteoarthritis clinical trial has some analgesic effect since its effects were always better than no treatment [4]. The fundamental question became; is it all placebo effects? Or is there a long-term benefit of lidocaine beyond its duration of action as a local anaesthetic? Or is it possible that control treatment agent (lidocaine or saline) has potential unknown therapeutic effects [5]?
To our knowledge, there are no available systematic reviews to elucidate whether the saline solution is a really inactive agent, or it might have some therapeutic effects when used to evaluate interventional treatment of discogenic pain. Therefore, our goal is to review the world literature of the published randomized controlled trials to treat discogenic pain involving the use of intradiscal injection of saline as a sham treatment arm. The hope is to clarify if saline is a real inactive/sham treatment, or does it have some therapeutic benefits that investigators should be aware of or even not to use saline as a sham treatment arm.
Methods
I Research Question
“Is intradiscal saline injection a real sham treatment?” A literature review was performed using Ovid EMBASE MEDLINE INFO from 1974 to July 17, 2020.
II Data Collection
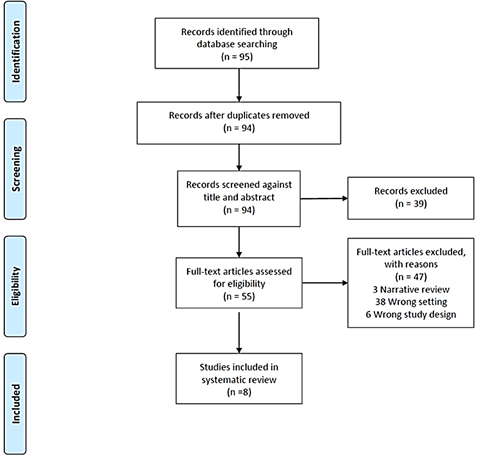
Inclusion criteria: Intradiscal electrothermal therapy, intradiscal drug administration, saline, and sodium chloride. All study designs limited to the English language were included. These studies were reviewed with regard to clinical application, dosage and route of administration, efficacy and potential side effects and complications. The level of evidence for each article selected for inclusion was determined based on the concept outlined by the Oxford Centre for Evidence-Based Medicine (CEBM) (Appendix 1). Results were filtered using the stepwise approach, as shown in the flowchart in (Figure 1).
Figure 1: PRISMA flowchart for study inclusion.
Results
We obtained 95 results after selecting the following keywords: intradiscal electrothermal therapy, intradiscal drug administration, saline, and sodium chloride. After title and abstract review, 39 studies were excluded. The remaining 55 studies were reviewed by two investigators. In case of disagreement, a third reviewer was used to break the tie. After full review, only 8 studies were included (see Figure 1 for inclusion and exclusion details). The level of evidence for each article selected for inclusion was determined based on the concept outlined by the Oxford Centre for Evidence-Based Medicine (CEBM), as shown in the (Appendix 1). Table 1 summarizes each of the studies meeting the inclusion criteria and the related level of evidence for each study according to Oxford CEBM.
Table 1: Summary of each of the
studies meeting the inclusion criteria and the related level of evidence for
each study according to Oxford CEBM.
|
Authors |
Study name |
Study design |
Patient population |
No. of patients |
Treatment groups |
Outcomes |
Level of evidence** |
|
Cao et al.
2011 [13] |
Intradiscal
injection therapy for degenerative chronic discogenic low back pain with end
plate Modic changes |
RCT |
Discogenic LBP
and end plate |
120 |
Intradiscal
injection of saline, diprospan, and diprospan+songmeile. |
No significant
pain relief within the groups receiving intradiscal saline. The groups that
received either diprospan or diprospan + songmeile injections significantly
improved their VAS and ODI scores. |
1b |
|
Peng et al. 2010 [6] |
A randomized placebo-controlled trial of intradiscal
methylene blue injection for the treatment of chronic discogenic low back
pain |
RCT |
Discogenic LBP longer than 6 months with no previous
lumbar surgery |
72 |
Intradiscal injection of methylene blue and isotonic
saline |
Mean reduction in NRS-101 of 52.50, and ODI of
35.58. As well as 91.6% patient satisfaction in the MB group vs 0.70%, 1.68%,
and 14.3% in the placebo group. |
1b |
|
Khot et al.
2004 [8] |
The Use of
Intradiscal Steroid Therapy for Lumbar |
RCT |
Chronic
discogenic LBP |
120 |
Intradiscal
injection of methylprednisolone and saline |
No difference
in outcomes measures (disability and pain scores) at 12 months |
1b |
|
Beall et al. 2020 [9] |
VAST Clinical Trial: Safely Supplementing Tissue
Lost to Degenerative Disc Disease |
RCT |
Disc degeneration at 1 or 2 vertebral levels from L1
to S1 with chronic low back pain for a minimum of 6 months |
220 |
Allograft, saline or continue nonsurgical management
(NSM) |
VAS improved at 6 months from 54.81 to 16.0 on the
allograft group and from 55.25 to 41 in the saline group. At 12 months the
allograft decreased to 12.27 and in the saline group decreased to 19.67. ODI
from 53.73 and 49.25 in the allograft and saline respectively to 18.47 at 6
months and 28.75 at 12 months in the allograft group. Saline group: 15.67 and
9.33 at 6 and 12 months, respectively. |
1b |
|
Kallewaard et
al. 2019 [7] |
A multicenter
randomized controlled trial on the efficacy of intradiscal methylene blue injection
for chronic discogenic low back pain: the IMBI study |
Double-blinded
RCT |
Chronic
discogenic low back pain for at least 6 months with poor response to
conservative therapy |
84 |
Intradiscal
injection of methylene blue and isotonic saline |
NRS between the
groups was statistically insignificant after 6 months with no change in the
PGIC |
1b |
|
Nguyen et al. 2017 [26] |
Intradiscal glucocorticoid injection for patients
with chronic low back pain (LBP) associated with active discopathy |
Double-blinded RCT |
Chronic lower back pain for at least 3 months with
discopathy on MRI |
135 |
glucocorticoids and iodixanol contrast vs iodixanol
contrast alone |
At 1 month 11-point NRS was higher in the GC IDI
(55.4%) vs control (33.3%), the improvement of LBP-related limitation
improved in the GC IDI group (84.6% VS 54.0%). At 3 months pain scores in the
GC IDI were higher than in the control and by 12 months, there were not
differences between the 2 groups |
1b |
|
Schwetschenau et al. 1976 [10] |
Double-blinded
evaluation of intradiscal chymopapain for herniated lumbar disc |
Double-blinded
RCT |
LBP with
radiculopathy and no improvement after 3 months of conservative treatment |
66 |
Chymopapain vs
placebo |
The successful
rate for the chymopapain group was 58% and for the placebo group was 49%,
with a p value of 0.14 |
1b |
|
Bae et al. 2014 [12] |
Is there clinical improvement associated with saline
injection for discogenic low back pain: comparison of RCTs |
Post-hoc comparison of RCT |
N/A |
N/A |
Intervertebral disc injection of saline vs
investigational drug |
At 12 months: saline patients had a 58.5% decreased
in VAS vs 36.6% decreased for the investigational group |
1a |
** level of evidence key.
According to Oxford Centre for Evidence-Based
Medicine (CEBM).
Peng and collaborators evaluated the treatment of chronic discogenic low back pain with intradiscal methylene blue (MB) injection in a double-blinded RCT [6]. 72 patients were confirmed with discogenic pain through a positive discography. Of those 72 patients, 36 patients received one ml of 1% MB injection; the remaining 36 patients received 1 ml of saline. The main outcome was pain alleviation and physical function improvement, assessed with 0-100 point’s numerical rating scale and ODI at 6, 12, and 24 months. There was a statistically significant difference between the MB and placebo (saline) groups when comparing NRS and ODI, with long-lasting results up to 24 months that favoured the MB group.
Kallewaard and collaborators replicated Peng et al. study in 2019 with a bigger sample size. 81 patients were enrolled in the study, 40 patients in the interventional group and 41 in the placebo (saline) group [6, 7]. The results did not support the findings of Peng et al. [6]. NRS between the groups was statistically insignificant after 6 months. Responders rate at 3 months, defined as >30% reduction in pain score, was 24.4% and 25% in placebo (saline) and treatment group, respectively. Patients’ global impression of change was also evaluated. In the placebo group, 26.8 and 24.4% reported improved PGIC at 3 and 6 months, respectively compared to 20 and 25% in the treatment group.
In a prospective, blinded RCT by Khot et al., 120 patients with chronic low back pain of discogenic origin, confirmed by discography, were randomized to receive an intradiscal injection of either saline (1 mL) or methylprednisolone (40 mg in 1 mL) after a positive discography [8]. The primary outcome was a change in disability scores at 1 year follow-up. Interestingly, there was no significant difference in disability scores between the groups. Patients in the steroid group reported a mean change of 2.28 in percentage disability compared to 3.42 with intradiscal saline injection. Moreover, there was no difference in changes of VAS among the groups even though the patients reported achieving pain relief with the administration of saline and steroids, indicating that no superiority was demonstrated between the two.
In a recent prospective, multicenter RCT, Beall and collaborators analysed the results of 220 patients with discogenic pain due to disc degeneration using MRI scoring, physical examination and pain evaluation [9]. Patients were randomized to receive intradiscal active allograft, non-surgical management (NSM) or saline as a placebo. Interim analysis of the first 24 patients was examined and clinical improvement was achieved at 6 months. VAS for back pain improved from 54.81 to 16.0 (70% improvement) for the allograft group and from 55.25 to 41.0 (26% improvement) for the saline group. At 12 months, VAS continued to decrease to 12.27 (78% improvement) and 19.67 (64% improvement) in allograft and saline group, respectively. More interestingly, average pain score and percentage reduction in VAS at 3 months were lower in the intradiscal saline group compared to intradiscal allograft, while in NSM average VAS score increased at 3 months. The ODI at 6 and 12 months improved by 66 and 76% for the allograft group, respectively and improved by 42 and 81% in placebo group at 6 and 12 months, respectively. Similarly, at 3 months, ODI increased from baseline for the NSM group. All NSM patients elected to cross over to the allograft group at 3 months.
In another double-blinded RCT, Nguyen et al. randomized 135 patients with low back pain (LBP) secondary to disc pathology to receive a single injection of either 1 mL of iodixanol contrast plus 1 mL (25 mg) of prednisolone acetate (2 mL total) versus 1 mL of iodixanol contrast only. Although the percentage of responders “defined to have LBP <40 on 0-100 NRS at 1 month” were statistically significant between the treatment and placebo group, 55% and 33%, respectively. 54% of the placebo group reported improvement in LBP-related limitations in activities at 1 month. This is, in addition to 33% of the placebo group achieving primary endpoint. After 3 months, the pain score started to increase in the treatment group, even higher than in the control group and at 12 months, no differences were seen between the two groups.
Schwetschenau and collaborators in 1976 studied chymopapain to treat lumbar herniated disc [10, 11]. 66 patients were enrolled in the double-blinded RCT. 35 patients received placebo (contrast diluted in water only) and 31 patients received contrast diluted in water plus chymopapain. The subjects were followed in 6 weeks, 3 months, 6 months and 12 months. The outcome of the study was classified as failure and successful response [‘success’ (if symptoms improved significantly)] or as [‘failure’ (if symptoms remained essentially unchanged or became worse)]. No statistically significant difference was found between the 2 groups. While chymopapain was successful in 53% of patients, intradiscal placebo injection showed 49% success rate. The authors conclude that there was no statistical significance and there was no advantage in using chymopapain.
Bae and collaborators performed a post hoc comparison using data from the results of four clinical trials assessing intervertebral disc injections. All trials were randomized, controlled trials utilizing intradiscal saline as a placebo. At 12 months, patients injected with intradiscal saline experienced a 58% reduction in their VAS score compared to only 36.6% VAS reduction in the treatment group. There was a statistically significant decrease in VAS for both groups across the four studies. The authors concluded that an intervertebral injection of saline could offer patients pain relief, decreased disability, diminish substance reaction and injection trauma [12].
On the other hand, there are some studies that contradict the possible therapeutic mechanism of action of intradiscal saline injection. In a double-blinded RCT, Cao and collaborators assessed the outcomes of intradiscal steroid therapy in patients with chronic discogenic pain [13]. They compared the effect of intradiscal saline, diprospan and diprospan+songmeile in patients with type I or type II Modic changes. In his RCT, there was no improvement in outcome measures with intradiscal normal saline injection while diprospan either alone or with songmeile resulted in statistically significant improvement in VAS and ODI at 3 and 6 months.
Discussion
Discogenic pain refers to pain originating from within the intervertebral disc due to derangement of the disc structure and the development of nociceptors as part of the degeneration that occurs with the aging process. Although it is an aging process, it is mistakenly called degenerative disc disease (DDD). Discogenic pain is a major cause of chronic low back pain in the United States. Degenerative changes of the disc include loss of water and proteoglycans and structural changes leading to imbalances between synthesis and degenerations favouring catabolism and disc degradation. Based on the degenerative process, one could conclude that the addition of an isotonic fluid e.g., normal saline solution, would aid the homeostasis maintenance of the structure hence decreasing the pain of such origin. If so, should we continue to use intradiscal saline injection as a sham treatment?
The potential therapeutic effect of saline injection has been studied previously in different interventions. In 1980, Frost and his colleagues randomized patients with myofascial trigger point pain into 2 groups to receive trigger point injections with local anaesthetics versus saline. It was surprising when they found that the group who received saline injection tended to have better pain relief in an experimental animal study, where authors injected rabbits with intradiscal hypertonic saline for the purpose of decreasing intradiscal pressure and relieving the pain generated by lumbar disc herniation through chemonucleolysis [14]. Intradiscal injections were administered in rabbits at 1, 4, 8, and 12 months. The authors concluded that 0.02 ml 10% hypertonic saline has the potential for reducing intradiscal pressure. Furthermore, an injection of a higher amount and concentration could be effective clinically [15].
An interesting, randomized control study conducted by Karppinen and his colleagues comparing transforaminal epidural methylprednisolone bupivacaine combination or saline found significant leg pain relief in favour of the steroid group but there was statistically significant more improvement in back pain in the saline group at 3 and 6 months [16]. Similarly, the use of intradiscal saline injection was found to have a positive effect in 6 out of 8 studies, demonstrating some improvement in pain and disability scores with sham treatment or at least no significant difference between sham and investigational treatment. One study showed no significant improvement in pain scores or functionality with intradiscal saline injection compared to methylene blue, while subjects who received intradiscal methylene blue reported statistically significant improvement in pain scores. Nonetheless, when the study was replicated by Kallewaard in 2019, it did not show a significant difference between intradiscal saline injections and intradiscal methylene blue [7]. Among subjects who received intradiscal saline injection, the responder rates were 17, 24.4 and 26.7 at 6 weeks, 3 and 6 months, respectively.
In animal models, the expression of pro-inflammatory mediators has been studied and compared between healthy versus degenerated intervertebral discs. It was found that induction of degenerative disc changes increases expression of Interleukin (IL) 1, 8 10 and Tumor necrosis factor α (TNF-α), with a more exaggerated response with repeated and prolonged injury [17, 18]. Similarly, in humans, IL-1β and TNF-α were elevated in degenerated and herniated intervertebral discs [19, 20]. Although the exact etiology for intervertebral disks (IVDs) degeneration is unclear, there are multiple hypotheses explaining potential mechanisms of IVDs degenerations. It includes up-regulation of proteolytic enzymes e.g., aggrecanases, alkaline phosphatase and inflammatory cytokines e.g., interleukin 1β (IL-1β) [21]. In another interesting study that was published in nature, Gilbert et al. found that acidic intervertebral disc media promotes disc degeneration. Another possible explanation of the therapeutic effect of intradiscal saline is neutralizing IVD acidic media which will slow down disc degeneration [22].
Although the mechanism of pain and disability improvement with intradiscal saline injection is yet unclear, there is some speculation that saline injection can potentially dilute/wash out inflammatory mediators, proteolytic enzymes and cytokines that in turn ameliorate nerve endings irritations or by neutralizing IVD acidic media [5]. A study investigating the effects of local anaesthetics in degenerated rabbit IVDs showed interesting results [23]. During the in vivo analysis, the number of cells in the nucleus pulposus was significantly decreased among the saline and local anaesthetics groups compared with the control and puncture-only groups. The results were confirmed with histologic analysis with no difference between the saline, puncture-only, bupivacaine, and lidocaine groups. In a prospective study, 20 out of 25 patients with low back pain due to disk herniation achieved tearing of the thinned posterior longitudinal ligament after undergoing a high-pressure injection of saline. These patients received a single high-pressure injection of 5-10 mL of normal saline into the nucleus of the disk. Even though patients experience immediate pain relief, long-term follow-up is pending [24]. In a double-blinded RCT comparing biacuplasty to sham treatment, there was no statistical significance in VAS scores and ODI at 8 weeks between the 2 groups. Nonetheless, VAS and ODI showed similarities and even showed slightly more improvement in the sham group. However, it might be a placebo effect. One cannot exclude a possible mechanical mechanism or similar mechanism of action to trigger point injection (TPI) which, in addition to local anaesthetic effect, could be secondary to mechanical disruption of muscle pain and release of local mediators [25]. On the other hand, there are some studies that contradict the possible therapeutic mechanical mechanism of action of intradiscal saline injection.
Conclusion
The use of saline possibly represents the result of a type II statistical error when used as in the control group vs active treatment for the management of chronic pain. Having pain relief in a control group is detrimental to the objectivity of the study and this error could pass unnoticed by investigators. On the other hand, the use of saline could be useful, pending further trials, as a treatment in the management of chronic pain.
Appendix 1: Level of evidence by
the Oxford Centre for Evidence-Based Medicine (CEBM).
|
Level |
Therapy/Prevention,
Aetiology/Harm |
Prognosis |
Diagnosis |
Differential
diagnosis/symptom prevalence study |
Economic
and decision analyses |
|
1a |
SR of RCTs |
SR of inception
cohort studies; CDR” validated in different populations |
SR of Level 1
diagnostic studies; CDR” with 1b studies from different clinical centers |
SR of prospective
cohort studies |
SR of Level 1
economic studies |
|
1b |
Individual RCT (with
narrow Confidence Interval) |
Individual inception
cohort study with > 80% follow-up; CDR” validated in a single population |
Validating** cohort
study with good” reference standards; or CDR” tested within one clinical
center |
Prospective cohort
study with good follow-up**** |
Analysis based on
clinically sensible costs or alternatives; systematic review(s) of the
evidence; and including multi-way sensitivity analyses |
|
1c |
All or none§ |
All or none
case-series |
Absolute SpPins and
SnNouts” “ |
All or none
case-series |
Absolute better-value
or worse-value analyses |
|
2a |
SR (with
homogeneity*) of cohort studies |
SR (with
homogeneity*) of either retrospective cohort studies or untreated control
groups in RCTs |
SR (with
homogeneity*) of Level >2 diagnostic studies |
SR (with
homogeneity*) of 2b and better studies |
SR (with
homogeneity*) of Level >2 economic studies |
|
2b |
Individual cohort
study (including low quality RCT, e.g., <80% follow-up) |
Retrospective cohort
study or follow-up of untreated control patients in an RCT; Derivation of
CDR” or validated on split-sample§§§ only |
Exploratory** cohort
study with good” reference standards; CDR” after derivation, or validated
only on split-sample§§§ or databases |
Retrospective cohort
study, or poor follow-up |
Analysis based on
clinically sensible costs or alternatives; limited review(s) of the evidence,
or single studies; and including multi-way sensitivity analyses |
|
2c |
“Outcomes” Research;
Ecological studies |
“Outcomes” Research |
|
Ecological studies |
Audit or outcomes
research |
|
3a |
SR (with
homogeneity*) of case-control studies |
|
SR (with
homogeneity*) of 3b and better studies |
SR (with
homogeneity*) of 3b and better studies |
SR (with homogeneity*)
of 3b and better studies |
|
3b |
Individual
Case-Control Study |
|
Non-consecutive
study; or without consistently applied reference standards |
Non-consecutive
cohort study, or very limited population |
Analysis based on
limited alternatives or costs, poor quality estimates of data, but including
sensitivity analyses incorporating clinically sensible variations. |
|
4 |
Case-series (and
poor-quality cohort and case-control studies§§) |
Case-series (and
poor-quality prognostic cohort studies***) |
Case-control study,
poor or non-independent reference standard |
Case-series or
superseded reference standards |
Analysis with no
sensitivity analysis |
|
5 |
Expert opinion
without explicit critical appraisal, or based on physiology, bench research
or “first principles” |
Expert opinion
without explicit critical appraisal, or based on physiology, bench research
or “first principles” |
Expert opinion
without explicit critical appraisal, or based on physiology, bench research
or “first principles” |
Expert opinion without
explicit critical appraisal, or based on physiology, bench research or “first
principles” |
Expert opinion
without explicit critical appraisal, or based on economic theory or “first
principles” |
Produced by Bob Phillips,
Chris Ball, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin
Dawes since November 1998. Updated by Jeremy Howick March 2009 (Link).
Article Info
Article Type
Original Research and Review of LiteraturePublication history
Received: Tue 29, Jun 2021Accepted: Wed 14, Jul 2021
Published: Fri 30, Jul 2021
Copyright
© 2023 Nagy Mekhail. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2021.02.04
Author Info
Nagy Mekhail Youssef Saweris Lou-Anne Acevedo-Moreno
Corresponding Author
Nagy MekhailEvidence-Based Pain Management Research, Cleveland Clinic, Cleveland, Ohio, USA
Figures & Tables
Table 1: Summary of each of the
studies meeting the inclusion criteria and the related level of evidence for
each study according to Oxford CEBM.
|
Authors |
Study name |
Study design |
Patient population |
No. of patients |
Treatment groups |
Outcomes |
Level of evidence** |
|
Cao et al.
2011 [13] |
Intradiscal
injection therapy for degenerative chronic discogenic low back pain with end
plate Modic changes |
RCT |
Discogenic LBP
and end plate |
120 |
Intradiscal
injection of saline, diprospan, and diprospan+songmeile. |
No significant
pain relief within the groups receiving intradiscal saline. The groups that
received either diprospan or diprospan + songmeile injections significantly
improved their VAS and ODI scores. |
1b |
|
Peng et al. 2010 [6] |
A randomized placebo-controlled trial of intradiscal
methylene blue injection for the treatment of chronic discogenic low back
pain |
RCT |
Discogenic LBP longer than 6 months with no previous
lumbar surgery |
72 |
Intradiscal injection of methylene blue and isotonic
saline |
Mean reduction in NRS-101 of 52.50, and ODI of
35.58. As well as 91.6% patient satisfaction in the MB group vs 0.70%, 1.68%,
and 14.3% in the placebo group. |
1b |
|
Khot et al.
2004 [8] |
The Use of
Intradiscal Steroid Therapy for Lumbar |
RCT |
Chronic
discogenic LBP |
120 |
Intradiscal
injection of methylprednisolone and saline |
No difference
in outcomes measures (disability and pain scores) at 12 months |
1b |
|
Beall et al. 2020 [9] |
VAST Clinical Trial: Safely Supplementing Tissue
Lost to Degenerative Disc Disease |
RCT |
Disc degeneration at 1 or 2 vertebral levels from L1
to S1 with chronic low back pain for a minimum of 6 months |
220 |
Allograft, saline or continue nonsurgical management
(NSM) |
VAS improved at 6 months from 54.81 to 16.0 on the
allograft group and from 55.25 to 41 in the saline group. At 12 months the
allograft decreased to 12.27 and in the saline group decreased to 19.67. ODI
from 53.73 and 49.25 in the allograft and saline respectively to 18.47 at 6
months and 28.75 at 12 months in the allograft group. Saline group: 15.67 and
9.33 at 6 and 12 months, respectively. |
1b |
|
Kallewaard et
al. 2019 [7] |
A multicenter
randomized controlled trial on the efficacy of intradiscal methylene blue injection
for chronic discogenic low back pain: the IMBI study |
Double-blinded
RCT |
Chronic
discogenic low back pain for at least 6 months with poor response to
conservative therapy |
84 |
Intradiscal
injection of methylene blue and isotonic saline |
NRS between the
groups was statistically insignificant after 6 months with no change in the
PGIC |
1b |
|
Nguyen et al. 2017 [26] |
Intradiscal glucocorticoid injection for patients
with chronic low back pain (LBP) associated with active discopathy |
Double-blinded RCT |
Chronic lower back pain for at least 3 months with
discopathy on MRI |
135 |
glucocorticoids and iodixanol contrast vs iodixanol
contrast alone |
At 1 month 11-point NRS was higher in the GC IDI
(55.4%) vs control (33.3%), the improvement of LBP-related limitation
improved in the GC IDI group (84.6% VS 54.0%). At 3 months pain scores in the
GC IDI were higher than in the control and by 12 months, there were not
differences between the 2 groups |
1b |
|
Schwetschenau et al. 1976 [10] |
Double-blinded
evaluation of intradiscal chymopapain for herniated lumbar disc |
Double-blinded
RCT |
LBP with
radiculopathy and no improvement after 3 months of conservative treatment |
66 |
Chymopapain vs
placebo |
The successful
rate for the chymopapain group was 58% and for the placebo group was 49%,
with a p value of 0.14 |
1b |
|
Bae et al. 2014 [12] |
Is there clinical improvement associated with saline
injection for discogenic low back pain: comparison of RCTs |
Post-hoc comparison of RCT |
N/A |
N/A |
Intervertebral disc injection of saline vs
investigational drug |
At 12 months: saline patients had a 58.5% decreased
in VAS vs 36.6% decreased for the investigational group |
1a |
** level of evidence key.
According to Oxford Centre for Evidence-Based
Medicine (CEBM).
Appendix 1: Level of evidence by
the Oxford Centre for Evidence-Based Medicine (CEBM).
|
Level |
Therapy/Prevention,
Aetiology/Harm |
Prognosis |
Diagnosis |
Differential
diagnosis/symptom prevalence study |
Economic
and decision analyses |
|
1a |
SR of RCTs |
SR of inception
cohort studies; CDR” validated in different populations |
SR of Level 1
diagnostic studies; CDR” with 1b studies from different clinical centers |
SR of prospective
cohort studies |
SR of Level 1
economic studies |
|
1b |
Individual RCT (with
narrow Confidence Interval) |
Individual inception
cohort study with > 80% follow-up; CDR” validated in a single population |
Validating** cohort
study with good” reference standards; or CDR” tested within one clinical
center |
Prospective cohort
study with good follow-up**** |
Analysis based on
clinically sensible costs or alternatives; systematic review(s) of the
evidence; and including multi-way sensitivity analyses |
|
1c |
All or none§ |
All or none
case-series |
Absolute SpPins and
SnNouts” “ |
All or none
case-series |
Absolute better-value
or worse-value analyses |
|
2a |
SR (with
homogeneity*) of cohort studies |
SR (with
homogeneity*) of either retrospective cohort studies or untreated control
groups in RCTs |
SR (with
homogeneity*) of Level >2 diagnostic studies |
SR (with
homogeneity*) of 2b and better studies |
SR (with
homogeneity*) of Level >2 economic studies |
|
2b |
Individual cohort
study (including low quality RCT, e.g., <80% follow-up) |
Retrospective cohort
study or follow-up of untreated control patients in an RCT; Derivation of
CDR” or validated on split-sample§§§ only |
Exploratory** cohort
study with good” reference standards; CDR” after derivation, or validated
only on split-sample§§§ or databases |
Retrospective cohort
study, or poor follow-up |
Analysis based on
clinically sensible costs or alternatives; limited review(s) of the evidence,
or single studies; and including multi-way sensitivity analyses |
|
2c |
“Outcomes” Research;
Ecological studies |
“Outcomes” Research |
|
Ecological studies |
Audit or outcomes
research |
|
3a |
SR (with
homogeneity*) of case-control studies |
|
SR (with
homogeneity*) of 3b and better studies |
SR (with
homogeneity*) of 3b and better studies |
SR (with homogeneity*)
of 3b and better studies |
|
3b |
Individual
Case-Control Study |
|
Non-consecutive
study; or without consistently applied reference standards |
Non-consecutive
cohort study, or very limited population |
Analysis based on
limited alternatives or costs, poor quality estimates of data, but including
sensitivity analyses incorporating clinically sensible variations. |
|
4 |
Case-series (and
poor-quality cohort and case-control studies§§) |
Case-series (and
poor-quality prognostic cohort studies***) |
Case-control study,
poor or non-independent reference standard |
Case-series or
superseded reference standards |
Analysis with no
sensitivity analysis |
|
5 |
Expert opinion
without explicit critical appraisal, or based on physiology, bench research
or “first principles” |
Expert opinion
without explicit critical appraisal, or based on physiology, bench research
or “first principles” |
Expert opinion
without explicit critical appraisal, or based on physiology, bench research
or “first principles” |
Expert opinion without
explicit critical appraisal, or based on physiology, bench research or “first
principles” |
Expert opinion
without explicit critical appraisal, or based on economic theory or “first
principles” |
Produced by Bob Phillips,
Chris Ball, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin
Dawes since November 1998. Updated by Jeremy Howick March 2009 (Link).
References
1. Kabisch M, Ruckes C, Seibert Grafe M, Blettner M (2011) Randomized
controlled trials: part 17 of a series on evaluation of scientific
publications. Dtsch Arztebl Int 108:
663-668. [Crossref]
2. Probst P, Grummich K, Heger P, Zaschke S, Knebel P et al. (2016) Blinding
in randomized controlled trials in general and abdominal surgery: protocol for
a systematic review and empirical study. Syst
Rev 5: 48. [Crossref]
3. Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA et al. (2014) A
randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med 371: 11-21. [Crossref]
4.
Bar Or D, Rael LT,
Brody EN (2017) Use of Saline as a Placebo in Intra-articular Injections in
Osteoarthritis: Potential Contributions to Nociceptive Pain Relief. Open
Rheumatol J 11: 16-22. [Crossref]
5.
Fukusaki M, Kobayashi
I, Hara T, Sumikawa K (1998) Symptoms of spinal stenosis do not improve after
epidural steroid injection. Clin J Pain
14: 148-151. [Crossref]
6.
Peng B, Pang X, Wu Y,
Zhao C, Song X (2010) A randomized placebo-controlled trial of intradiscal
methylene blue injection for the treatment of chronic discogenic low back pain.
Pain 149: 124-129. [Crossref]
7.
Kallewaard JW,
Wintraecken VM, Geurts JW, Willems PC, van Santbrink H et al. (2019) A
multicenter randomized controlled trial on the efficacy of intradiscal
methylene blue injection for chronic discogenic low back pain: the IMBI study. Pain 160: 945-953. [Crossref]
8.
Khot A, Bowditch M,
Powell J, Sharp D (2004) The use of intradiscal steroid therapy for lumbar
spinal discogenic pain: a randomized controlled trial. Spine (Phila Pa 1976) 29: 833-836. [Crossref]
9.
Beall DP, Wilson GL,
Bishop R, Tally W (2020) VAST Clinical Trial: Safely Supplementing Tissue Lost
to Degenerative Disc Disease. Int J Spine
Surg 14: 239-253. [Crossref]
10. Schwetschenau PR, Ramirez A, Johnston J, Wiggs C, Martins AN (1976)
Double-blind evaluation of intradiscal chymopapain for herniated lumbar discs.
Early results. J Neurosurg 45:
622-627. [Crossref]
11. Martins AN, Ramirez A, Johnston J, Schwetschenau PR (1978) Double-blind
evaluation of chemonucleolysis for herniated lumbar discs. Late results. J Neurosurg 49: 816-827. [Crossref]
12. Bae HW, Kanim L, Kim J, Provenzano NJ, Thordarson SR (2014) Is There
Clinical Improvement Associated with Saline Injection for Discogenic Low Back
Pain: Comparison of RCTs. Spine J 14:
S32.
13. Cao P, Jiang L, Zhuang C, Yang Y, Zhang Z et al. (2011) Intradiscal
injection therapy for degenerative chronic discogenic low back pain with end
plate Modic changes. Spine J 11:
100-106. [Crossref]
14. Frost FA, Jessen B, Siggaard Andersen J (1980) A control, double-blind
comparison of mepivacaine injection versus saline injection for myofascial
pain. Lancet 1: 499-500. [Crossref]
15. Sato K, Nagata K, Hirohashi T (2002) Intradiscal pressure after repeat
intradiscal injection of hypertonic saline: an experimental study. Eur Spine J 11: 52-56. [Crossref]
16.
Manchikanti L, Nampiaparampil DE, Manchikanti KN, Falco FJE, Singh V et
al. (2015) Comparison of the efficacy of saline,
local anesthetics, and steroids in epidural and facet joint injections for the
management of spinal pain: A systematic review of randomized controlled trials.
Surg Neurol Int 6: S194-S235. [Crossref]
17. Rousseau MAA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ et al. (2007) Stab
incision for inducing intervertebral disc degeneration in the rat. Spine (Phila Pa 1976) 32: 17-24. [Crossref]
18. Holm S, Mackiewicz Z, Holm AK, Konttinen YT, Kouri VP et al. (2009)
Pro-inflammatory, pleiotropic, and anti-inflammatory TNF-alpha, IL-6, and IL-10
in experimental porcine intervertebral disk degeneration. Vet Pathol 46: 1292-1300. [Crossref]
19. Le Maitre CL, Freemont AJ, Hoyland JA (2005) The role of interleukin-1 in
the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 7: R732-R745. [Crossref]
20. Kokubo Y, Uchida K, Kobayashi S, Yayama T, Sato R et al. (2008) Herniated
and spondylotic intervertebral discs of the human cervical spine: histological
and immunohistological findings in 500 en bloc surgical samples. Laboratory
investigation. J Neurosurg Spine 9:
285-295. [Crossref]
21. Risbud MV, Shapiro IM (2014) Role of cytokines in intervertebral disc
degeneration: pain and disc content. Nat Rev Rheumatol 10: 44-56. [Crossref]
22. Gilbert HTJ, Hodson N, Baird P, Richardson SM,
Hoyland JA (2016) Acidic pH promotes intervertebral disc degeneration:
Acid-sensing ion channel -3 as a potential therapeutic target. Sci Rep
6: 37360. [Crossref]
23. Ura K, Sudo H, Iwasaki K, Tsujimoto T, Ukeba D et al. (2019) Effects of
Intradiscal Injection of Local Anesthetics on Intervertebral Disc Degeneration
in Rabbit Degenerated Intervertebral Disc. J
Orthop Res 37: 1963-1971. [Crossref]
24. Kanai A (2009) Treatment of lumbar disk herniation by percutaneous
intradiscal high-pressure injection of saline. Pain Med 10: 76-84. [Crossref]
25. Wong CSM, Wong SHS (2012) A new look at trigger point injections. Anesthesiol Res Pract 2012: 492452. [Crossref]
26. Nguyen C, Boutron I, Baron G, Sanchez K, Palazzo C et al. (2017) Intradiscal Glucocorticoid Injection for Patients With Chronic Low Back Pain Associated With Active Discopathy: A Randomized Trial. Ann Intern Med 166: 547-556. [Crossref]
