Journals
Inhibitory effect of quercetin on protein expression CtBP1 and migration of hepatocellular carcinoma cell SMMC-7721
A B S T R A C T
Our study aimed to investigate the potential role of quercetin in the migration of hepatocellular carcinoma cells, SMMC-7721, and its underlying mechanism. Cell monolayer scratching assay was performed to explore the role of quercetin in the migration ability of SMMC-7721 cells. Western Blot and immunofluorescence assay were conducted to detect the protein expression of transcriptional co-suppressor, CtBP1, and epithelial adhesion molecule, E-cadherin. The migration ability of SMMC-7721 cells was decreased by the addition of quercetin in a time and concentration dependent manner. Results of western blot and immunofluorescence assay demonstrated that quercetin inhibited the expression of CtBP1, while up-regulated the expression of E-cadherin. These findings suggest that quercetin contributed to the up-regulation of E-cadherin through inhibition of CtBP1 expression. This in turn hinders the underlying EMT process, and finally prevents the invasiveness and metastasis of HCC cells. However, the mechanism how quercetin up-regulation CtBP1 in HCC cells still need to be elucidated.
K E Y W O R D S
Quercetin, CtBP1, E-cadherin, EMT, Hepatocellular carcinoma
I N T R O D U C T I O N
Hepatocellular Carcinoma (HCC) is a common malignancy of the digestive system. Majority of the HCC cases are prevalent in areas with high hepatitis virus infection, which include China and Africa [1]. HCC is associated with strong ability of invasiveness and metastasis and high mortality [2, 3]. C-terminal binding protein 1 (CtBP1) is a transcriptional co-activator that suppress the expression of epithelial adhesion molecules, cancer suppressor genes and pro-apoptotic proteins. This consequently promotes epithelial-mesenchymal transition (EMT), cell cycle and inhibits apoptosis [4]. As a co-activator of transcriptional factors, such as ZEB1 and SNAIL, CtBP1 can inhibit the expression of E-cadherin, and promote the invasiveness and metastasis of colon cancer [5]. Our previous study proved that the expression of CtBP1 was up-regulated in HCC patients, while downregulated its targeted gene, E-cadherin, which was associated with invasiveness and metastasis of HCC [6].
Quercetin (Q) is the most common flavonoid in nature, that is mostly present in green leafy vegetables, apples, onions, cauliflower, tea and berries, with anti-inflammatory, anti-allergic, antiviral, anti-tumor and antioxidant biological activities [7]. Quercetin hinders cell cycle and induces apoptosis, and its anti-tumor effects inhibit the invasiveness and metastasis of cancer cells [8, 9]. This acts by repressing the invasiveness and metastasis of cancer cells by blocking the metastasis-associated signaling pathways and protein expression [10-15]. In the present study, we firstly reported that quercetin can inhibit the expression of CtBP1 and up-regulate the expression of E-cadherin, which consequently explains the underlying mechanism of metastasis inhibition of HCC cells.
Materials and Methods
Materials and Reagents
Quercetin was purchased from Shanghai Yaji Biotechnology Co., Ltd. It was dissolved with dimethyl sulfoxide (DMSO) and diluted with serum-free medium (Eagle's essential medium, DMEM) to the desired concentration. Fetal bovine serum (FBS) was purchased from Hangzhou Four Seasons Green Bioengineering Materials Co., Ltd. DMEM medium was purchased from the GIBCO company (USA). Trypsin was purchased from Sigma company (USA). Penicillin-streptomycin mixture (100X) was purchased from Beijing Solarbio Life Science Co., Ltd. DMSO was purchased from Beijing Solarbio Life Science Co., Ltd. HCC cell line, SMMC-7721, was purchased from Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
Cell line and cell culture
SMMC-7721 cells were maintained in DMEM supplemented with 10% FBS. Cells were cultured at 37°C in a humidified incubator with 5% CO2.
Cell monolayer scratching assay
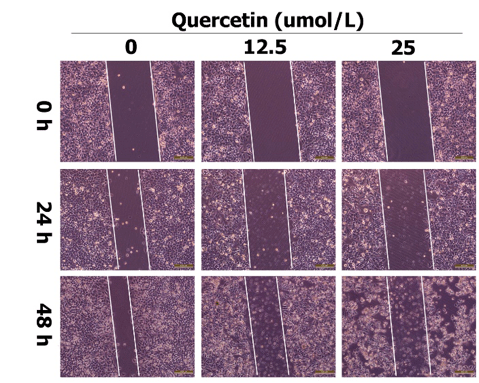
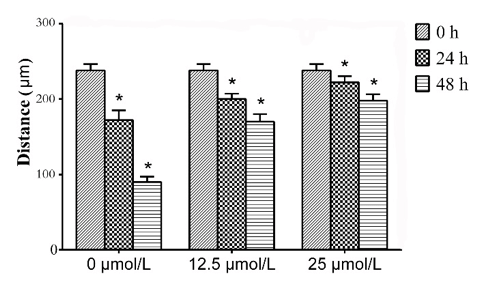
SMMC-7721 cells were grown in 6-well plates with DMEM medium supplemented with 10% FBS until 80% confluence. Wounds were created by scratching the cell surface with a 10μL pipette tip. After washing with PBS, quercetin solution (12.5μmol/L and 25μmol/L) was added. Quercetin were disolved in DMSO and the final concentration were 0.1 % (Vol/Vol) in all groups including two quercetin treated group and the blank control group. Every group were triplicated in three wells. After 24h and 48h, cells moving and growing across the scratched lines were monitored using an inverted microscope (TS100-F, Japan Nikon Corporation). The migratory ability of the cells was quantified as the gap distance recovered compared with the original gap.
Western blotting
The SMMC-7721 cells were treated with quercetin of different concentrations and collected after 6, 12, and 24 hours. SMMC-7721 cells were lysed with radio immunoprecipitation assay (RIPA) Lysis Buffer supplemented with protease inhibitors, PMSF. The lysate was centrifuged for 30min at 12 000 rpm and 4°C. The protein was quantified by BCA protein concentration assay. The total proteins (20 μg) in the supernatant was separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% evaporated milk for 1h and subsequently incubated with a mouse anti-human CtBP1 monoclonal antibody (Santa Cruz, USA; Cat.No. sc-17805; Dilution: 1:1000) for overnight at 4°C. The membranes were then incubated with an HRP conjugated goat anti-mouse IgG antibody (ZSBIO, Beijing; Cat.No. ZDR5307; Dilution: 1:2000) for 1h at room temperature. Chemiluminescence was detected using SuperSignal West Femto Maximum Sensitivity Substrate. The ImageJ software was used to detect the grayscale values of each band.
Immunofluorescence assay
SMMC-7721 Cells (n=8x103) were seeded on the cover slides in 24-well plates in DMEM medium supplemented with 10% FBS and grown for 24-48 hours. The cells were then treated with different concentrations of Quercetin for 6 hours and fixed in 4% paraformaldehyde for 20 min at room temperature. After washing with PBS, the cells were blocked by 5% BSA for 30-60 min at room temperature. Then the blocked fluid was removed, sufficient amount of diluted primary antibody was added, and placed in a wet box, incubated for overnight at 4°C. After washing with PBST, the cells were stained with fluorescence secondary antibody and incubated at room temperature for 1 hour. The cells were washed by PBST for three times and stained by DAPI. Fluorescent images were obtained using a confocal microscope.
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed using the SPSS 17.0 software. Statistical comparisons between groups were made with one-way analysis of variance (ANOVA); Bonferroni's post hoc test was subsequently used to compare individual means. P < 0.05 was considered statistically significant.
Figure 1: The average size of control Transferosomal nano-carriers. Particle size was measured by Zeta Sizer. The average size of Nano-Transferosomal HU was 52.09±4.6 nm (Fig. 2), and the zeta potential was -8.07±0.7.
Figure 2: The average size of Nano-Transferosomal HU. Particle size was measured by Zeta Sizer.
Figure 3: Drug release rate of nanotransferosomal HU. Results were expressed as mean ± 5% values. Curve initiated with a burst release, in following by the mild ascending slope with the maximal release after 48 hours.
Figure 4: Cytotoxic effects of nanotransferosomal HU and standard HU on the viability (MTT assay) of mouse breast cancer cell line 4T1. Results were expressed as mean ± SD values of two independent experiments.
Figure 5: Cytotoxic effects of nanotransferosomal HU and standard HU on the viability (MTT assay) of mouse breast cancer cell line 4T1. Results were expressed as mean ± SD values of two independent experiments.
Results
Quercetin inhibited the migration of SMMC-7721 cells. Cell monolayer scratching assay was performed to evaluate the effect of quercetin on migration of SMMC-7221 cells. Results revealed that the distance of cell migration was significantly decreased with the increase of time and concentration of quercetin (P < 0.05, Figures 1 & 2).
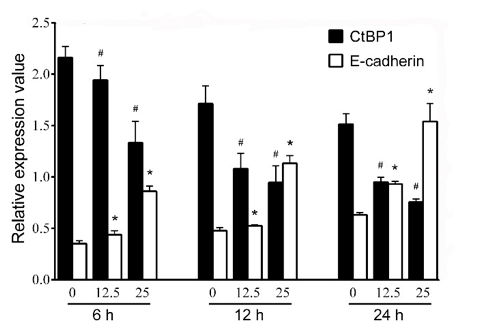
Quercetin inhibited the expression of CtBP1 and promoted the expression of E-cadherin. Western blotting analysis was performed to evaluate the expressions of CtBP1 and E-cadherin. Results showed that the expression of CtBP1 was decreased and the expression of E-cadherin was increased with the change of time and concentration of quercetin (Figures 3 & 4). Furthermore, quercetin demonstrated significant inhibitory effect at 6h.
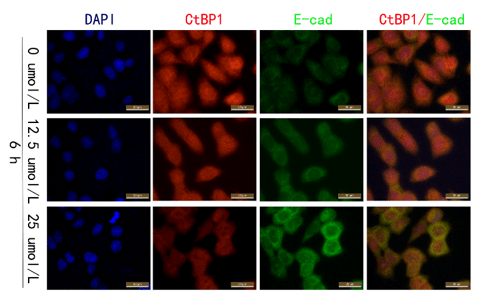
Immunofluorescence assay indicated that the expression of CtBP1 in SMMC-7721 gradually decreased, while the expression of E-cadherin was increased with increased concentrations of quercetin (Figure 5).
Discussion
Quercetin is one of the richest flavonoid seen in foods, and is abundant in fruits, green leafy vegetables and seeds, buckwheat, nuts, flowers, bark, broccoli, olive oil, apples, onions, green tea, red grapes, red wine, black cherries, blueberries and cranberries. Quercetin is used as a nutritional supplement and beneficial against a variety of diseases, including cardiovascular protection, anticancer, antitumor, anti-ulcer, anti-allergy, anti-viral, anti-inflammatory, anti-diabetic, anti-hypertensive, immune regulatory, and anti-infective [16].
According to an epidemiological survey, vegetables and fruits in daily diet prevents people from cancer. The potential anti-tumor effects of quercetin include inhibition of proliferation, growth factors and antioxidants [17]. Quercetin can induce apoptosis, inhibit tumor growth and spread of brain, liver, and colon cells [18, 19]. In addition, quercetin can suppress the invasiveness and metastasis of cancer cells [20, 21]. The present study aimed to investigate the function of quercetin in HCC metastasis and its underlying mechanism.
HCC is one of the most common malignant tumors of the digestive system and has a strong invasion and metastasis ability. Intrahepatic blood metastasis usually occurs in the early stages of HCC, resulting in the poor prognosis. We used the low-toxic dosage of quercetin (12.5, 25μmol/L) to treat SMMC-7721 cells, and cell monolayer scratching assay demonstrated that it could inhibit the migration, which was in accordance with the results of lung cancer and colon cancer studies [20, 21]. Meanwhile, evenly distributed cells were observed within the middle of the scratches. They are obviously not the cells migrated from the neighboring cells. These cells maybe derive from the floating cells, which show weakened adhesion ability induced by higher concertation of quection, attaching to the dishes again. But the mechanism still need further investigation.
EMT is a pathophysiological and physiological process, where epithelial cells achieve interstitial phenotype to increase the abilities of invasion and migration, and played a key role in the development and progression of cancer [22]. In the EMT process, the expression of epithelial markers (such as E-cadherin and β-catenin) was down-regulated, while the mesenchymal markers (such as VIM and N-cadherin) was up-regulated [22].
In lung cancer, quercetin inhibited the migration of cancer cells through suppression of mesenchymal cytoskeletal protein expression, VIM and N-cadherin [20].
In colorectal cancer, non-toxic dosage of quercetin could inhibit the EMT process by regulating the expression of E-cadherin, N-cadherin, β-catenin and snail, thus arresting the metastasis [21].
Previous studies showed that CtBP1 could bind to EMT-related transcription factors, including ZEB1, SNAIL, and SLUG to form a transcriptional inhibitory complex, which inhibits the expression of E-cadherin and promotes the process of EMT [5]. CtBP1 was proved to be up-regulated in HCC tissues and negatively correlated with E-cadherin expression [6]. CtBP1 in HCC cells suppressed the expression of E-cadherin and promoted the invasiveness and metastasis [6].
The above discussed results showed that quercetin inhibited the expression of CtBP1 in hepatocellular carcinoma cells and up-regulated the expression of E-cadherin, suggesting that quercetin could inhibit the invasion and metastasis of hepatocellular carcinoma through CtBP1/E-cadherin pathway. In conclusion, quercetin can inhibit the migration of hepatocellular carcinoma cells, suggesting its potential application value in the treatment of metastatic liver cancer.
Acknowledgments
This work was supported by grants from Guangxi health and Family Planning Commission (No. Z2015404).
Article Info
Article Type
Research ArticlePublication history
Received: Fri 25, May 2018Accepted: Mon 04, Jun 2018
Published: Mon 11, Jun 2018
Copyright
© 2023 Xiaoling Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2018.02.001
Author Info
Jianhong Tang Lihui Bian Shengjun Xiao Xiaoling Zhang Xiaoyu Wang Yueli Yang
Corresponding Author
Xiaoling ZhangDepartment of Physiology, Faculty of Basic Medical Sciences, Guilin Medical University, Guilin, Guangxi 541004, People's Republic of China
Figures & Tables
Figures & Tables

References
1. Zheng R, Zeng H, Zhang S, Chen W (2017) Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 36: 66. [Crossref]
2. Chen JL, Lin XJ, Zhou Q, Shi M, Li SP, et al. (2016) Association of HBV DNA replication with antiviral treatment outcomes in the patients with early-stage HBV-related hepatocellular carcinoma undergoing curative resection. Chin J Cancer 35: 28. [Crossref]
3. Oliveri RS, Wetterslev J and Gluud C (2012) Hepatocellular carcinoma. Lancet 380: 470; author reply 470-471.
4. Chinnadurai G (2009) The transcriptional corepressor CtBP: a foe of multiple tumor suppressors. Cancer Res 69: 731-734. [Crossref]
5. Pena C, Garcia JM, Garcia V, Silva J, Dominguez G, et al. (2006) The expression levels of the transcriptional regulators p300 and CtBP modulate the correlations between SNAIL, ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas. Int J Cancer 119: 2098-2104. [Crossref]
6. Zhang XL, Huang CX, Zhang J, Inoue A, Zeng SE, et al. (2013) CtBP1 is involved in epithelial-mesenchymal transition and is a potential therapeutic target for hepatocellular carcinoma. Oncol Rep 30: 809-814. [Crossref]
7. Cai X, Fang Z, Dou J, Yu A and Zhai G (2013) Bioavailability of quercetin: problems and promises. Curr Med Chem 20: 2572-2582. [Crossref]
8. Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, et al. (2010) The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-kappaB inhibition. Eur J Pharmacol 649: 84-91. [Crossref]
9. Zhang W and Zhang F (2009) Effects of quercetin on proliferation, apoptosis, adhesion and migration, and invasion of HeLa cells. Eur J Gynaecol Oncol 30: 60-64. [Crossref]
10. Rivera Rivera A, Castillo-Pichardo L, Gerena Y and Dharmawardhane S (2016) Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS One 11: 0157251. [Crossref]
11. Han M, Song Y and Zhang X (2016) Quercetin Suppresses the Migration and Invasion in Human Colon Cancer Caco-2 Cells Through Regulating Toll-like Receptor 4/Nuclear Factor-kappa B Pathway. Pharmacogn Mag 12: 237-244. [Crossref]
12. Cao HH, Cheng CY, Su T, Fu XQ, Guo H, et al. (2015) Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer 14: 103. [Crossref]
13. Michaud-Levesque J, Bousquet-Gagnon N, Beliveau R (2012) Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res 318: 925-935. [Crossref]
14. Zhou J, Liang S, Fang L, Chen L, Tang M, et al. (2009) Quantitative proteomic analysis of HepG2 cells treated with quercetin suggests IQGAP1 involved in quercetin-induced regulation of cell proliferation and migration. Omics 13: 93-103. [Crossref]
15. Sonoki H, Sato T, Endo S, Matsunaga T, Yamaguchi M, et al. (2015) Quercetin Decreases Claudin-2 Expression Mediated by Up-Regulation of microRNA miR-16 in Lung Adenocarcinoma A549 Cells. Nutrients 7: 4578-4592. [Crossref]
16. Anand David AV, Arulmoli R, Parasuraman S (2016) Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev 10: 84-89. [Crossref]
17. Lamson DW, Brignall MS (2000) Antioxidants and cancer, part 3: quercetin. Altern Med Rev 5: 196-208. [Crossref]
18. Akan Z, Garip AI (2013) Antioxidants may protect cancer cells from apoptosis signals and enhance cell viability. Asian Pac J Cancer Prev 14: 4611-4614. [Crossref]
19. Vasquez-Garzon VR, Arellanes-Robledo J, Garcia-Roman R, Aparicio-Rautista DI, Villa-Trevino S (2009) Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free Radic Res 43: 128-137. [Crossref]
20. Klimaszewska-Wisniewska A, Halas-Wisniewska M, Izdebska M, Gagat M, Grzanka A et al. (2017) Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochem 119: 99-112. [Crossref]
21. Kee JY, Han YH, Kim DS, Mun JG, Park J, et al. (2016) Inhibitory effect of quercetin on colorectal lung metastasis through inducing apoptosis, and suppression of metastatic ability. Phytomedicine 23: 1680-1690. [Crossref]
22. Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131-142. [Crossref]
