In Vitro Investigation of Renal Cell Carcinoma Response to Combination Sorafenib and Cryoablation Treatment
A B S T R A C T
The 5-year survival rate for localized kidney cancer is 93%, but only 13% for those presenting with metastatic disease (2019 SEER data). Cryosurgery is an established treatment modality for renal cell cancer (RCC), with outcomes showing equipoise to radiofrequency ablation (RFA) and partial nephrectomy. Sorafenib is a targeted therapy for RCC utilized in more advanced stage diseases. Given the success of both cryoablation and sorafenib as monotherapies for RCC, in this study, we investigated the cellular response of RCC to combinatorial sorafenib pre-treatment and cryoablation in vitro using cell culture and tissue-engineered tumor models. In vitro samples were exposed to a single or repeat (double) 5-minute freeze at -10°C, -15°C, or -20°C representing temperatures within the periphery of a cryolesion. A repeat freeze to -20°C was necessary to fully ablate samples yielding day 1 viability of 2.9% (±0.2) with no recovery observed over the 7 days post-treatment culture. These findings were consistent with published data on the lethal temperature in RCC, suggesting that -25°C is necessary to destroy RCC following a single freeze event. Pre-treatment of samples with sorafenib at concentrations of 10.61 and 21.21 µM (½ clinical and clinical dose, respectively) was combined with a single or repeat 5-minute freeze to -10°C, -15°C, or -20°C. At the time of drug removal (day 0/pre-freeze), 10.61 µM sorafenib treated samples yielded 25.3% (±0.4) viability, yet samples regrew to control levels by day 7. Following combination freeze and sorafenib exposure, sample viability was found to be 27.5% (±0.7), 2.9% (±0.4), and 0.2% (±0.02) following a single freeze and 15.6% (±0.5), 0.7% (±0.1), and 0.1% (±0.01) following a repeat (double freeze), respectively. Regrowth was observed over the 7-day assessment period in samples exposed to a -10°C single or double freeze and a -15°C single freeze, but not in the -20°C single freeze or -15°C double freeze conditions. Thus, pre-treatment with 10.61 µM sorafenib was found to increase the minimum lethal temperature from the reported -25°C to -20°C following a single freeze event and from -20°C to -15°C following a double freeze. Results of the cell culture studies were confirmed in the 3D tissue-engineered tumor model, wherein the combination of 10.61 µM sorafenib and freezing was found to further increase the lethal temperature from <-20°C to -15°C following a single freeze event. This increased freeze susceptibility yielded a 32% improvement in the overall ablative volume of the ice ball following combinatorial treatment versus freezing alone. These in vitro results suggest that the combination of sorafenib and cryoablation may provide a possible combinatorial treatment path for RCC.
Keywords
Cryosurgery, freeze dose, renal cancer, sorafenib, combination therapy
Introduction
Renal cell carcinoma (RCC) accounts for about 90% of kidney cancers and is the most commonly diagnosed malignancy of the kidney [1]. The American Cancer Society estimates that about 76,080 new cases of kidney cancer will be diagnosed in the United States in 2021 and that about 13,780 will die from the disease [1]. The 5-year survival rate for localized disease is 93%, 70% for regional disease and 13% for distant cancers that have spread to other organs [2]. Various treatment options exist for RCC and are dependent on the stage and grade of the disease. These include radical or partial nephrectomy (RN, PN), thermal ablation (TA), including cryoablation and radiofrequency ablation (RFA), immunotherapies, and targeted therapies. The nephron-sparing approaches of PN and TA preserve a greater portion of renal function compared to RN and are recommended for clinically localized masses [3]. Both cryoablation and RFA have been reported to have similar outcomes, according to a number of retrospective studies [4-9]. In 2019, Wu et al. reported that cryoablation provided for superior outcome compared to RFA in tumors >2cm, and in tumors ≤2cm, no difference in overall survival was noted [10]. A prospective 5-year study was conducted on percutaneous cryoablation of stage T1a and T1b tumors and concluded that the procedure was safe and effective with an overall 5-year survival rate of 97% [11]. Today, the AUA recommends both cryoablation and RFA as primary treatment options for patients with clinical T1a tumors <3cm due to their lower incidence of postoperative complications compared to RN [12].
As cryoablation has proven to be an effective treatment option for RCC coupled with the continued desire for improved therapeutic options, the combination of cryoablation with other treatment modalities, such as anticancer agents, has emerged. To this end, there is a growing body of literature showing the benefits of combinatorial anti-cancer agent and freezing approaches in a number of cancers, including prostate, kidney, lung, and bladder [13-21]. More recently, targeted therapies in combination with cryoablation have garnered interest. Targeted therapies are often used in more advanced stages of kidney cancer, requiring systemic therapy and sometimes in conjunction with surgery or ablation. One such targeted drug therapy is sorafenib, a multi-kinase inhibitor that acts on a number of receptor tyrosine kinases (RTK’s) involved in cell proliferation and angiogenesis [22]. Sorafenib is administered orally and approved for the treatment of hepatocellular carcinoma (HCC), renal carcinoma (RCC), and thyroid carcinomas [23]. Several studies have shown that sorafenib in conjunction with freezing provides good efficacy and tolerability for the treatment of unresectable liver cancer [24, 25]. The only known study to date on sorafenib and cryoablation for the treatment of RCC is a 2019 report by Liu et al. in which the authors evaluated oral sorafenib alone at 400mg twice daily, in comparison to the combination of cryoablation followed by oral sorafenib 1-week post freezing [26].
The authors reported progression-free survival (PFS) and overall survival (OS) of 20 months and 36 months, respectively, in the combination group compared to 12 and 29 months in the sorafenib only group [26]. Within the combination group, 38.8% of patients underwent complete primary tumor ablation (mean tumor size 5.6 cm) while 61.2% underwent partial primary tumor ablation (mean tumor size 8.7 cm); patients with complete ablations scored significantly higher PFS and OS. The authors also compared serum levels of immune function indicators before and 6-8 weeks after treatment, finding elevated proportions of CD3+ T, CD4+ T, CD4+T/CD8+ T, and NK cells in the combination group that were not observed in the sorafenib only group. Overall, the results of the study found cryoablation followed by sorafenib treatment to be safe and effective, with superior OS in tumors <6 cm, possibly due to enhanced tumor immunity.
While Liu et al. reported a positive outcome with sorafenib treatment following cryoablation, the majority of the literature on adjunctive cryoablation/drug combination treatment suggests that pre-treatment with anti-cancer agents followed by cryoablation yields superior cancer cell destruction. As such, in this study, we investigated the impact of the combination of sorafenib pre-treatment and freezing using the 786-O cell line, a model of clear cell renal carcinoma, the most common RCC diagnosed in the US. As cryolesions are dynamic, we evaluated a range of temperatures representing the transition zone from lethal to non-lethal temperatures within a cryogenic lesion experienced in vivo [27]. Single and repeat (double) freeze-thaw exposures were employed to evaluate their impact on ablative efficacy (cell death). Studies also included 3-dimensional tissue-engineered models (TEM) to evaluate the efficacy of combination treatment compared to cryoablation alone in an ex vivo like tumor model. We have previously published the benefits of utilizing TEM models as a bridge between 2D in vitro culture and in vivo models [28-31]. TEMs provide the benefits of more complex cellular signaling and in vivo like morphology while maintaining the ease and reproducibility associated with in vitro testing. This type of cryosurgical modeling further enables the identification of cellular responses to precise conditions, thus providing guidance data for clinical translation.
This in vitro study was designed as a first step investigation into the sensitivity of RCC to freezing as well as to evaluate the potential benefit of the combination of sorafenib (sub-clinical and clinical doses) and freezing. Results demonstrate that exposing 786-O cells to a single freezing event <-20°C is necessary for complete cell destruction. Application of a repeat or double freeze to -20°C was found to yield complete cell destruction with no recovery. The combination of 10.61 µM sorafenib (sub-clinical dose) and a single freeze resulted in complete cell death at -20°C, whereas when a double freeze was employed in combination with a sub-clinical dose of sorafenib (10.61 µM or 1/2 clinical dose) pre-treatment complete cell death was attained at -15°C. TEM studies confirmed these findings, further supporting the potential of the combination of sorafenib pre-treatment followed by cryoablation as a viable treatment option for renal cancer.
Materials and Methods
I Cell Culture
Renal cancer cells (786-O; ATCC CRL-1932) were cultured in T-75 flasks (Cell Treat, Shirley, MA, USA) in RPMI-1640 (ATCC, #30-2001) supplemented with 10% FBS (Peak Serum) and 1% penicillin/streptomycin (Lonza). Cells were lifted using TrypLE Express (Gibco/Life Technologies, Grand Island, NY), centrifuged and plated into Costar strip well plates (Corning, Tewksbury, MA, USA) at 1,600 cells per well and cultured for 24 hours prior to experimentation.
II Tissue-Engineered Model Generation
Rat tail type I collagen solution (BD Bioscience, Bedford, MA) was used to form 0.2% w/v gel matrices as per SOP. Cells (0.75-1 x 106 cells/mL) were suspended in the collagen solution prior to solidification in 3mm x 40 mm TEM ring fixtures and then placed in 100 mm petri dishes and allowed to solidify for 30 minutes in a 37°C hybridization oven as per Robilotto et al. and Baust et al. [28, 31-33]. Following gelation, 15 mL of cell culture medium was added to the dish to cover the TEMs and the dishes were placed in the incubator. The TEM cell containing matrices were cultured for 24 hours prior to 48-hour drug exposure where indicated, or 72 hours total, prior to utilization.
III Sorafenib Treatment
Sorafenib (AChemBlock, #G6085, Burlingame, CA) was prepared fresh prior to each use in DMSO. Stock solution was diluted to final concentrations of 5.30, 7.07, 10.61 and 21.21 µM in media. These concentrations correlated to 125, 166.67, 250, and 500 mg/m2, respectively, with 500 mg/m2 representing the typical clinical dose of sorafenib for RCC. Samples were exposed to a single application of sorafenib for 48 hours. The drug was removed, and fresh medium was applied just prior to freezing.
IV Cell Culture Freezing Protocol
Samples in Costar 8-well strips (75 µL medium/well) were exposed to freezing temperatures of -10°C, -15°C or -20°C in a refrigerated circulating bath (Neslab/Thermo Scientific, Waltham, MA) for 5 minutes. 30 minutes prior to freezing, the culture medium was aspirated and replaced with 75 µL per well of the appropriate culture medium. Strips were placed into aluminum blocks containing a thin coating of ethanol to facilitate complete contact and thermal exchange, with each well, within the baths. Sample temperature was monitored in a cell-free well and ice nucleation was initiated at -2°C using liquid nitrogen vapor to prevent supercooling. Sample temperature was recorded at 1-second intervals using a type T thermocouple (Omega HH806AU, Omega, Samford, CT). For single freeze conditions, samples were held for a total time of 5 minutes in the freezing bath, passively thawed at room temperature for 10 minutes under a laminar flow hood and then placed at 37°C for recovery and assessment. For repeat (double) freeze conditions, samples were held for 5 minutes, passively thawed for 10 minutes, and then frozen again for an additional 5 minutes (5/10/5 protocol). Following the second freeze interval, samples were passively thawed at room temperature for 10 minutes and then placed at 37°C for recovery and assessment.
V 2D Viability Assessment
The metabolic activity indicator alamarBlue (Invitrogen, Carlsbad, CA) was utilized to assess cell viability. Stock alamarBlue was diluted 1:20 in Hank’s Balanced Salt Solution (HBSS, Corning/Mediatech) and applied to samples for 60 minutes (±1 minute) at 37°C. Raw fluorescent units were obtained using a TECAN Infinite plate reader (excitation 530 nm and emission 590 nm, Tecan Austria GmBH, Grodig, Austria) and analysed using Microsoft Excel. Raw fluorescence units were converted to percentages based upon pre-freeze control values (±SEM). Assessments were conducted on days 1, 3, 5 and 7 of recovery. A minimum of 3 experimental repeats with an intra experimental repeat of 7 wells was performed in each condition (n≥ 21). Statistical significance was determined by single-factor ANOVA where p < 0.01 was applied as the significance threshold.
VI TEM Freeze Procedure
All tests were performed in a laminar flow hood to ensure sample sterility. Freezing was conducted using the PSN (Pressurized Sub-Cooled Nitrogen) Cryosystem (CPSI Biotech, Owego, NY) with an input N2 pressure of 1,500 psi [34]. The cryoablation probe utilized in these studies was a 1.5 mm diameter cryoprobe with a 3 cm long freeze zone at the distal end (CPSI Biotech).
Individual cell-seeded TEM’s were stacked into the 3-D configuration detailed in Robilotto et al. and submerged in a warm circulating culture medium within an acrylic box [35]. The box was placed on a heat pad and stir table and the cryoprobe and thermocouple array consisting of four type T thermocouples were inserted into the fixture. Samples were held until TEM and bath temperatures equilibrated at 32°C (±2°C). TEM models were frozen using a single 5-minute freeze protocol. Temperature of the bath and within the TEM were monitored throughout the freezing process at the midpoint of the freeze zone using a type-T multipoint thermocouple array at fixed distances of 7.5 mm, 10.5 mm, 13 mm, and 16 mm extending radially from the surface of the cryoprobe. Temperatures were recorded using an Omega TempScan at 10-second intervals throughout the entire freeze cycle. At the completion of the freeze cycle, TEMs were allowed to passively thaw in the warm circulating bath for 30 minutes prior to disassembly, at which time the individual TEM layers were returned to culture for recovery and assessment.
VII TEM Viability Assessment
Following thawing, individual TEM layers were measured via calipers to determine the diameter of the iceball created following the freeze-thaw cycle. Iceball radii were measured at cardinal locations around the probe surface to determine the symmetry of the freeze zone created. TEMs were then placed into the culture to assess at 24 hrs and 72 hrs post-freezing recovery. In situ sample viability assessment (live/dead assay) was performed using the fluorescent probes Calcein-AM and Propidium Iodide (Cal/PI; Molecular Probes). Briefly, the culture medium was decanted from the TEM samples and a working solution of 5 µg/mL Calcein-AM (live cells; Molecular Probes, Eugene, OR) and 4 µg/mL propidium iodide (necrotic cells; Molecular Probes) in 1X PBS (Corning) was added directly to each sample. Samples were incubated in the dark at 37°C for 60 minutes (±1 minute). Fluorescent staining was visualized using a Zeiss Axio Observer 7 with ZEN software (Carl Zeiss AG, Oberkochen, Germany). Panoramic digital images spanning the center of the freeze zone were acquired using a 10X objective and stitched together from a 6 x 30 set of overlapping images. Following acquisition, a scale bar was imprinted onto each of the images to enable direct image comparison. The diameters of the necrotic zones were then measured using the ZEN software measurement tool. All experiments were repeated a minimum of 3 times. Following testing, data were combined and averaged (± standard deviation) to determine mean iceball size, isotherm distribution, and ablative diameter. Statistical significance was determined using single-factor ANOVA where noted.
Results
I Single Freeze Exposure Response of Renal Cancer Cells
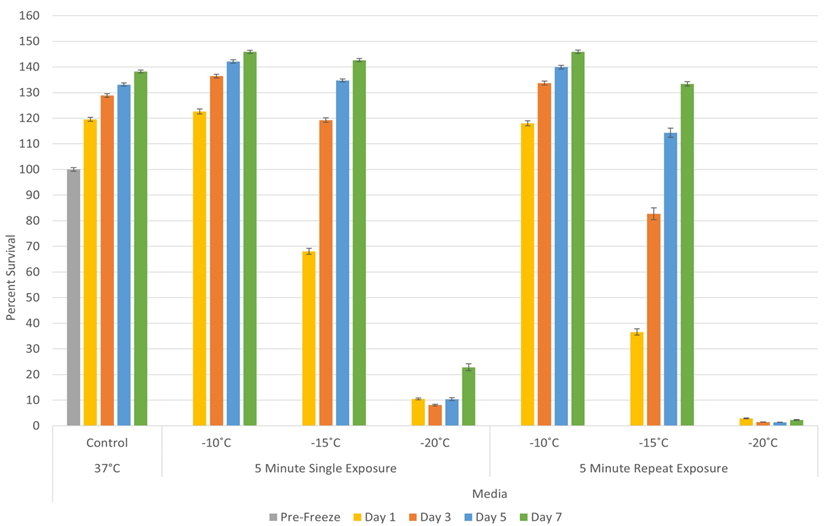
To assess the impact of freezing on RCC, 786-O cells were exposed to a single 5-minute freeze at -10°C, -15°C or -20°C, thawed, allowed to recover in culture and assessed for initial cell viability (24 hours) as well as recovery over a 7-day period. Analysis of 786-O samples revealed minimal death following exposure to a -10°C single freeze (Figure 1). Exposure to -15°C resulted in a significant decline in 786-O viability at day 1 post-freeze to 68.0% (±1.2) compared to controls (p<0.01). The surviving cells, however, rapidly recovered, reaching non-treated control levels by day 5. Exposure to -20°C resulted in a further reduction in 786-O viability to 10.5% (±0.4) 1-day post freeze. A low but significant level of regrowth was observed over the 7-day post-thaw analysis interval (D7= 22.8% (±1.3) vs. D1= 10.5% (±0.4); P<0.01).
Figure 1: Assessment of renal cancer cell viability and recovery following a single or double freeze event. 786-O cells were subjected to a single 5-minute or repeat 5/10/5 (5-minute freeze, 10-minute thaw, 5-minute freeze) exposure to the indicated temperatures. Sample viability decreased in a thermal dose-dependent manner, with recovery in all single freeze conditions and repeat freezing to -10°C and -15°C. A repeat -20°C freeze resulted in complete 786-O cell destruction in vitro.
II Repeat (Double) Freeze Exposure Response of Renal Cancer Cells
Studies were then conducted to assess the impact of a repeat (double) freeze exposure on sample viability and recovery. To this end, samples were exposed to repeat freezing at -10°C, -15°C and -20°C. Repeat freeze exposure (double 5-minute freezes) to -10°C yielded a similar outcome as the single freeze exposure with minimal cell death observed in 786-O cells (Figure 1). Repeat freezing at -15°C resulted in a significant increase in cell death in 786-O samples at day 1 post freeze compared to a single freeze event (repeat vs. single = 36.6% (±2.1) vs. 68.8% (±1.2), P<0.01). Despite the increased cell death, the repeat -15°C samples were found to recover to untreated control levels over the 7-day recovery interval. Repeat exposure to -20°C resulted in complete cell destruction with no recovery over the 7-day assessment interval (day 1: 2.9% (±0.2) vs. day 7: 2.3% (±0.2)). This was significantly different from single freeze samples, where a slight but significant recovery was observed by day 7 (D7: repeat vs. single = 2.3% (±0.2) vs. 22.8% (±1.3)). The results from the double freeze experiments suggested that the repeat freeze exposure yields complete destruction at -20°C, representing an elevation of the minimal lethal temperature to -20°C from the previously reported -25°C [18].
III Assessment of Sorafenib Exposure on 786-O Cells
A dose-response study was conducted to determine the impact of a 48-hour exposure of 786-O cells to sorafenib at concentrations of 5.30, 7.07, 10.61 and 21.21 µM (Figure 2). The sorafenib concentrations evaluated equate to clinical doses of 125, 166.67, 250, and 500 mg/m2, respectively. A typical clinical dose for sorafenib for RCC is 500 mg/m2; as such, the evaluated concentrations represented a quarter, third, half and full clinical dose [23]. DMSO controls were included using the highest concentration (0.08%), which 786-O cells were exposed to. Assessment of 786-O sample viability following 48 hours exposure to 5.30, 7.07, 10.61 and 21.21 µM sorafenib revealed sample viability of 74.5% (±0.6), 46.9% (±1.3), 25.3% (±0.4), and 8.5% (±0.2), respectively, compared to untreated control samples (100% (±0.7)). DMSO solution control samples revealed that the 0.08% DMSO had a minimal impact on cell viability (95.4% (1.4) vs. 100% (±0.7)). Assessment over the 7-day recovery period revealed that sample viability recovered in the 5.30, 7.07, and 10.61 µM sorafenib conditions (130.9% (±0.3), 129.4% (±1.3), 126.3% (±1.1), respectively), whereas samples continued to decline to 1.2% (±0.1) in the 21.21 µM condition (clinical dose) compared to matched non-treated control and DMSO treated samples (138.2% (±0.7) and 137.8% (±0.9)).
Figure 2: Assessment of the impact of sorafenib exposure on renal cancer cell viability in vitro. 786-O samples were exposed to 5.30 µM, 7.07 µM, 10.61 µM, and 21.21 µM sorafenib for 48h (concentrations one quarter, one third, one half of clinical, and clinical dose) along with DMSO carrier control. Upon drug removal, sample viability was 75%, 47%, 25%, and 8%. Sample conditions of ½ clinical or less recovered over the assessment period, while 21.21 µM treated samples yielded complete cell death.
IV Impact of Adjunctive Sorafenib Pre-Treatment and Freezing Treatment
Given the observed initial decline in 786-O viability and subsequent recovery following exposure to 10.61 µM sorafenib (1/2 clinical) and near complete destruction following 21.21 µM sorafenib (250 vs. 500 mg/m2, respectively), we explored the impact of combining sorafenib pre-treatment at these concentrations followed by freezing on cell survival. The 10.61 µM sorafenib (½ clinical) condition was selected in an effort to evaluate the potential of reducing the drug concentration, thereby reducing the negative side effects experienced clinically while still yielding enhanced cell death when combined with freezing. As such, samples were pre-treated with 10.61 µM sorafenib for 48 hours and then frozen with a single or repeat 5-minute freeze to -10°C, -15°C, or -20°C (Figure 3).
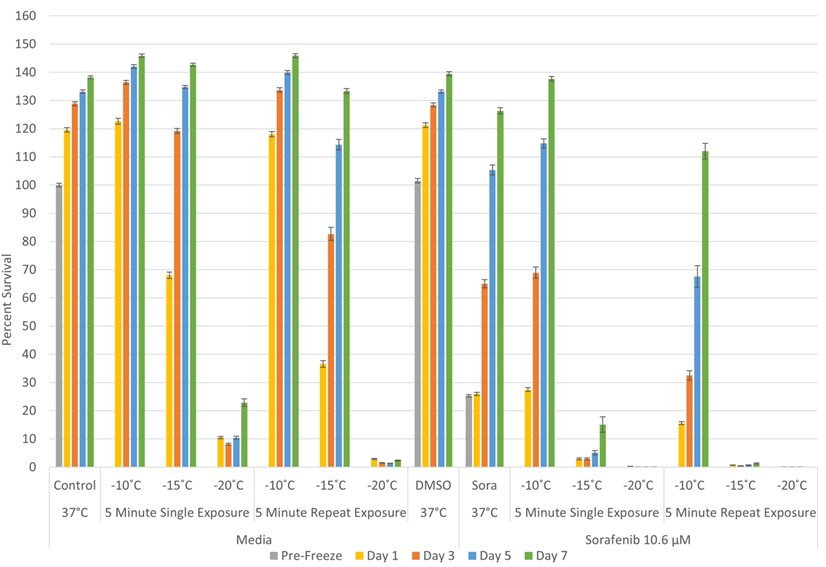
The combination of 10.6 µM sorafenib and freezing yielded a significant increase in 786-O cell death. Specifically, 786-O cells exposed to 10.6 µM sorafenib (S) for 48 hours followed by a single freeze at -10°C (S/-10) had a minimal impact. 786-O samples exposed to 10.6 µM sorafenib and freezing to -15 and -20°C resulted in an increase in cell death compared to freeze alone samples (S/-15: 2.9% (± 0.4) vs. -15: 68.0% (±1.2); P<0.01) and S/-20: 0.02% (±0.02) vs. -20°C: 10.5% (±0.4)). There was also a significant increase in 786-O cell death compared to sorafenib alone samples (S alone: 25.3% (±0.4)) (Figure 4). While a significant increase in cell death was observed at day 1, regrowth was observed over the 7-day assessment period in combination samples frozen to -15°C using a single freeze protocol. In the 10.6 µM sorafenib/ -20°C single freeze samples, the combination was found to result in complete cell death and no recovery in vitro over the 7-day assessment period.
Figure 3: Effect of adjunctive sorafenib pre-treatment at a sub-clinical dose in combination with freezing on renal cancer cell survival. Samples were pre-treated with 10.6 µM sorafenib for 48h and the drug was removed prior to freezing. Sample viability was compared to freezing without sorafenib and significant improvements in cell death were observed following combination treatment. A single -20°C or repeat -15°C freeze induced total cell death in combination treatment, compared to repeat -20°C in freezing alone.
When a repeat freeze exposure protocol was utilized in combination with 10.6 µM sorafenib pre-treatment, sample viability was found to be 15.6% (±0.5), 0.7% (±0.1), and 0.1% (±0.01) following freezing to -10°C, -15°C and -20°C, respectively (Figure 3). This represented a significant increase in cell death compared to both repeat freeze exposure alone and the sorafenib/single freeze combination for the -10°C and -15°C conditions. The combination of ½ clinical 10.61 µM sorafenib pre-treatment and a double freeze to -15°C resulted in near complete destruction of 786-O cells at day 1 and significantly reduced recovery at day 7 compared to -15°C repeat freeze or sorafenib treatment alone (day 1= S/repeat -15°C: 0.7% (±0.1) vs. S: 26.0% (±0.6) and S/single -15°C: 2.9% (±0.4) and Day 7 = S/repeat -15°C: 1.9% (±0.2) vs. S: 126.3% (±1.1) and S/single -15°C: 15.1% (±2.1)). While a continued decrease in viability was noted following 10.6 µM sorafenib pre-treatment and repeat freezing at -20°C, this was not significant as the combination of sorafenib pre-treatment and freezing (single or repeat) resulted in complete cell death with no recovery.
In addition to ½ clinical dose/ freeze combination, 786-O samples were also pre-treated with 21.21 µM sorafenib (a clinical dose equivalent to 500 mg/m2) for 48 hours prior to a single or repeat 5-minute freeze (Figure 4). As exposure to 21.21 µM sorafenib was found to be lethal in the dose-response studies, these studies were conducted to evaluate if there was any negative impact of the combination on overall cell destruction. As with dose-response studies, 48-hour treatment with 21.21 µM sorafenib resulted in near complete destruction of 786-O cells yielding post-treatment day 1 viability of 8.5% (±0.2), which continued to decline to 1.2% (±0.1) on day 7. When samples were pre-treated with 21.21 µM sorafenib and then exposed to freezing, a further increase in cell death was noted in all conditions compared to drug or freeze treatment alone. Specifically, following the combination of 21.21 µM sorafenib exposure then a single freeze to -10°C, -15°C or -20°C, sample viability at day 1 was found to be 2.6% (±0.1), 0.5% (±0.1) and 0.1% (±0.1), respectively. The combination of sorafenib pre-treatment and a double freeze to -10°C or -15°C resulted in a further decrease to 1.5% (±0.1), and 0.2% (±0.1), respectively. This represented a significant decrease in sample viability (increase in cell death) for all conditions compared to matched sorafenib or freeze-only conditions (p<0.01 for all matched conditions). Samples treated with DMSO alone (0.08% vehicle control) combined with freezing yielded similar results to freezing alone. When samples were allowed to recover in culture, it was found that the benefit of the combination of 21.21 µM sorafenib pre-treatment and freezing (single or double) was maintained with an observed continual decline and no sample recovery over the 7-day interval.
Figure 4: Impact of clinical dose sorafenib on renal cancer cell survival following combination treatment. 786-O exposure to 21.21 µM sorafenib for 48h resulted in complete cell death over the assessment period. The combination of sorafenib and freezing accelerated the rate of decline, resulting in less than 1% sample viability on day 1 in samples exposed to temperatures or -15°C or below.
V TEM Response to Freezing
Acellular hydrogels are often utilized to assess the generation and spread of critical isotherms and the cooling power of cryoprobes. While useful, these models do not provide information on the response of cells or tissues to the freezing regime. To bridge this gap, we have developed and previously reported on the use of in vitro, 3-dimensional tissue constructs (TEM) as a clinically analogous test setup to provide for evaluation of the thermal performance of a cryoprobe as well as the assessment of the cellular responses (level of cell destruction) associated with a given interventional protocol [28-33, 35]. This model has been shown to provide vital information on in vivo response while reducing the expense and burden of exploratory animal studies [31, 34, 35].
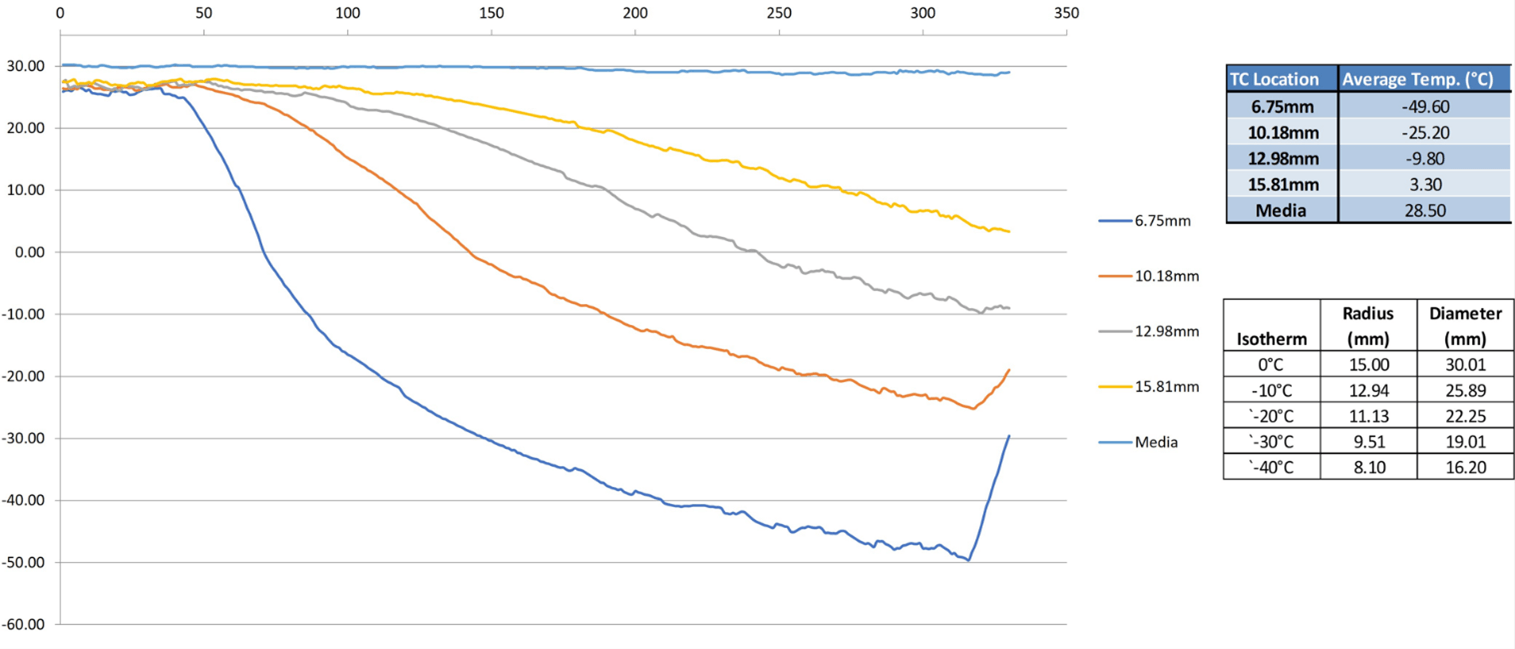
TEMs consisting of 786-O cells were prepared and then treated with either a single freeze only, 10.61 µM sorafenib (½ clinical), or the combination of 10.6 µM sorafenib and freezing. TEMs were frozen using a 1.5mm x 3cm PSN cryoneedle under a single 5-minute freeze protocol in a circulating heat-loaded model. The thermal profile within the TEM during the freeze procedure was measured in real-time at fixed positions radiating from the center of the cryoprobe freeze zone (Figure 5). TEMs were stained with Calcein-AM (green, live) and Propidium Iodide (red, necrotic) at 24 hours and 72 hours post-thaw to determine the extent of cell death within the frozen volume (Figure 6). As Calcein-AM and Propidium Iodide are single end-point assessments, sister samples were used to assess viability following 24 hours and 72 hours of recovery. Representative fluorescent images in (Figure 6) illustrate necrotic (red) versus live (green) regions within the TEMS for each condition following treatment. Isotherms from the thermal monitoring are imprinted over the image and the orange line represents the transition from variable survival to complete necrosis. Non-frozen control and 10.61 µM sorafenib only TEMs revealed no cell death over the course of the 72-hour incubation period (Figure 6).
Measurements of the iceball diameter and the extent of cell death in frozen/thawed TEMs were made using the ZEN software and results were averaged from triplicate experiments (Table 1). Measurements were obtained 24 hours and 72 hours post-freeze in sister samples for each condition and converted to the volume of an ellipsoid using the length of the cryoprobe freeze zone. The percentage of the frozen mass that was completely ablated was then calculated for each condition (Table 1). Following a single 5-minute freeze, the freeze diameter was 2.85 cm (±0.03 cm), equating to a volume of 19.6 cm3 (±0.3). The average size of the frozen areas measured on the micrographs corresponded to within 1 mm of those obtained with the thermal profile data collected during the freeze procedure. Following 24 hours of recovery, TEMs had a zone of necrosis 2.13 cm (± 0.03) in diameter equating to a volume of 9.5 cm3 (±0.4) after a single freeze. This area of destruction correlated with the -20°C isotherm and when compared to the overall frozen volume, this equated to ~48.5% of the frozen mass being destroyed (necrotic). After 72 hours of recovery, the necrotic diameter was found to decrease to 2 cm (± 0.7) equating to 8.4 cm3 (±0.6). We have previously reported that this decrease in the necrotic volume at day 3 is a result of cellular infiltration and regrowth within the periphery of the cryogenic lesion in the region where temperatures are above the minimal critical temperature [28, 31, 32, 34, 35].
Figure 5: Real-time monitoring of the isothermal profile generated by the PSN 1.5mm cryoprobe at the center of the ablation segment within a TEM during a 5-minute freezing protocol. The temperature of the bath (“media”) and within the TEM were monitored throughout the freezing process at the midpoint of the freeze zone using a type-T multipoint thermocouple array at fixed distances of 7.5 mm, 10.5 mm, 13 mm, and 16 mm extending radially from the surface of the cryoprobe. Actual distances were measured using Zen software from images acquired of the visualized thermocouples within each TEM layer. Isotherms (0°C, -10°C, -20°C -30°C, -40°C) were extrapolated from measured data points.
Table 1: Analysis of iceball size and lethality following freezing or combination sorafenib/freeze treatment.
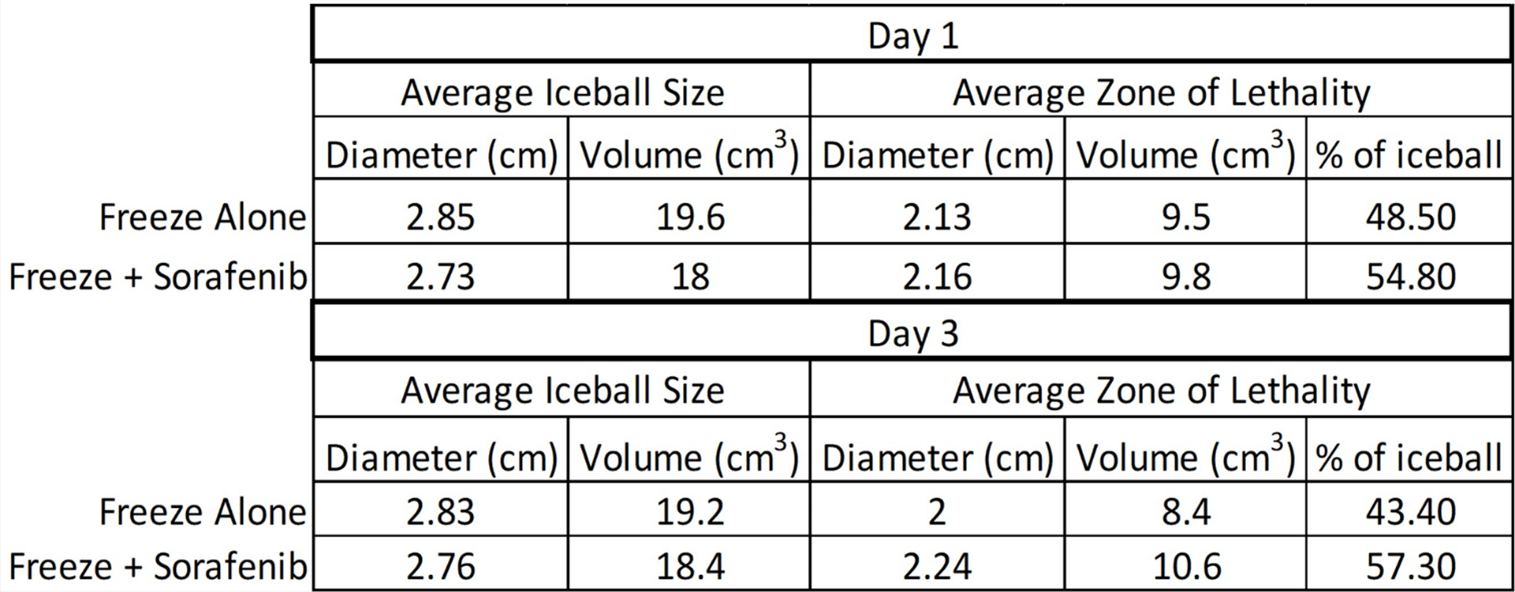
In combination 10.61 µM sorafenib and freeze TEM samples, the freeze diameter was found to be 2.73 cm (±0.2) equating to a volume of 18 cm3 (±1.3) following a single 5-minute freeze. At 24 hours, the combination treated TEMs had a zone of necrosis 2.16 cm (± 0.2) in diameter equating to a volume of 9.8 cm3 (±1.8). When compared to the overall frozen volume, this equated to 54.8% of the frozen mass being necrotic. After 72 hours of recovery, the necrotic diameter increased to 2.24 cm (± 0.25), corresponding to a volume of 10.6 cm3 (±2.2). This represented an increase in the overall ablated volume at 72 hours post-treatment to 57.3% of the frozen mass versus 43.4% in TEMs without sorafenib pre-treatment. When comparing the percent ablated mass in the freeze only versus combination TEMs, following 24 hours recovery, there was a 13% increase in ablative volume in the combination samples over freeze alone (p=0.06). Interestingly, at 72 hours post-treatment, the 10.6 µM sorafenib/ freeze combination samples were found to have a ~32% increase in ablative volume over freeze alone (p < 0.01). The increased necrotic area within the combination samples correlated with the -15°C isotherm thereby representing an increase in the minimal lethal isotherm from <-20°C with freezing alone to -15°C in 10.61 µM sorafenib/ freeze combination TEM samples, representing a further improvement compared to cell culture studies.
Figure 6: A & B) Fluorescent micrographs of the 786-O TEMs following single 5-minute freeze with and without sorafenib pre-treatment. A) At 24 hours and B) 72 hours post-thaw, unfrozen controls, single freeze, and combination sorafenib/freeze TEMs were probed with Calcein-AM (green, live) and Propidium Iodide (red, dead) and visualized using fluorescent microscopy to determine the extent of cell death. Images were stitched together from a 6 x 30 set of overlapping images using a 10X objective. Measurements were made using the Zeiss ZEN software and temperatures from the corresponding IR images were mapped to the micrographs. Isotherms were extrapolated from thermal monitoring and imprinted onto images for assessment. The yellow line represents the edge of the iceball and the orange line represents the edge of necrotic cell death.
Discussion
This study may have important clinical implications when treating patients with kidney cancer. Although the interior isotherm core (~<-30 to -40°C) of a cryotherapeutic freeze results in immediate cell death and subsequent necrosis, the scenario that leads to tumor persistence commonly occurs at the periphery of the ice ball where temperatures are sublethal (>-30°C). The ice ball edge is 0°C, yet the initial isotherm that has some potential to kill cells is typically located 3-4 mm within the ice edge where temperatures approach -15°C to -20°C. This results in three distinct (potentially more) zones of tissue damage within a frozen tissue mass, including the outer 3-4mm of minimal damage, 4-5mm increasing partial damage (apoptotic zone) and then complete destruction in the core [31]. This is significant as in the case of a 3cm tumor, volumetrically, these zones (minimal, apoptotic and complete damage) represent in the range of 34 to 46%, 32 to 45% and 22% of the frozen mass, respectively, reported to be generated by a typical argon cryoprobe [31]. This is the apoptotic zone where cells can either die or repopulate, depending on factors influencing the cells’ ability to recover or successfully enter the death pathway [14, 27, 32]. In an effort to prevent cancer recurrence after ablation, clinicians can add additional cryotherapy probes [if feasible] to ensure sufficient overlap of lethal ice between probes or utilize adjuncts to influence the zone of apoptosis, such as sorafenib as described. In routine clinical practice, it is common to use 2-3 ice probes for small lesions [<2.5-3 cm; additional probes for larger lesions] and to employ a double freeze-thaw cycle to maximize cancer death. There is a clinical need to develop cryotherapy influencers that induce cells to die in the periphery of the iceball. This study shows the potential of employing a common pharmacologic agent used to treat RCC when combined with the destructive effects of cryotherapy.
This study investigated the response of 786-O renal cancer cells following a freezing insult in an effort to identify the minimal lethal temperature (dose) necessary for complete cell destruction in vitro. Further, studies assessed the combination of sorafenib pre-treatment and freezing on cell destruction. Combination treatment studies were conducted using sub-clinical and clinical doses of sorafenib alone or in combination with freezing at temperatures associated with the periphery of a cryolesion (-10°C to -25°C). Previous reports have suggested that the minimal critical temperature (lethal dose) for renal cancer is around -25°C following a single freeze event [18]. Initial freeze dose-response studies examined 786-O cell survival following exposure to temperatures ranging from -10°C to -20°C. These studies revealed that 786-O cells were completely destroyed following a double 5-minute freeze at -20°C whereas a double freeze to -10°C resulted in minimal to no cell death (Figure 1). Following a single freeze event at -20°C, ~10% survival was found 1-day post-treatment and samples were observed to recover over the 7-day assessment interval. Single or double freeze exposure to the intermediate temperature of -15°C resulted in a significant level of cell death at 1-day post-freeze, yet samples were found to recover over the 7 days post-treatment assessment interval.
A number of studies by our group and others have detailed the benefit of combining chemotherapy or other cytotoxic agents pre-pre-treatment with freezing to enhance cell death in a number of cancers, including bladder, prostate, breast and kidney [13, 14, 16, 18, 20, 21, 27, 36-38]. Studies have shown the ability to elevate the minimal lethal temperature (ablative dose) for hormone-refractory prostate cancer from -40°C to -20°C via pre-treatment with sub-clinical (non-toxic) doses of 5-fluorouracil, Taxotere, cisplatin and even calcitriol (vitamin D3) [15, 16, 37, 39, 40]. Other studies have demonstrated that the combination of cisplatin/freezing can elevate the minimal lethal temperature for bladder cancer to -15°C21 and gemcitabine/freezing combination can elevate the minimal target temperature for liver cancer [41]. An important factor to note in these studies is that the benefits of the combination were noted using the anticancer agent (cisplatin, 5-fluorouracil, Taxotere, gemcitabine) at concentrations less than half the typical clinical dose. This may have important patient implications as it is understood that the side effects associated with chemo are drug and dose-dependent [42]. Based on these reports and the current usage of sorafenib as a primary treatment for renal cancer, we investigated the potential of combining low dose (sub-clinical) sorafenib pre-treatment with mild freezing. In this study, we investigated sorafenib doses of 5.30, 7.07, 10.61 and 21.21 µM (125, 166.67, 250, and 500 mg/m2, respectively). The typical clinical dose of sorafenib for RCC is 500 mg/m2 [23]. Sub-clinical doses were studied in an effort to increase cancer susceptibility to freezing injury while reducing the negative toxic side effects associated with higher drug levels.
Pre-treatment with sorafenib alone for 48 hours resulted in a decline in sample viability at day 1 post-treatment at all concentrations. Interestingly, following exposure to the three sub-clinical concentrations (5.30, 7.07 and 10.61 µM), samples were found to recover to untreated controls within 3-5 days (Figure 2). 786-O samples exposed to 21.21 µM sorafenib (500 mg/m2, clinical dose) alone yielded near complete cell death at day 1 with no recovery noted. Given the differential response of cell death followed by recovery and complete cell death observed between the 10.61 and 21.21 µM sorafenib conditions (250 and 500 mg/m2 (½ clinical and clinical dose, respectively)), these conditions were selected for evaluation in combination with freezing. To this end, 786-O samples were exposed to 10.61 µM sorafenib (½ clinical) for 48 hours then frozen to -10°C, 15°C and -20°C (Figure 3). Pre-treatment of 786-O samples with 10.61 µM sorafenib (½ clinical dose) 48 hours prior to freezing resulted in a decrease in cell survival at day 1 post-freeze compared to freeze alone at all temperatures as well as 10.61 µM sorafenib alone. Most notably, the combination of sorafenib pre-treatment and a single -20°C freeze resulted in complete cell death with no recovery (Figure 3). Similarly, the combination of 10.61 µM sorafenib pre-treatment and a double -15°C freeze yielded complete cell death. This differed significantly compared to matched freeze or sorafenib alone samples.
With the observed benefit of sub-clinical sorafenib and freezing, studies were conducted using a clinical dose (21.21 µM) of sorafenib to confirm that the combination did not have a negative impact on cell death at any temperature. Increasing the concentration of sorafenib during pre-treatment to 21.21 µM followed by freezing was found to further increase cell death compared to either sorafenib or freezing alone. Specifically, 1 day following 48 hours pre-treatment with 21.21 µM sorafenib, 786-O survival was found to be ~10% whereas when samples were pre-treated with 21.21 µM sorafenib and then frozen to -10°C or colder, day 1 survival was found to be <2% (Figure 4).
With the benefit of the combinatorial approach observed in cull culture, studies using a tissue-engineered RCC tumor model were conducted. TEM studies revealed similar yet slightly improved results to that of the in vitro cell culture studies. Correlation of the thermal monitoring data collected during TEM freezing with fluorescent imaging of the TEMs post-thaw revealed the ablative zone within the TEM corresponded to -20°C following a single 5-minute freeze protocol. At 24 hours post-thaw, the ablative area was found to be ~ 49% of the total frozen volume, indicating margins of incomplete cell death of ~ 3.5 mm (Figure 6). Due to a combination of the recovery following transient cell injury and cellular regrowth in the warmer, non-lethal isotherms of the freeze zone periphery, a reduction in the necrotic zone was observed over the recovery period and by 72 hours post-thaw, the ablative area had decreased to ~43% of the frozen volume (Figure 6, Table 1). This phenomenon is typically addressed in vivo through the application of a positive freeze margin to ensure that the entirety of the cancerous lesion is contained within the lethal zone of the frozen mass. Regardless of the recovery, the isotherm and 72-hour viability data obtained with the TEM studies revealed complete cell death in the range of -20°C following a single 5-minute freeze. These findings correlated well with our in vitro data and previous studies, which have suggested -20°C to -25°C as the minimal critical temperature for RCC cells [18].
The results from the combination 10.61 µM sorafenib (½ clinical) and freezing TEM studies also confirmed the in vitro findings of the elevation of the minimal critical temperature (lethal dose) following combination treatment. Isotherm assessment during the freeze procedure within the TEMs, when correlated with fluorescence imaging post-treatment, indicated that the combination of 10.61 µM sorafenib pre-treatment and freezing increased the lethal temperature (ablative dose) to between -10°C and -15°C (Figure 6). Volumetric assessment of ablation zones revealed a ~13% increase in the necrotic volume in combination treated TEMs compared to freezing alone 1 day following treatment. Importantly, the combination was found to increase the ablative volume by ~32% at day 3, indicating the drug may inhibit the repair and recovery process post-freeze. Originally designed as a Raf inhibitor, sorafenib has been reported to act primarily on vascular endothelial growth factor (VEGFR) and platelet-derived growth factor (PDGFR) kinases in RCC [43]. Future studies investigating the specific pathways activated in combination therapy are warranted. Regardless, combinatorial approaches using sorafenib and freezing may be able to utilize sub-clinical doses to minimize side effects, given that ½ clinical dose when combined with freezing to -15°C or -20°C yielded the same result of complete ablation as the clinical dose in our studies. However, the exact dosing requires further investigation when translated to an in vivo model, given that the pharmacokinetics of sorafenib at standard dosing of 400mg twice daily are reported to yield an average serum level of 10mg/l or 16 µM [44, 45].
While promising, this in vitro study has several limitations. One limitation is that in vitro models create ideal conditions for cellular recovery, and hence, a bias toward survival. Another limitation is that in vitro modeling cannot account for the effects of vascular stasis and host immune response during the recovery process following freezing. Cryoablation results in a large volume of necrotic tissue that must be cleared by the immune system, an additional variable that can affect an individual’s response to treatment. To this end, Liu et al. assessed RCC response to cryoablation followed by sorafenib treatment and reported significant changes in serum indicators of immune function, including T lymphocyte subsets and Treg cells, that were not observed in the sorafenib only group [26]. These results support a growing interest in the exploration into the immunologic effects associated with cryoablation, in which investigators have hypothesized that antigens released during the freeze/thaw process can prime the immune system against future or distant tumors of the same type [46-48]. Indeed, there have been a number of case reports that document resolution or improvement of distant metastases following cryoablation of a primary tumor; however, elucidating the particular mechanisms of action is difficult [49-54]. As such, in vivo response has the potential to be more successful than the in vitro results reported herein.
In conclusion, our findings suggest that the minimal lethal temperature for renal cancer is <-20°C for a single freeze event, whereas a repeat freeze protocol results in an increase of the minimal lethal temperature to -20°C in vitro. Pre-treatment with low dose sorafenib (10.16 µM) in combination with freezing resulted in an increase in the level of cell death and inhibition of repopulation of surviving cells. Specifically, combination treatment resulted in the elevation of the minimal critical temperature (lethal dose) ~5°C regardless of whether a single or double freeze protocol was applied (e.g., sorafenib/single freeze = ~-20°C and sorafenib/double freeze = ~-15°C). Further, when higher “clinical doses” of 21.21 µM sorafenib (500mg/m2) were applied in combination with freezing, complete 786-O cell death was observed within 1-day post-treatment at all temperatures assessed (e.g., ≤-10°C). The data from the TEM studies confirmed these findings, further suggesting that the combination resulted in a shift of the minimum lethal temperature for renal cancer from the -20°C to -25°C for freezing alone to around -15°C for combination treatment with ½ clinical dose sorafenib pre-treatment followed by a single freeze event. Extrapolating these in vitro findings to an in vivo scenario, the data suggest that freezing alone and in combination with sorafenib pre-treatment may provide benefit in the treatment of renal cancer. This, in turn, has the potential to improve the outcome, reduce comorbidities associated with either treatment alone, thereby providing for an effective minimally invasive treatment strategy. In combination with previous in vitro and in vivo reports, these data suggest cryoablation alone or in combination with low-dose sorafenib may provide an improved path for the treatment of renal cancer.
Acknowledgement
The authors would like to thank Ms. Courtney McLaughlin and Ms. Gabrielle String, MS for their contributions to data acquisition for this manuscript.
Funding
This study was supported in part by funding from the National Institutes of Health National Cancer Institute Grant No. 1R43CA224431-01A awarded to CPSI Biotech.
Conflicts of Interest
Financial: JMB, KLS, KKS. ATR and RVB are employees of CPSI Biotech. Non-Financial: JGB and TP served in an advisory role to CPSI as collaborators on this project. JMB and JGB are related.
Author Contributions
Conception: JMB, KLS, and KKS designed the studies. RVB, ATR, TP and JGB provided review and feedback on study design. Performance of Work: KLS, JMB and ATR performed experimentation for this study. Interpretation or Analysis of Data: KLS, JMB, ATR and KKS conducted data analysis and interpretation. RVB, TP and JGB reviewed and provided guidance and feedback on data analysis and interpretation. Writing the Article: KLS and JMB prepared the draft manuscript. JMB prepared the revised manuscript. KLS, KKS, ATR, RVB, TP and JGB provided revision input for the manuscript.
Availability of Data and Materials
The data that support the findings of this study are available from CPSI Biotech but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of CPSI Biotech.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 20, Dec 2021Accepted: Mon 17, Jan 2022
Published: Mon 24, Jan 2022
Copyright
© 2023 John M. Baust. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2022.01.01
Author Info
Kimberly L. Santucci Kristi K. Snyder Anthony Robilotto John G. Baust Robert G. Van Buskirk Thomas J. Polascik John M. Baust
Corresponding Author
John M. BaustCPSI Biotech, Owego, New York, USA
Figures & Tables
Table 1: Analysis of iceball size and lethality following freezing or combination sorafenib/freeze treatment.




References
1.
Cancer Facts & Figures 2021. Atlanta, GA: American
Cancer Society.
2.
SEER Cancer Statistics Review (CSR) 1975-2016.
Bethesda, MD: National Cancer Institute; 2019.
3.
Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK
et al. (2009) Guideline for management of the clinical T1 renal mass. J Urol
182: 1271-1279. [Crossref]
4.
Atwell TD, Schmit GD, Boorjian SA, Mandrekar J, Kurup
AN et al. (2013) Percutaneous ablation of renal masses measuring 3.0 cm and
smaller: comparative local control and complications after radiofrequency ablation
and cryoablation. AJR Am J Roentgenol 200: 461-466. [Crossref]
5.
Pirasteh A, Snyder L, Boncher N, Passalacqua M, Rosenblum D et al. (2011)
Cryoablation vs. radiofrequency ablation for small renal masses. Acad Radiol
18: 97-100. [Crossref]
6.
Wagstaff P, Ingels A, Zondervan P, de la Rosette JJMCH,
Laguna MP (2014) Thermal ablation in renal cell carcinoma management: a
comprehensive review. Curr Opin Urol 24: 474-482. [Crossref]
7.
Klatte T, Kroeger N, Zimmermann U, Burchardt M,
Belldegrun AS et al. (2014) The contemporary role of ablative treatment
approaches in the management of renal cell carcinoma (RCC): focus on
radiofrequency ablation (RFA), high-intensity focused ultrasound (HIFU), and
cryoablation. World J Urol 32: 597-605. [Crossref]
8.
Kunkle DA, Uzzo RG (2008) Cryoablation or
radiofrequency ablation of the small renal mass : a meta-analysis. Cancer
113: 2671-2680. [Crossref]
9.
El Dib R, Touma NJ, Kapoor A (2012) Cryoablation vs
radiofrequency ablation for the treatment of renal cell carcinoma: a
meta-analysis of case series studies. BJU Int 110: 510-516. [Crossref]
10. Wu
J, Chang J, Bai HX, Su C, Zhang PJ et al. (2019) A Comparison of Cryoablation
with Heat-Based Thermal Ablation for Treatment of Clinical T1a Renal Cell
Carcinoma: A National Cancer Database Study. J Vasc Interv Radiol 30:
1027-1033.e3. [Crossref]
11. Georgiades
CS, Rodriguez R (2014) Efficacy and safety of percutaneous cryoablation for
stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year
study. Cardiovasc Intervent Radiol 37: 1494-1499. [Crossref]
12. Campbell
S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA et al. (2017) Renal Mass and
Localized Renal Cancer: AUA Guideline. J Urol 198: 520-529. [Crossref]
13. Mir
LM, Rubinsky B (2002) Treatment of cancer with cryochemotherapy. Br J Cancer
86: 1658-1660. [Crossref]
14. Goel
R, Anderson K, Slaton J, Schmidlin F, Vercellotti G et al. (2009) Adjuvant
approaches to enhance cryosurgery. J Biomech Eng 131: 074003. [Crossref]
15. Clarke
DM, Baust JM, Buskirk RGV, Baust JG (2001) Chemo-cryo combination therapy: an
adjunctive model for the treatment of prostate cancer. Cryobiology 42:
274-285. [Crossref]
16. Clarke
DM, Baust JM, Van Buskirk RG, Baust JG (2004) Addition of anticancer agents
enhances freezing-induced prostate cancer cell death: implications of
mitochondrial involvement. Cryobiology 49: 45-61. [Crossref]
17. Baust
JM, Klossner DP, Robilotto AT, Van Buskirk RG, Gage AA et al. (2014) Vitamin D3
therapy increases cryoablation efficacy: A novel strategy for the treatment of
prostate cancer. Cryobiology 69: 198.
18. Clarke
DM, Robilotto AT, Rhee E, VanBuskirk RG, Baust JG et al. (2007) Cryoablation of
renal cancer: variables involved in freezing-induced cell death. Technol
Cancer Res Treat 6: 69-79. [Crossref]
19. Gu
XY, Jiang Z, Fang W (2011) Cryoablation combined with molecular target therapy
improves the curative effect in patients with advanced non-small cell lung
cancer. J Int Med Res 39: 1736-1743. [Crossref]
20. Forest
V, Peoc'h M, Campos L, Guyotat D, Vergnon JM (2006) Benefit of a combined
treatment of cryotherapy and chemotherapy on tumour growth and late
cryo-induced angiogenesis in a non-small-cell lung cancer model. Lung Cancer
54: 79-86. [Crossref]
21. Santucci
KL, Baust JM, Snyder KK, Van Buskirk RG, Katz A et al. (2020) Investigation of
Bladder Cancer Cell Response to Cryoablation and Adjunctive Cisplatin Based
Cryo/Chemotherapy. Clin Res Open Access 6.
22. Wilhelm
SM, Adnane L, Newell P, Villanueva A, Llovet JM et al. (2008) Preclinical
overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF
and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 7:
3129-3140. [Crossref]
23. NEXAVAR
(sorafenib) tablets for oral use. Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 2018.
24. Ni
H, Yang M, Guo Z, Zhang T (2011) Sorafenib combined with cryoablation to treat
unresectable hepatocellular carcinoma. Chin J Cancer Res 23: 188-193. [Crossref]
25. Yang
Y, Lu Y, Wang C, Bai W, Qu J et al. (2012) Cryotherapy is associated with
improved clinical outcomes of Sorafenib therapy for advanced hepatocellular
carcinoma. Cell Biochem Biophys 63: 159-169. [Crossref]
26. Liu
C, Cao F, Xing W, Si T, Yu H et al. (2019) Efficacy of cryoablation combined
with sorafenib for the treatment of advanced renal cell carcinoma. Int J
Hyperthermia 36: 220-228. [Crossref]
27. Baust
JG, Bischof JC, Jiang-Hughes S, Polascik TJ, Rukstalis DB et al. (2015)
Re-purposing cryoablation: a combinatorial 'therapy' for the destruction of
tissue. Prostate Cancer Prostatic Dis 18: 87-95. [Crossref]
28. Robilotto
AT, Clarke D, Baust JM, Van Buskirk RG, Gage AA et al. (2007) Development of a
tissue engineered human prostate tumor equivalent for use in the evaluation of
cryoablative techniques. Technol Cancer Res Treat 6: 81-89. [Crossref]
29. Robilotto
AT, Baust JM, Van Buskirk RG, Gage AA, Baust JG (2013) Rapid induction of
apoptosis at ultra low temperatures enhances the efficacy of prostate cancer
cryoablation. Cryobiology.
30. Baust
JM, Snyder KK, Santucci KL, Robilitto AT, Smith JT et al. (2014) Assessment of
SCN and argon cryoablation devices in an in vivo like 3-D tissue engineered
prostate and renal cancer model. Advances in Thermal Ablative Therapy and
Biopreservation, Annual Meeting of the American College of Cryosurgery; Key
Largo, FL.
31. Baust
JM, Robilotto A, Snyder KK, Santucci K, Stewart J et al. (2017) Assessment of
Cryosurgical Device Performance Using a 3D Tissue-Engineered Cancer Model.
Technol Cancer Res Treat. Technol Cancer Res Treat 16: 900-909. [Crossref]
32. Robilotto
AT, Baust JM, Van Buskirk RG, Gage AA, Baust JG (2013) Temperature-dependent
activation of differential apoptotic pathways during cryoablation in a human
prostate cancer model. Prostate Cancer Prostatic Dis 16: 41-49. [Crossref]
33. Baust
JG, Smith Joshua T, Santucci Kimberly L, Snyder Kristi K, Robilotto Anthony T
et al. Tissue Engineered Model and Method of Use. US patent 9,213,0252015.
34. Baust
JM, Robilotto AT, Santucci KL, Snyder KK, Van Buskirk RG et al. (2020)
Evaluation of a Novel Cystoscopic Compatible Cryocatheter for the Treatment of
Bladder Cancer. Bladder Cancer 6: 303-318.
35. Robilotto
AT, Baust, JM, Santucci KL, Snyder KK, Van Buskirk RG (2019) Assessment of a
novel supercritical nitrogen cryosurgical device using prostate and renal
cancer tissue engineered models. Medical Devices and Diagnostic Engineering
5: 1-8.
36. Ikekawa
S, Ishihara K, Tanaka S, Ikeda S (1985) Basic studies of cryochemotherapy in a
murine tumor system. Cryobiology 22: 477-483. [Crossref]
37. Santucci
KL, Baust JM, Snyder KK, Van Buskirk RG, Baust JG (2018) Dose Escalation of
Vitamin D3 Yields Similar Cryosurgical Outcome to Single Dose Exposure in a
Prostate Cancer Model. Cancer Control 25: 1073274818757418. [Crossref]
38. Jiang
J, Goel R, Schmechel S, Vercellotti G, Forster C et al. (2010) Pre-conditioning
cryosurgery: cellular and molecular mechanisms and dynamics of TNF-alpha
enhanced cryotherapy in an in vivo prostate cancer model system. Cryobiology
61: 280-288. [Crossref]
39. Baust
JM, Klossner DP, Robilotto AT, Vanbuskirk RG, Gage AA et al. (2012) Vitamin
D(3) cryosensitization increases prostate cancer susceptibility to cryoablation
via mitochondrial-mediated apoptosis and necrosis. BJU Int 109: 949-958.
[Crossref]
40. Le
Pivert P, Haddad RS, Aller A, Titus K, Doulat J et al. (2004) Ultrasound guided
combined cryoablation and microencapsulated 5-Fluorouracil inhibits growth of
human prostate tumors in xenogenic mouse model assessed by luminescence imaging.
Technol Cancer Res Treat 3: 135-142. [Crossref]
41. Santucci
KL, Snyder KK, Baust JG, Van Buskirk RG, Baust JM (2020) Investigation of Liver
Cancer Cell Response to Cryoablation and Adjunctive Based Cryo/Chemotherapy. Br
J Cancer Res 3: 407-414.
42. Cancer.Net
https://www.cancer.net/navigating-cancer-care/how-cancer-treated/chemotherapy/side-effects-chemotherapy.
43. Motzer
RJ, Bukowski RM (2006) Targeted therapy for metastatic renal cell carcinoma. J
Clin Oncol 24: 5601-5608. [Crossref]
44. Awada
A, Hendlisz A, Gil T, Bartholomeus S, Mano M et al. (2005) Phase I safety and
pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in
patients with advanced, refractory solid tumours. Br J Cancer 92:
1855-1861. [Crossref]
45. Strumberg
D, Richly H, Hilger RA, Schleucher N, Korfee S et al. (2005) Phase I clinical
and pharmacokinetic study of the Novel Raf kinase and vascular endothelial
growth factor receptor inhibitor BAY 43-9006 in patients with advanced
refractory solid tumors. J Clin Oncol 23: 965-972. [Crossref]
46. Yakkala
C, Chiang CLL, Kandalaft L, Denys A, Duran R (2019) Cryoablation and
Immunotherapy: An Enthralling Synergy to Confront the Tumors. Front Immunol
10: 2283. [Crossref]
47. Baust
JG, Snyder KK, Santucci KL, Robilotto AT, Van Buskirk RG et al. (2019)
Cryoablation: physical and molecular basis with putative immunological
consequences. Int J Hyperthermia 36: 10-16. [Crossref]
48. Abdo
J, Cornell DL, Mittal SK, Agrawal DK (2018) Immunotherapy Plus Cryotherapy:
Potential Augmented Abscopal Effect for Advanced Cancers. Front Oncol 8:
85. [Crossref]
49. Gursel
E, Roberts M, Veenema RJ (1972) Regression of prostatic cancer following
sequential cryotherapy to the prostate. J Urol 108: 928-932. [Crossref]
50. Soanes
WA, Gonder MJ, Ablin RJ (1970) A possible immuno-cryothermic response in
prostatic cancer. Clin Radiol 21: 253-255. [Crossref]
51. Uhlschmid
G, Kolb E, Largiader F (1979) Cryosurgery of pulmonary metastases. Cryobiology
16: 171-178. [Crossref]
52. Misao
A, Sakata K, Saji S, Kunieda T (1981) Late appearance of resistance to tumor
rechallenge following cryosurgery. A study in an experimental mammary tumor of
the rat. Cryobiology 18: 386-389. [Crossref]
53. Neel HB 3rd, Ketcham AS, Hammond WG (1973) Experimental evaluation of in situ oncocide for primary tumor therapy: comparison of tumor-specific immunity after complete excision, cryonecrosis and ligation. Laryngoscope 83: 376-387. [Crossref]
54. Sabel MS, Arora A, Su G, Chang AE (2006) Adoptive immunotherapy of breast cancer with lymph node cells primed by cryoablation of the primary tumor. Cryobiology 53: 360-366. [Crossref]
