Immunotherapy, Immunobiomarkers and Gene Analysis Role in the Improvement of Lung Cancer Treatment
A B S T R A C T
Lung cancer is the second most common cancer with a poor survival rate (18.6%). Recently treatment of lung cancer has been improved starting from choosing appropriate treatment by gene and immunobiomarkers analysis to immunotherapy which played a major role in the treatment of lung cancer and showed improvement in overall survival (OS), progression-free survival (PFS) and objective response rate (ORR) with less adverse effects. Through this triad Immunotherapy, immunobiomarkers and Gene analysis oncologists can provide better treatment for lung cancer patients.
The most important cause that makes immunotherapy shows good results in the treatment of lung cancer is P53 gene mutation which is present in more than half of lung cancer patients. P53 gene mutation causes high genetic instability so causes more mutations in cancer cells and more neoantigens are formed. These neoantigens make cancer cells more immunogenic and respond better to the treatment of lung cancer by immunotherapy.
The most common immunotherapy drug family that is effective in the treatment of lung cancer is Immune checkpoint inhibitors. Throughout the journey of treatment Immune checkpoint inhibitors can develop resistance by multiple mechanisms that are discussed in the review and to overcome these mechanisms Immune checkpoint inhibitors can be used in combination with other immunotherapies or radiotherapy.
Keywords
Lung cancer, immune checkpoint inhibitor, immunobiomarkers, overall survival, progression-free survival, objective response rate
Introduction
Lung cancer constitutes nearly 18.5% of all cancer-related deaths and the second most common cancer (after excluding skin cancer) [1]. Breast cancer is the most commonly diagnosed cancer in women, while prostate cancer is the most commonly diagnosed cancer in men. Primary Lung cancers have two types of small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC represents 85% of all primary lung cancers, and most patients present with unresectable tumors and in advanced stages at the time of diagnosis [2]. NSCLC has three main types which are squamous cell, adenocarcinoma and large-cell undifferentiated carcinoma. Adenocarcinoma accounts 30% of all lung cancers (most common subtype of lung cancer); and about 40% of NSCLC occurrences [3]. SCLC grows quickly and sends metastasis to other parts of the body. This type of lung cancer represents fewer than 20% of lung cancers and usually associated with tobacco smoking. Two main types of SCLC are small cell carcinoma (most common type of SCLC) and combined small cell carcinoma [4].
For several years, chemotherapy was the only treatment that could prolong survival in patients with advanced NSCLC but recently immunotherapy played a major role in lung cancer treatment [5]. Immunotherapy is a treatment modality that induces the immune system to recognize and destroy cancer cells. Immune checkpoint inhibitors are the most common type of immune therapy which are used in lung cancer treatment. Immune checkpoint inhibitors showed good results even better than chemotherapy in the treatment of lung cancer [5, 6]. Immune checkpoint inhibitors are either agonistic or antagonistic antibodies that activate T- cells and work on immune checkpoints. Immune checkpoints are proteins expressed on cancer cells or immune cells that prevent the immune system from attacking cancer. Immune checkpoints are identified by immunohistochemistry and the main immune checkpoints are Cytotoxic T-lymphocyte antigen-4 (CTLA-4) and Programmed death ligand 1 (PDL1) or receptor on T- cell as PD-1 receptors [7].
Toxicity profile of immunotherapy is different from cytotoxic chemotherapeutic agents. Toxicity profile is due to immune system activation. This activation may cause an immune attack on normal tissues. Signs and symptoms which appear from this activation are similar to autoimmune diseases. Any organ in the body can be attacked, so that a wide variety of possible immune-related adverse events may occur, depending on which organ is affected [8]. It is difficult to predict patients who will suffer from these adverse effects. These adverse effects have been related to gut microbiome; interleukin 17 and healthy tissue expression of CTLA-4. Adverse effects that can occur are Colitis, Pneumonitis, Hepatitis, Thyroid disorder, Hypophysitis and Skin toxicity. Each side effect has four different grades [9]. Hypothyroidism is the most common immune-related adverse event that has been associated with PDL1 and PD1 in treatment of NSCLC Hypothyroidism is treated by thyroid hormone replacement therapy [10]. Therefore, monitoring of TSH level is necessary every 4–6 weeks and If TSH > 10, levothyroxine should be used to make TSH reach the normal range (normal range according to patient age) [11]. It was reported that patients who are treated with PD-1 inhibitors have higher incidence of pneumonitis compared to patients who receive PD-L1 treatment (4 vs. 2%; P = 0.01). Therefore, clinicians should be more aware of lung inflammation in NSCLC patients receiving PD-1 inhibitors therapy [10]. The incidence of adverse effects of PD-1/PD-L1 inhibitors monotherapy are lower than that of combination therapy. For example, Treatment-related adverse effects (TRAEs) occurrence with pembrolizumab monotherapy were 70.9%, while TRAEs that occur with pembrolizumab combination with chemotherapy were (98.2%) [12, 13].
The incidence of pneumonia is generally 7.4-24.3 months after the start of treatment. Clinical symptoms are mainly dyspnea, dry cough, chest pain and fever [14]. Patients with mild (grade I) pneumonia need to re-assess arterial oxygen saturation in both resting and active states and repeat chest CT in one month [15, 16]. For grade II or higher physicians should suspect bacterial or viral infections. So nasal swab can be done for possible viral infections and sputum culture, blood culture, and urine antigen test are used to detect pneumococcus and legionella (Bacterial infection) [16]. Bronchoscopy and bronchoalveolar lavage are essential. If the infections are not completely excluded, empiric antibiotics can be used. Other than ruling out infections there must be management of clinical symptoms. It can be done by taking oral or intravenous prednisone/methylprednisolone 1–2 mg/kg/day for grade II pneumonia patients [16, 17]. Severe cases (stage three and four) need hospitalization and intravenous methylprednisolone 1-2 mg/kg/day. If corticosteroids remain ineffective after 2 days from starting treatment other immunosuppression drugs can be used such as infliximab, mycophenolate mofetil or intravenous immunoglobulin [16, 17].
Gene analysis by NGS and immunobiomarkers (PDL1, CTLA-4) identification by immunohistochemistry guide us to choose the best combination of treatment for lung cancer patients either immunotherapy alone or in combination with chemotherapy [18-20].
Immune Checkpoint Inhibitor (Immunotherapy)
I CTLA-4 Inhibition
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) protein competes withCD28 for B7 binding in an inhibitory fashion and suppresses T-cell. Antibodies to CTLA-4 block the inhibition of CD28/B7 which causes T-cell activation and in turn prolong anti-tumor activity. CTLA-4inhibitors don’t show great effect when used as monotherapy and only seem to be working in lung cancer when used in combination with chemotherapy [21, 22].
II Ipilimumab
FDA approved nivolumab with ipilimumab and two cycles of platinum-doublet chemotherapy as the first line of treatment for patients with metastatic or recurrent NSCLC with no EGFR or anaplastic lymphoma kinase (ALK) genomic mutation. This approval was based on results from Study CA2099LA (CheckMate 9LA) in which there was an improvement in OS for patients receiving nivolumab with ipilimumab and chemotherapy (median 14.1 months) compared to chemotherapy alone (median 10.7 months). PFS and ORR demonstrated also statistically significant differences [23].
III PD-1 Checkpoint Blockade
PD-L1 and PDL-2 are the most studied membrane inhibitory ligands in NSCLC. Approximately half of NSCLC patients express PD-L1 and PDL-2 and their expression contribute to poor prognosis by suppressing T-cell function and promoting tumor cell immune escape [24]. PD-1 inhibitors are considered the best immune checkpoint blockade in the treatment of lung cancer. Anti-PD1 and chemotherapy were associated with better OS and PFS when compared with anti-PDL1 plus chemotherapy in the treatment of lung cancer this is because anti-PD-1 works on PD-1 receptors so inhibits both PD-L1 and PD-L2 not only PD-L1 [25, 26]. Unlike CTLA-4 inhibitors anti-PD1 shows good results as a monotherapy. PD-1 results depend on PDL-1 expression on tumor cells.
IV Pembrolizumab
Pembrolizumab is the first-line therapy for patients with PD-L1 50% or greater as when pembrolizumab is used as monotherapy there were no significant differences in PFS, OS and respond rate (RR) compared to pembrolizumab combined with chemotherapy and pembrolizumab (2 mg/kg every 3 weeks or 10 mg/kg every 2 or 3 weeks) showed 3-year OS rate in 35% of patients who received pembrolizumab compared to13% of patients who received docetaxel (chemotherapy) [27, 28]. Pembrolizumab showed 5-year OS for patients who have PD-L1 50% or greater either these patients are previously treated patients or treatment-naive patients and only grade 3 adverse events occurred, and grade 4 adverse events aren’t documented [29]. Pembrolizumab standard dose which is approved by FDA is 200 mg every three, but it is documented that there was no difference in PFS and OS for patients who receive pembrolizumab with a dose of 100 mg every three weeks (low dose). This dose is applied either pembrolizumab is used as a monotherapy or in combination with chemotherapy. Grade three adverse effect or more was observed on both doses, so 100 mg of pembrolizumab appears to be effective and with less cost [30].
V Nivolumab
Advanced pretreated NSCLC Patients who received nivolumab 1, 3, or 10 mg/kg every 2 weeks in 8-week cycles for up to 96 weeks showed 5-year overall survival. Moreover, nivolumab improved survival over docetaxel in the treatment of squamous and non-squamous cell NSCLC. Trials for previously treated, advanced squamous cell NSCLC patients showed OS: 9.2 vs. 6.0 months and for non-squamous NSCLC patients OS was 12.2 months vs 9.4 months and adverse effects were observed for patients treated with Nivolumab was ≤10% of patients compared to docetaxel was ~ 55% [31, 32]. Therefore, nivolumab was approved by the FDA, at a dose of 240 mg IV every 2 weeks, for patients with previously treated, metastatic squamous and non-squamous cell lung carcinoma who have progressed on platinum-containing therapy. Nivolumab at 480 mg IV every 4 weeks was approved for NSCLC patients [33].
Anti PD-L1 Antibody
I Atezolizumab
Atezolizumab is usually used as the second line of NSCLC treatment as atezolizumab in combination with chemotherapy is not cost-effective when it is used as first-line therapy of advanced non-squamous NSCLC but atezolizumab can be used as the first line of treatment of ES‐SCLC patients when combining with durvalumab and platinum‐based chemotherapy [34]. These approvals were based on meaningful improvements in overall survival [35].
II Avelumab
Avelumab (New anti PD-L1 antibody) dose is10 mg/kg IV over one hour every 2 weeks until progression or unacceptable toxicity. Patients have to take acetaminophen and antihistamine before the first four infusions, and continuation of premedication is primarily based on clinical cases [36]. The Phase I trial investigated avelumab as the main therapy for NSCLC. The trial included patients suffering from recurrent NSCLC (adenocarcinoma and squamous cell carcinoma) or patients in stage IV and no ALK fusion or activating EGFR mutation. Avelumab had acceptable results for patients had PD-L1-positive tumors based on ≥1% expression. ORR was19.9% (95% CI, 13.9% – 27.0%). ORR For patients with squamous histology was17.4% and 20.9% for patients with non-squamous histology. Disease control rate was 64% as 45% of patients had stable disease [37, 38].
III Durvalumab
Durvalumab (10 mg /kg of body weight every 2 weeks for up to 12 months) was validated for locally advanced unresected stage 3 NSCLC after chemoradiation therapy, regardless of PD-L1 expression. It appears that patients with PD-L1 expression higher than 25% have the highest benefit. This was shown in phase 3 trial durvalumab significantly improve PFS compared to placebo results. Treatment with durvalumab started 1 to 42 days after chemo radiotherapy was given to patients. Durvalumab significantly prolonged OS compared to placebo results (stratified hazard ratio for death, 0.68; 99.73% CI, 0.47 to 0.997; P=0.0025). The median time to distant metastasis or death was 28.3 months in the durvalumab group and 16.2 months in the placebo group [39].
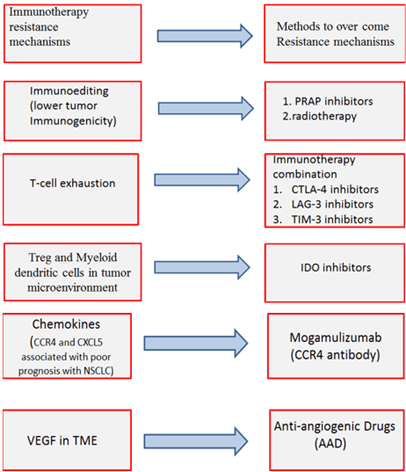
Figure 1: Mechanisms of Immunotherapy resistance and methods to overcome these mechanisms.
Unlucky immunotherapy can develop resistance by different mechanisms. Intrinsic mechanisms as cell signaling, immune recognition, gene expression, DNA damage response and extrinsic mechanisms such as T cell activation and neo-angiogenesis [40].
IV Intrinsic Resistance Mechanisms:
i. Cancer cells: Neo-antigen burden of cancer cells and tumor immunogenicity depend mainly on genetic instability. Increasing genetic instability of cancer cells will increase neo-antigen burden of cancer cells and tumor immunogenicity. Therefore, Tumor tries to repair their DNA damage by Poly adenosine diphosphate ribose polymerase (PARP) enzyme to decrease genetic instability and a result will decrease immunotherapy efficacy. PARP inhibitors can be used in case immune checkpoint inhibitors develop resistance to increase genetic instability and increase tumor immunogenicity [41].
ii. T cell resistance: Multiple ligands are expressed on cancer cells not only PD-L1and CTLA-4 . T cells can respond to these ligands through receptors on it. These receptors are immunoglobin mucin-3 (TIM-3) and lymphocyte activation gene 3 (LAG-3) [42]. Multiple ligand expression usually causes severe T cell exhaustion consequently leading to Immunotherapy resistance [43].
V Extrinsic Resistance Mechanism
i. Treg and Myeloid dendritic cells (MDSC) cause immunosuppression in tumor microenvironment (TME) and facilitate tumor cell growth. Immunotherapy efficacy has been linked to lower Treg and MDSC infiltration in preclinical studies but cancer cells and their stroma (fibroblast and endothelial cells) express Indoleamine 2, 3-dioxygenase (IDO) that promotes Treg and MDSC proliferation and activation [44, 45].
ii. Chemokine: Inflammation is an important component of TME and Chemokines are a family of small cytokines that has a crucial role in inflammation and immunity. Chemokines induce tumor angiogenesis, tumor growth, metastatic spreading and leukocytes recruitment. Chemokines recruit CCR2+ monocytes and CXCR2+ neutrophils at the tumor site. This recruitment differentiates monocytes and neutrophils to tumor-associated macrophages (TAMs) and tumor- associated neutrophils (TANs). TAM and TAN create an immunosuppressive tumor microenvironment .chemokine receptors are expressed by cancer and stromal cells [46].
iii. Vascular endothelial growth factor (VEGF): VEGF is expressed in TME due to hypoxia. Although, VEGF increases immunogenicity of the tumor as VEGF is driver of tumor neoangiogenesis it exerts an immunosuppressive effect [47]. Anti-PD-1 non-responders showed higher VEGF levels compared to responders, suggesting a role of VEGF in immunotherapy resistance so anti-VEGF use is considered an important strategy to overcome resistance [48].
There are different methods to overcome immunotherapy resistance mechanisms:
i. First method to overcome immunotherapy resistance is immunotherapy combination. Immunotherapy combination is done to overcome T-cell exhaustion. LAG-3 receptor is expressed on T-cells and binds to Fibrinogen-like protein 1(FGL1) (ligand on cancer cells). This binding causes T-cells exhaustion as it blocks indirectly TCR pathway resulting in cytokines inhibition [49, 50]. When Lymphocytes express PD-1 and LAG-3 this make severe exhaustion in T cells, unlike T cells which express PD-1 only mild to non-significant exhaustion occurs [51]. Anti-LAG3 antibody (e.g., IMP321, relatlimab) plus a PD-1/PD-L1 inhibitor revealed favourable preclinical results in different tumors that express these two types of immune checkpoints (FGL1and PDL-1). Preclinical results were observed in clinical phase I/II trials for LAG-3 and PD-1 positive tumors when treated with relatlimab plus nivolumab [52].
Similar to LAG-3 TIM-3 negatively regulates T cell activation and severe exhaustion was observed with TIM-3 positive CD8+ T cells. Galectin-9, HMGB1 orCEACAM-1 are TIM-3 ligands expressed by cancer cells [53]. In Phase I trial anti-TIM3 antibody TSR-022 in combination with a PD-1 inhibitor increase clinical activity in anti-PD-1 refractory NSCLC and melanoma.
T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) is a co-inhibitory receptor up regulated by immune cells, including activated T cells, natural killer cells, and regulatory T cells. TIGIT has a direct immunosuppressive effect when binds to CD155 so TIGIT inhibitors improve anti-tumor T cell responses. Tiragolumab (TIGIT-inhibitor) plus atezolizumab meaningfully improve ORR compared to atezolizumab monotherapy in PD-L1 positive metastatic NSCLC patients (55.2% vs 17.2%) [54].
Finally, CTLA4 inhibitors combination with PD-1 and PDL-1 prolong OS benefit for first line ipilimumab plus nivolumab in advanced-stage disease (median OS 17.1 vs. 13.9 months with chemotherapy, 2-year OS of 40% vs. 32.8% (HR 0.79, 97.72% CI 0.65-0.96; P = 0.007)), independent of TMB or PD-L1 expression. Treatment-related serious adverse events (AE) of any grade were more frequent with ipilimumab plus nivolumab than with chemotherapy (24.5% vs. 13.9%) [55].
ii. VEGF inhibitors called anti-Angiogenic Drugs (AAD) can normalize tumor vasculature and restore blood flow, therefore decreasing tumor hypoxia and facilitating immune cell infiltration [56]. VEGF is measured with R&D Systems kit Examination or sandwich enzyme-linked immunosorbent assay. Anti-VEGF radiotherapy and anti-PD-L1 therapy resulted in the generation of prolonged protective T cell immunity. A significant improvement in survival (p=0.003) was observed in Mice were given Lewis lung carcinoma (LLC) that were treated with radiotherapy +anti-PD-L1+anti-VEGF therapies combination compared to radiotherapy alone. Anti-VEGF therapy in combination with cancer immunotherapy has promising results in both preclinical and clinical settings. Combined anti-VEGF and PD-L1 inhibitors have a synergistic effect that was shown in the treatment of mice have small cell lung cancer and tumor associated with PD-1/TIM-3 double positive T cell [57]. Therapeutic combinations of AAD and immunotherapy have already been approved for endometrial cancer and renal cell carcinoma. IMpower150 trial in the treatment of NSCLC showed an OS benefit for the first-line quadruple (atezolizumab/bevacicumab/carboplatin/paclitaxel) therapy versus AAD/doublet-chemotherapy with a particular benefit in patients who have EGFR-mutant/ALK-positive tumors [58].
iii. PARP inhibitors (Olaparib, Niraparib, Rucaparib, talazoparib) work on PARP enzyme that acts in DNA Damage Response (DDR) pathways. These pathways repair damaged DNA which occurs during cell replication [59]. PARP enzyme repairs single-strand DNA mutations. NSCLC with BRCA mutation doesn’t show a good response to PARP inhibitors monotherapy. However, numerous clinical studies showed synergistic effects of PARP inhibitors and immunotherapy in many solid malignancies with BRCA mutation. PARP inhibitors induce genetic instability, increase TMB and neoantigens burden via DDR deficiency and may be involved in PD-L1 up-regulation by cancer cells [60]. Schlafen family member 11 (SLFN11) encourages cell death in response to DNA damage. Recently SLFN11was identified as a prognostic biomarker for SCLC sensitivity to PARP inhibitors [61]. PARP inhibitors are established in the treatment of BRCA-mutated breast and ovarian cancer but for lung cancer PARP inhibitors did not show good results in NSCLC treatment but showed good results in the treatment of SCLC [62, 63]. Talazoparib 1.0 mg daily exhibits anti-tumor activity and the highest bioavailability in patients presenting with BRCA mutations. The median PFS of these patients was 11.1 weeks [95% confidence interval (CI): 4.3–13.0 weeks] [64]. PARP inhibitors such as Olaparib when combined with chemotherapeutics like cisplatin and etoposide enhance anti-tumor effects in SCLC as several preclinical reported that PARP inhibitors can also increase the response of SCLC patients to other chemotherapeutics [65]. This response depends on SLFN11 expression on tumor cells [66]. Multiple groups have examined combining novel targeted therapies with PARP inhibitors [67]. Olaparib and WEE1 inhibitor adavosertib (AZD1775) can significantly improve the efficacy of the single-agent activity of Olaparib in SCLC circulating tumor cell patient-derived xenograft [68]. Olaparib increases the cytotoxic effects of chemotherapies such as ATR inhibitor (AZD-6738) in SCLC treatment [69]. It is reported that PARP inhibitors overcome resistance occurred to tyrosine kinase inhibitors in the treatment of Lung cancers with epidermal growth factor receptor (EGFR) mutation [70].
iv. Another method to increase tumor immunogenicity other than PARP inhibitors is immunotherapy and radiotherapy combination. Radiation cytotoxic activity comes from inducing Caspase-driven genomic and mitochondrial DNA fragmentation in tumor cells, promoting the release of cytochrome c from mitochondria to activate caspase 9 (CASP9) which initiates intrinsic apoptosis. Radiotherapy also increases IFN I production and activation of anticancer immune responses [71]. Usually, irradiated tumor cells often fail to produce IFN I and this is due to CASP9 involvement so emricasan (Caspase Inhibitor) plus PD-L1 inhibitor enhanced radiation effects [72].
v. Indoleamine 2, 3-dioxygenase 1 and 2 (IDO1 and 2) and tryptophan-2, 3-dioxygenase (TDO2) are important metabolic pathways in cancer progression. IDO is IFN induced in cancer, stromal non-immune and immune cells that metabolize tryptophan to kynurenine. This metabolic convergence induces an immunosuppressive effect in TME. Kynurenine accumulation and tryptophan decrease promote the generation of Treg and MDSCs and inhibit T cells proliferation and activation [73]. IDO1 up-regulation has been reported in different cancer types including NSCLC and associated with poor prognosis and immunotherapy resistance. IDO1 inhibitors increase T cell proliferation and tumor infiltration as well as IL-2 up-regulation [74]. IDO1 inhibitors have been tested in multiple phases I/II trials in combination with PD-1/PD-L1/ CTLA-4 inhibitors with promising results [75]. However, the first large phase III ECHO-301 trial evaluating the selective IDO inhibitors in combination with pembrolizumab in advanced melanoma demonstrated improvement in PFS compared to pembrolizumab [76].
vi. The CC chemokine receptor type 4 (CCR4) is expressed on Treg cells and other circulating/tumor-infiltrating T cells and binds to ligands expressed on cancer cells asCCL17, CCL2 .This binding promotes the recruitment of immunosuppressive Treg cells. Furthermore, the CXCL5/CXCR2-axis encourages myeloid cell recruitment.CXCR2 blockade significantly reduces the presence of MDSC in murine tumors [77]. CCR4 and CXCL5 expression have been associated with poor prognosis in various cancer types including NSCLC [78]. The monoclonal anti-CCR4 antibody mogamulizumab exerts Treg-depleting effects and it is FDA-approved for refractory T cell lymphoma. First results from phase I solid tumor trials in combination with PD-1/PD-L1/ CTLA-4 inhibitors suggest an acceptable safety profile. Mogamulizumab has an antitumor effect in NSCLC [79].
Choosing Best Treatment
I Immunobiomarkers
i PD-L1
It is very important to identify the percent of cells in the tumor which express PDL-1. Lung tumors that express PDL-1 <1% don’t show responses with anti-PD1/PD-L1 therapies [80]. Lung tumors that express PDL-1 >1% show good results with anti-PD1/PD-L1specially pembrolizumab. Pembrolizumab is used as monotherapy in the treatment of lung tumors with≥50% PDL-1 and for tumors with <50% PDL-1 expression pembrolizumab needs to be combined with chemotherapy [81, 82]. After different cohorts combination of carboplatin + nab-paclitaxel/paclitaxel is the best combination of chemotherapy that can be used with pembrolizumab in the treatment of advanced, squamous cell NSCLC. Carboplatin + nab-paclitaxel/paclitaxel demonstrated an increase in OS (median 7.8 months) but gene analysis gives information about which gene has been mutated and through this analysis, we can identify the best chemotherapy that can be used in treatment [83].
ii Tumor Mutational Burden (TMB)
TMB is driven by several factors including DNA replication errors and mutations mediated by defects in tumor suppressor genes (e. g. TP53) and deficient DNA mismatch repair (dMMR) mechanisms (generally indicated by high microsatellite instability [MSI-H]) [84, 85]. TMB is evaluated by using targeted NGS, which measures the number of mutations on a portion of the coding region and simultaneously provides data on specific DNA alterations which have been validated [86]. Tumors with high levels of TMB express more cancer-specific antigens (neoantigens) that increase tumor response to immunotherapy [87, 88].
Nivolumab plus ipilimumab showed promising efficacy in the treatment of NSCLC with a high tumor mutational burden (≥10 mutations per megabase) and these results are better than chemotherapy results. The median PFS was 7.2 months (95% confidence interval [CI], 5.5 to 13.2) versus 5.5 months (95% CI, 4.4 to 5.8) (hazard ratio for disease death or progression, 0.58; 97.5% CI, 0.41 to 0.81; P<0.001) and PFS rate was 42.6% with nivolumab plus ipilimumab versus 13.2% with chemotherapy. ORR was 45.3% with nivolumab plus ipilimumab and 26.9% with chemotherapy. The benefit of nivolumab plus ipilimumab over chemotherapy did not depend on PDL-1 expression. Treatment-related adverse effects grade 3 or 4 were 31.2% with nivolumab plus ipilimumab and 36.1% with chemotherapy [89].
Other immunobiomarkers can be checked if PDL-1 or PD-1 develops resistance. These immunobiomarkers are Ligands expressed by cancer cells and identified by immunohistochemistry as FGL1, TIM-3 ligands (galectin-9, HMGB1 and CEACAM), CTLA4, TIGIT ligands (CD155 (PVR) and CD112 (PVRL2, nectin-2)), SLFN11and IDO1.All these immunobiomarkers are expressed on tumor surrounding stroma not only cancer cells.
iii Gene Analysis
Integrate next-generation sequencing (NGS) and mass spectrometry (MS)-based technologies are used to identify molecular mechanisms of lung cancer subtypes, according to mutated genes we can select the best treatment [90].
a. P53 gene mutation accounts for half of lung cancer cases (microcellular lung cancer (70%) and adenoid lung cancer (33%)): Tumor protein 53(TP53) mutations cause chromosomal instability and usually associated with other gene mutations [91]. Auranofin (AF) anti-rheumatic drug with anticancer properties that acts as a thioredoxin reductase 1 (TrxR) inhibitor. AF radicating NSCLC cells via distinct molecular mechanisms, including ferroptotic cell death and apoptotic directed by the overexpression of mutant p53 protein [92].
b. EGFR mutation represents 10-15% of lung cancer generally appears in adenocarcinoma: osimertinib is the first line of treatment and shows improvement in PFS, ORR [93]. BRAF V600E mutation can occur with EGFR mutation and develop resistance to osimertinib and make lung cancer more metastatic so dabrafenib 75 mg twice daily (BID), trametinib 1 mg once every day (OD) and osimertinib 80 mg OD Within 2 weeks of treatment, the patient didn’t need more opioid administration as there is complete relieve of the severe bone pain in the hips, as well as marked improvement in quality of life and appetite gain, which turned possible for him to resume his daily activities. As adverse events (AE), he experienced grade 1 fatigue, fever, and nausea, all managed with symptomatic medication tumor reduction within 6 weeks of treatment [94].
c. KRAS mutation causes adenocarcinoma: a synergistic effect of the trametinib/FGFR inhibitor (over- come resistance) combination inhibits proliferation of lung cells with KRAS mutation. There must be careful attention to synergistic or additive toxicities [95].
d. ALK mutation about 5% percent of patients have NSCLC commonly adenocarcinoma: lorlatinib has the best results including PFS and ORR but has the highest probability of grade 3-5 adverse effects when used as first-line of treatment in ALK-positive NSCLC, followed by low-dose 300 mg two times daily alectinib which had the best safety profile, high-dose (600 mg twice daily) alectinib, brigatinib, ensartinib, and ceritinib [96].
e. BRAF mutation has been reported in about 4% of NSCLC usually adenocarcinoma: KRAS and BRAF are two key oncogenes in the MAPK/ERK pathway. Trametinib in combination with dabrafenib (BRAF inhibitor) represents the first MEK1/2 inhibitor that is approved for advanced BRAFV600E-mutant NSCLC [97].
f. ROS1 oncogenic fusion is present in 1%–2% of NSCLC and 4% of lung adenocarcinoma: Crizotinib is durable in ROS1-positive patients 12.5% of patients treated with Crizotinib achieved complete response (CR) and remained in CR at follow-up and 81% had a partial response as the best response and follow- up showed progress [98].
g. HER2 mutation causes1–2% of lung adenocarcinomas: trastuzumab deruxtecan (6.4 mg per kilogram of body weight) was administered to patients who had metastatic HER2-mutant NSCLC that was refractory to standard treatment. Patients confirm objective response (95% confidence interval [CI], 44 to 65) and median duration of response was 9.3 months (95% CI, 5.7 to 14.7), median PFS was 8.2 months (95% CI, 6.0 to 11.9) and median OS was 17.8 months (95% CI, 13.8 to 22.1) [99].
h. Rearranged During Transfection (RET) mutation: The RET proto-oncogene that encodes for tyrosine kinase receptor which is activated by gene fusion in 1%–2% of NSCLC. Patients are usually female young Asian non-smokers patients. Pralsetinib and Selpercatinib and (RET-selective inhibitors) 20 mg once daily to 240 mg two times daily during phase one and 160 mg two times daily during phase two. They offer manageable adverse events with long-lasting efficacy so they have changed the treatment of patients with RET-altered tumors based on their phase I/II trials [100].
i. MET mutation causes adenocarcinoma and squamous NSCLC and about 5% of lung cancer: poor prognosis was observed in patients who have tumors with a MET exon 14 skipping mutation as they didn’t show good responses to different types of therapies, including immunotherapies [101]. Capmatinib therapy is efficient for NSCLC patients with a MET exon 14 skipping mutation. The safety profile shows low-grade and reversible adverse events. Results suggest that capmatinib may be a new therapeutic line for patients with advanced NSCLC with a MET exon 14 skipping mutation [102].
j. AKT1 mutation present in 0.69% of all squamous cell lung carcinoma patients: pan-AKT inhibitor AZD5363 orally provides tumor regressions and durable responses across different tumor types harboring the mutation [103].
k. FGFR1 amplification is found in approximately 20% of squamous cell cancers: combination therapy of FGFR/PLK1 inhibitors has good potential in treating lung cancer with FGFR alterations and may be extended to other cancer types with the same type of mutation. Significantly, co-targeting FGFR1/PLK1 causes no additional toxicities [104].
Table 1: Mutated genes that cause lung cancer and best therapy
for each mutated gene.
|
GENE MUTATED |
LUNG CANCER CAUSED BY THIS MUTATION |
BEST THERAPY
|
|
p53 genes |
Half of lung cancer cases (microcellular lung
cancer (70%) and adenoid lung cancer (33%) |
Auranofin |
|
EGFR mutation |
10-15% of lung cancer generally appears in
adenocarcinoma |
Osimertinib and if resistance is developed use
dabrafenib and trametinib |
|
KRAS mutation |
adenocarcinoma |
trametinib/FGFR inhibitor |
|
ALK mutation |
5% percent of patients have NSCLC commonly
adenocarcinoma |
Lorlatinib (best results) Alectinib (safest profile) |
|
BRAF mutation |
4% of NSCLC usually adenocarcinoma |
Trametinib in combination with dabrafenib (BRAF
inhibitor) |
|
ROS1 oncogenic
fusion |
1%–2% of NSCLC and 4% of lung adenocarcinoma |
Crizotinib |
|
HER2 mutation |
1–2% of lung adenocarcinomas |
trastuzumab deruxtecan |
|
Rearranged during transfection (RET)
mutation |
1%–2% of NSCLC patients are usually associated
with female young Asian non-smokers patients |
Pralsetinib and Selpercatinib |
|
MET mutation |
adenocarcinoma and squamous NSCLC and about 5% of
lung cancer |
Capmatinib |
|
AKT1 mutation |
0.69% of all squamous cell lung carcinoma |
pan-AKT inhibitor AZD5363 |
|
FGFR1 amplification |
20% of squamous cell cancers |
combination therapy of FGFR/PLK1 inhibitors |
Conclusion
Immunotherapy is an important line of lung cancer treatment. Immunotherapy can be used in different lung cancer types and stages. Immunobiomarkers and gene analysis role is not limited only in choosing whether immunotherapy is going to be used as monotherapy or in combination with chemotherapy therapy. They also help in choosing appropriate immunotherapy that can be used if PD-1 and PDL-1 develop resistance. Therefore, lung cancer prognosis has improved in the last years and increase is observed in 5-year overall survival rates.
Finally screening programmes by CT scan are highly recommended for people age ranges from 55 to 74 years old who are currently smoking or have quit in the past 15 years or have positive history of lung cancer in the family [105].
Funding
None.
Author Contributions
Mostafa Wael conceived of the presented idea. Mostafa Wael and Mostafa Hosam wrote the manuscript. Arwa Youssef and Abdelrahman Alzahabi collected the data. Rawan Ellackany made the figure and the table. Arwa Youssef and Mostafa Wael made final approval of the version to be published. Mostafa Wael supervised the project. All authors reviewed the manuscript.
Competing Interests
None.
Conflicts of Interest
None.
Article Info
Article Type
Review ArticlePublication history
Received: Thu 27, Oct 2022Accepted: Thu 10, Nov 2022
Published: Thu 15, Dec 2022
Copyright
© 2023 Mostafa Wael. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RCO.2022.01.01
Author Info
Mostafa Wael Mostafa Hosam Arwa Youssef Rawan Ellackany Abdelrahman Alzahabi
Corresponding Author
Mostafa WaelFaculty of Medicine, Modern University for Technology and Information, Cairo, Egypt
Figures & Tables
Table 1: Mutated genes that cause lung cancer and best therapy
for each mutated gene.
|
GENE MUTATED |
LUNG CANCER CAUSED BY THIS MUTATION |
BEST THERAPY
|
|
p53 genes |
Half of lung cancer cases (microcellular lung
cancer (70%) and adenoid lung cancer (33%) |
Auranofin |
|
EGFR mutation |
10-15% of lung cancer generally appears in
adenocarcinoma |
Osimertinib and if resistance is developed use
dabrafenib and trametinib |
|
KRAS mutation |
adenocarcinoma |
trametinib/FGFR inhibitor |
|
ALK mutation |
5% percent of patients have NSCLC commonly
adenocarcinoma |
Lorlatinib (best results) Alectinib (safest profile) |
|
BRAF mutation |
4% of NSCLC usually adenocarcinoma |
Trametinib in combination with dabrafenib (BRAF
inhibitor) |
|
ROS1 oncogenic
fusion |
1%–2% of NSCLC and 4% of lung adenocarcinoma |
Crizotinib |
|
HER2 mutation |
1–2% of lung adenocarcinomas |
trastuzumab deruxtecan |
|
Rearranged during transfection (RET)
mutation |
1%–2% of NSCLC patients are usually associated
with female young Asian non-smokers patients |
Pralsetinib and Selpercatinib |
|
MET mutation |
adenocarcinoma and squamous NSCLC and about 5% of
lung cancer |
Capmatinib |
|
AKT1 mutation |
0.69% of all squamous cell lung carcinoma |
pan-AKT inhibitor AZD5363 |
|
FGFR1 amplification |
20% of squamous cell cancers |
combination therapy of FGFR/PLK1 inhibitors |
References
1. Bray F, Ferlay J,
Soerjomataram I, Siegel RL, Torre LA et al. (2018) Global cancer statistics
2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin 68: 394-424. [Crossref]
2. Zago G, Muller M,
van den Heuvel M, Baas P (2016) New targeted treatments for non-small-cell lung
cancer - role of nivolumab. Biologics 10: 103-117. [Crossref]
3. Midha A, Dearden S,
McCormack R (2015) EGFR mutation incidence in non-small-cell lung cancer of
adenocarcinoma histology: a systematic review and global map by ethnicity. Am
J Cancer Res 5: 2892-2911. [Crossref]
4. Gibbons DL, Byers
LA, Kurie JM (2014) Smoking, p53 mutation, and lung cancer. Mol Cancer Res
12: 3-13. [Crossref]
5. Brahmer JR,
Govindan R, Anders RA, Antonia SJ, Sagorsky S et al. (2018) The Society for
Immunotherapy of Cancer consensus statement on immunotherapy for the treatment
of non-small cell lung cancer (NSCLC). J Immunother Cancer 6: 75. [Crossref]
6. Hoos A (2016)
Development of immuno-oncology drugs - from CTLA4 to PD1 to the next
generations. Nat Rev Drug Discov 15: 235-247. [Crossref]
7. Peterson JJ, Moses
SKS (2016) Update on New Therapies With Immune Checkpoint Inhibitors. Clin J
Oncol Nurs 20: 405-410. [Crossref]
8. Sgambato A,
Casaluce F, Sacco PC, Palazzolo G, Maione P et al. (2016) Anti PD-1 and PDL-1
Immunotherapy in the Treatment of Advanced Non- Small Cell Lung Cancer (NSCLC):
A Review on Toxicity Profile and its Management. Curr Drug Saf 11:
62-68. [Crossref]
9. Naidoo J, Page DB,
Li BT, Connell LC, Schindler K et al. (2015) Toxicities of the anti-PD-1 and
anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26: 2375-2391. [Crossref]
10. Pillai RN, Behera
M, Owonikoko TK, Kamphorst AO, Pakkala S et al. (2018) Comparison of the
toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer:
A systematic analysis of the literature. Cancer 124: 271-277. [Crossref]
11. Brahmer JR,
Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ et al. (2018) Management of
Immune-Related Adverse Events in Patients Treated With Immune Checkpoint
Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice
Guideline. J Clin Oncol 36: 1714-1768. [Crossref]
12. Garon EB, Rizvi NA,
Hui R, Leighl N, Balmanoukian AS et al. (2015) Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med 372: 2018-2028. [Crossref]
13. Nishijima TF,
Shachar SS, Nyrop KA, Muss HB (2017) Safety and tolerability of PD-1/PD-L1
inhibitors compared with chemotherapy in patients with advanced cancer: a
meta-analysis. Oncologist 22: 470-479. [Crossref]
14. Michot JM,
Bigenwald C, Champiat S, Collins M, Carbonnel F et al. (2016) Immune-related
adverse events with immune checkpoint blockade: a comprehensive review. Eur
J Cancer 54: 139-148. [Crossref]
15. Brahmer JR,
Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ et al. (2018) Management of
Immune-Related Adverse Events in Patients Treated With Immune Checkpoint
Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice
Guideline. J Clin Oncol 36: 1714-1768. [Crossref]
16. Hampton JEB, Bazzell
AF, Dains JE (2018) Clinical Management of Pneumonitis in Patients Receiving
Anti-PD-1/PD-L1 Therapy. J Adv Pract Oncol 9: 422-428. [Crossref]
17. Su Q, Zhu EC, Wu
JB, Li T, Hou YL et al. (2019) Risk of Pneumonitis and Pneumonia Associated
With Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and
Meta-Analysis. Front Immunol 10: 108. [Crossref]
18. Lindeman NI, Cagle
PT, Aisner DL, Arcila ME, Beasley MB et al. (2018) Updated Molecular Testing
Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted
Tyrosine Kinase Inhibitors: Guideline From the College of American
Pathologists, the International Association for the Study of Lung Cancer, and
the Association for Molecular Pathology. Arch Pathol Lab Med 142:
321-346. [Crossref]
19. Novello S, Barlesi
V, Califano R, Cufer T, Ekman S et al. (2018) Metastatic non–small-cell lung
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol 27: v1-v27. [Crossref]
20. Ettinge DS, Wood
DE, Aisner DL, Akerley W, Bauman J et al. (2017) Non-Small Cell Lung Cancer,
Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr
Canc Netw 15: 504-535. [Crossref]
21. Ribas A, Camacho
LH, Berestein GL, Pavlov D, Bulanhagui CA et al. (2005) Antitumor activity in
melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T
lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol
23: 8968-8977. [Crossref]
22. Fong L, Small EJ
(2008) Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging
class of immunomodulatory antibodies for cancer treatment. J Clin Oncol
26: 5275-5283. [Crossref]
23. Vellanki PJ, Mulkey
F, Jaigirdar AA, Rodriguez L, Wang Y et al. (2021) FDA Approval Summary:
Nivolumab with Ipilimumab and Chemotherapy for Metastatic Non-small Cell Lung
Cancer, A Collaborative Project Orbis Review. Clin Cancer Res 27:
3522-3527. [Crossref]
24. Zinner RG, Obasaju
CK, Spigel DR, Weaver RW, Beck JT et al. (2015) PRONOUNCE: randomized,
open-label, phase III study of first-line pemetrexed + carboplatin followed by
maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed
by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell
lung cancer. J Thorac Oncol 10: 134-142. [Crossref]
25. Latchman Y, Wood CR,
Chernova T, Chaudhary D, Borde M et al. (2001) PD-L2 is a second ligand for
PD-1 and inhibits T cell activation. Nat Immunol 2: 261-268. [Crossref]
26. Lisberg A, Garon EB
(2019) Does Platinum-Based Chemotherapy Still Have a Role in First-Line
Treatment of Advanced Non-Small-Cell Lung Cancer? J Clin Oncol 37:
529-536. [Crossref]
27. Matsumoto H,
Kobayashi N, Somekawa K, Fukuda N, Kaneko A et al. (2022) Pembrolizumab
monotherapy versus pembrolizumab plus chemotherapy in patients with
non-small-cell lung cancer: A multicenter retrospective trial. Thorac Cancer
13: 228-235. [Crossref]
28. Herbst RS, Garon
EB, Kim DW, Cho BC, Gracia JLP et al. (2018) Long-term survival in patients
(pts) with advanced NSCLC in the KEYNOTE-010 study overall and in pts who
completed two years of pembrolizumab (pembro). Ann Oncol 29.
29. Garon EB, Hellmann
MD, Rizvi NA, Carcereny E, Leighl NB et al. (2019) Five-Year Overall Survival
for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With
Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol
37: 2518-2527. [Crossref]
30. Low JL, Huang Y,
Sooi K, Ang Y, Chan ZY et al. (2021) Low-dose pembrolizumab in the treatment of
advanced non-smallcell lung cancer. Int J Cancer 149: 169-176. [Crossref]
31. Borghaei H, Ares
LP, Horn L, Spigel DR, Steins M et al. (2015) Nivolumab versus Docetaxel in
Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373:
1627-1639. [Crossref]
32. Gettinger S, Horn
L, Jackman D, Spigel D, Antonia S et al. (2018) Five-Year Follow-Up of
Nivolumab in Previously Treated Advanced Non–Small-Cell Lung Cancer: Results
From the CA209-003 Study. J Clin Oncol 36: 1675-1684. [Crossref]
33. Bristol-Myers
Squibb Company: Nivolumab [package insert]. In. Princeton; 2018.
34. Yang Z, Zhu Y,
Xiang G, Hua T, Ni J et al. (2021) First-line atezolizumab plus chemotherapy in
advanced non-squamous non-small cell lung cancer: a cost-effectiveness analysis
from China. Expert Review Pharmacoeconomics Outcomes Res 21: 1061-1067.
[Crossref]
35. Mathieu L, Shah S,
Scherf LP, Larkins E, Vallejo J et al. (2021) FDA Approval Summary:
Atezolizumab and Durvalumab in Combination with Platinum‐Based
Chemotherapy in Extensive Stage Small Cell Lung Cancer. Oncologist 26:
433-438. [Crossref]
36. Barlesi F,
Vansteenkiste J, Spigel D, Ishii H, Garassino M et al. (2018) Avelumab versus
docetaxel in patients with platinum-treated advanced non-small-cell lung cancer
(JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol
19: 1468-1479. [Crossref]
37. Jerusalem G, Chen
F, Spigel D et al. (2016) JAVELIN solid tumor: Safety and clinical activity of
avelumab (Anti-PD-L1) as first-line treatment in patients with advanced NSCLC. J
Thorac Oncol 12: S252.
38. Shafique MR, Fisher
TL, Evans EE, Leonard JE, Pastore DRE et al. (2019) Preliminary results from
CLASSICAL-Lung, a phase 1b/2 study of pepinemab (VX15/2503) in combination with
avelumab in advanced NSCLC. J Clin Oncol 37.
39. Antonia SJ,
Villegas A, Daniel D, Vicente D, Murakami S et al. (2018) Overall survival with
durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379:
2342-2350. [Crossref]
40. Fares CM, Allen
EMV, Drake CG, Allison JP, Lieskovan SH (2019) Mechanisms of Resistance to
Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not
Work for All Patients? Am Soc Clin Oncol Educ Book 39: 147-164. [Crossref]
41. Schreiber RD, Old
LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity's roles in
cancer suppression and promotion. Science 331: 1565-1570. [Crossref]
42. Huang RY, Francois
A, McGray AR, Miliotto A, Odunsi K (2017) Compensatory upregulation of PD-1,
LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in
metastatic ovarian cancer. Oncoimmunology 6: e1249561. [Crossref]
43. Thommen DS,
Schreiner J, Müller P, Herzig P, Roller A et al. (2015) Progression of Lung
Cancer Is Associated with Increased Dysfunction of T Cells Defined by
Coexpression of Multiple Inhibitory Receptors. Cancer Immunol Res 3:
1344-1355. [Crossref]
44. Highfill SL, Cui Y,
Giles AJ, Smith JP, Zhang H et al. (2014) Disruption of CXCR2-mediated MDSC
tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med 6: 237ra67.
[Crossref]
45. Opitz CA, Patterson
LFS, Mohapatra SR, Dewi DL, Sadik A et al. (2020) The therapeutic potential of
targeting tryptophan catabolism in cancer. Br J Cancer 122: 30-44. [Crossref]
46. Do HTT, Lee CH, Cho
J (2020) Chemokines and their Receptors: Multifaceted Roles in Cancer
Progression and Potential Value as Cancer Prognostic Markers. Cancers
(Basel) 12: 287. [Crossref]
47. Yi M, Jiao D, Qin
S, Chu Q, Wu K et al. (2019) Synergistic effect of immune checkpoint blockade
and anti-angiogenesis in cancer treatment. Mol Cancer 18: 60. [Crossref]
48. Chen PL, Roh W,
Reuben A, Cooper ZA, Spencer CN et al. (2016) Analysis of Immune Signatures in
Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and
Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov 6:
827-837. [Crossref]
49. Wang J, Sanmamed
MF, Datar I, Su TT, Ji L et al. (2019) Fibrinogen-likeProtein 1 Is a Major
Immune Inhibitory Ligand of LAG-3. Cell 176: 334-47e12. [Crossref]
50. Long L, Zhang X,
Chen F, Pan Q, Phiphatwatchara P et al. (2018) The promising immune checkpoint
LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer
9: 176-189. [Crossref]
51. He Y, Yu H,
Rozeboom L, Rivard CJ, Ellison K et al. (2017) LAG-3 Protein Expression in
Non-Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and
Tumor-Infiltrating Lymphocytes. J Thorac Oncol 12: 814-823. [Crossref]
52. Barrueto L,
Caminero F, Cash L, Makris C, Lamichhane P et al. (2020) Resistance to
Checkpoint Inhibition in Cancer Immunotherapy. Transl Oncol 13: 100738.
[Crossref]
53. Wolf Y, Anderson
AC, Kuchroo VK (2020) TIM3 comes of age as an inhibitory receptor. Nat Rev
Immunol 20: 173-185. [Crossref]
54. Abreu DR, Johnson
ML, Hussein MA, Cobo M, Patel AJ et al. (2020) Primary analysis of a
randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab
(tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L)
treatment in patients with PD-L1- selected NSCLC (CITYSCAPE). J Clin Oncol
38: 9503-9503.
55. Hellmann MD, Ares
LP, Caro RB, Zurawski B, Kim SW et al. (2019) Nivolumab plus Ipilimumab in
advanced non-small-cell lung Cancer. N Engl J Med 381: 2020-2031. [Crossref]
56. Huang Y, Yuan J,
Righi E, Kamoun WS, Ancukiewicz M et al. (2012) Vascular normalizing doses of
antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment
and enhance immunotherapy. Proc Natl Acad Sci U S A. 109: 17561-17566. [Crossref]
57. Chen JLY, Pan CK,
Huang YS, Tsai CY, Wang CW et al. (2021) Evaluation of antitumor immunity by a
combination treatment of high-dose irradiation, anti-PDL1, and anti-angiogenic
therapy in murine lung tumors. Cancer Immunol Immunother 70: 391-404. [Crossref]
58. Socinski MA, Nishio
M, Jotte RM, Cappuzzo F, Orlandi F et al. (2021) IMpower150 Final Overall
Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in
First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol 16: 1909-1924. [Crossref]
59. Stewart RA, Pilié
PG, Yap TA (2018) Development of PARP and Immune-Checkpoint Inhibitor
CombinationsPARP and Immune-Checkpoint Inhibitor Combinations. Cancer Res
78: 6717-6725. [Crossref]
60. Li A, Yi M, Qin S,
Chu Q, Luo S et al. (2019) Prospects for combining immune checkpoint blockade
with PARP inhibition. J Hematol Oncol 12: 98. [Crossref]
61. Thomas A, Murai J,
Pommier Y (2018) The evolving landscape of predictive biomarkers of response to
PARP inhibitors. J Clin Invest 128: 1727-1730. [Crossref]
62. Gourley C, Balmaña
J, Ledermann JA, Serra V, Dent R et al. (2019) Moving From Poly (ADP-Ribose)
Polymerase Inhibition to Targeting DNA Repair and DNA Damage Response in Cancer
Therapy. J Clin Oncol 37: 2257-2269. [Crossref]
63. Knelson EH, Patel SA,
Sands JM (2021) PARP Inhibitors in Small-Cell Lung Cancer: Rational
Combinations to Improve Responses. Cancer (Basel) 13: 727. [Crossref]
64. de Bono J,
Ramanathan RK, Mina L, Chugh R, Glaspy J et al (2017) Phase I, Dose-Escalation,
Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced
Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov
7: 620-629. [Crossref]
65. Byers LA, Wang J,
Nilsson MB, Fujimoto J, Saintigny P et al. (2012) Proteomic profiling
identifies dysregulated pathways in small cell lung cancer and novel
therapeutic targets including PARP1. Cancer Discov 2: 798-811. [Crossref]
66. Murai J, Feng Y, Yu
GK, Ru Y, Tang SW et al. (2016) Resistance to PARP inhibitors by SLFN11
inactivation can be overcome by ATR inhibition. Oncotarget 7:
76534-76550. [Crossref]
67. Boussios S,
Karihtala P, Moschetta M, Karathanasi A, Sadauskaite A et al. (2019) Combined
strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for the
treatment of ovarian cancer: a literature review. Diagnostics (Basel) 9:
87. [Crossref]
68. Lallo A, Frese KK,
Morrow CJ, Sloane R, Gulati S et al. (2018) The Combination of the PARP
Inhibitor Olaparib and the WEE1 Inhibitor AZD1775 as a New Therapeutic Option
for Small Cell Lung Cancer. Clin Cancer Res 24: 5153-5164. [Crossref]
69. Gay CM, Tong P, Li
L, Stewart CA, Sen T et al. (2018) Abstract 2822: ATR inhibitors are active as
single agents and in combination with PARP1 and ATM inhibitors in molecularly
distinct subsets of small cell lung cancer models. Cancer Res 78:
2822-2822.
70. Marcar L, Bardhan
K, Gheorghiu L, Dinkelborg P, Pfäffle H et al. (2019) Acquired Resistance of
EGFR-Mutated Lung Cancer to Tyrosine Kinase Inhibitor Treatment Promotes PARP
Inhibitor Sensitivity. Cell Rep 27: 3422-3432.e4. [Crossref]
71. Deng L, Liang H, Xu
M, Yang X, Burnette B et al. (2014) STING-Dependent Cytosolic DNA Sensing
Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in
Immunogenic Tumors. Immunity 41: 843-852. [Crossref]
72. Han C, Liu Z, Zhang
Y, Shen A, Dong C et al. (2020) Tumor cells suppress radiation-induced immunity
by hijacking caspase 9 signaling. Nat Immunol 21: 546-554. [Crossref]
73. Labadie BW, Bao R,
Luke JJ (2019) Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via
Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis. Clin
Cancer Res 25: 1462-1471. [Crossref]
74. Yentz S, Smith D
(2018) Indoleamine 2,3-Dioxygenase (IDO) inhibition as a strategy to augment
Cancer Immunotherapy. BioDrugs 32: 311-317. [Crossref]
75. Prendergast GC,
Malachowski WP, DuHadaway JB, Muller AJ (2017) Discovery of IDO1 inhibitors:
From Bench to Bedside. Cancer Res 77: 6795-6811. [Crossref]
76. Long GV, Dummer R,
Hamid O, Gajewski TF, Caglevic C et al. (2019) Epacadostat plus pembrolizumab
versus placebo plus pembrolizumab inpatients with unresectable or metastatic
melanoma (ECHO-301/KEYNOTE252): a phase 3, randomised, double-blind study. Lancet
Oncol 20: 1083-1097. [Crossref]
77. Viveiros P,
Sukhadia B, Nunna P, Park KW, Chuang J et al. (2019) Abstract 523:Implications
of the A2a receptor (A2aR) on tumor microenvironment in nonsmall cell lung
cancer (NSCLC). Cancer Res 79: 523-523.
78. Cheng Y, Ma XL, Wei
YQ, Wei XW (2019) Potential roles and targeted therapy of the CXCLs/CXCR2 axis
in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer 1871:
289-312. [Crossref]
79. Doi T, Muro K,
Ishii H, Kato T, Tsushima T et al. (2019) A phase I study of the anti-CC
chemokine receptor 4 antibody, Mogamulizumab, incombination with Nivolumab in
patients with advanced or metastatic solid tumors. Clin Cancer Res 25:
6614-6622. [Crossref]
80. Brahmer J, Reckamp
KL, Baas P, Crinò L, Eberhardt WEE et al. (2015) Nivolumab versus docetaxel in
advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:
123-135. [Crossref]
81. Reck M, Abreu DR,
Robinson AG, Hui R, Csőszi T et al. (2016) Pembrolizumab versus Chemotherapy
for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375:
1823-1833. [Crossref]
82. Herbst RS, Baas P,
Kim DW, Felip E, Gracia JLP et al. (2016) Pembrolizumab versus docetaxel for
previously treated, PD-L1-positive, advanced non-small-cell lung cancer
(KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540-1550. [Crossref]
83. Ares LGP, Luft A,
Tafreshi A, Gumus M, Mazieres J et al. (2018) Phase 3 study of
carboplatinpaclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab
(Pembro)for patients (Pts) with metastatic squamous (Sq) non-small cell lung
cancer (NSCLC). J Clin Oncol 105-105.
84. Llorca FP, Robin NR
(2018) Tumor mutational burden in non-small cell lung cancer-the pathologist’s
point of view. Transl Lung Cancer Res 7: 716-721. [Crossref]
85. Chalmers ZR,
Connelly CF, Fabrizio D, Gay L, Ali SM et al. (2017) Analysis of 100,000 human
cancer genomes reveals the landscape of tumor mutational burden. Genome Med
9: 34. [Crossref]
86. Frampton GM,
Fichtenholtz A, Otto GA, Wang K, Downing SR et al. (2013) Development and
validation of a clinical cancer genomic profiling test based on massively
parallel DNA sequencing. Nat Biotechnol 31: 1023-1031. [Crossref]
87. Rizvi NA, Hellmann
MD, Snyder A, Kvistborg P, Makarov V et al. (2015) Cancer immunology.
Mutational landscape determines sensitivity to PD-1 blockade in non-small cell
lung cancer. Science 348: 124-128. [Crossref]
88. Schumacher TN,
Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348:
69-74. [Crossref]
89. Hellmann MD,
Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA et al. (2018) Nivolumab plus
Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med
378: 2093-2104. [Crossref]
90. Nishimura T,
Nakamura H, Végvári Á, Varga GM, Furuya N (2019) Current status of clinical
proteogenomics in lung cancer. Expert Rev Proteomics 16: 761-772. [Crossref]
91. Alidousty C, Baar
T, Martelotto LG, Heydt C, Wagener S et al. (2018) Genetic instability and
recurrent MYC amplification in ALK-translocated NSCLC: a central role of TP53
mutations. J Pathol 246: 67-76. [Crossref]
92. Boullosa LF,
Loenhout JV, Flieswasser T, Waele JD, Hermans C et al. (2021) Auranofin reveals
therapeutic anticancer potential by triggering distinct molecular cell death
mechanisms and innate immunity in mutant p53 non-small cell lung cancer. Redox
Biol 42: 101949. [Crossref]
93. Bulbul A, Husain H
(2018) First-Line Treatment in EGFR Mutant Non-Small Cell Lung Cancer: Is There
a Best Option? Front Oncol 8: 94. [Crossref]
94. Meng P, Koopman B,
Kok K, Elst AT, Schuuring E et al. (2020) Combined osimertinib, dabrafenib and
trametinib treatment for advanced non-small-cell lung cancer patients with an
osimertinib-induced BRAF V600E mutation. Lung Cancer 146: 358-361. [Crossref]
95. Manchado E,
Weissmueller S, Morris JP, Chen CC, Wullenkord R et al. (2016) A combinatorial
strategy for treating KRAS-mutant lung cancer. Nature 534: 647-651. [Crossref]
96. Chuang CH, Chen HL,
Chang HM, Tsai YC, Wu KL et al. (2021) Systematic Review and Network
Meta-Analysis of Anaplastic Lymphoma Kinase (ALK) Inhibitors for
Treatment-Naïve ALK-Positive Lung Cancer. Cancers (Basel)13: 1966.
[Crossref]
97. Chul Kim, Giaccone
G (2018) MEK inhibitors under development for treatment of non-small-cell lung
cancer. Expert Opin Investig Drugs 27: 17-30. [Crossref]
98. Joshi A, Pande N,
Noronha V, Patil V, Kumar R et al. (2019) ROS1 mutation non-small cell lung
cancer-access to optimal treatment and outcomes. Ecancermedicalscience
13: 900. [Crossref]
99. Li BT, Smit EF,
Goto Y, Nakagawa K, Udagawa H et al. (2022) Trastuzumab Deruxtecan in
HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 386: 241-251. [Crossref]
100.
Liu
AW, Liang C, Lee CS (2022) A contemporary review of rearranged during
transfection-selective inhibitors. J Oncol Pharm Pract 28: 175-184. [Crossref]
101.
Wolf
J, Baik C, Heist RS, Neal JW, Mansfield AS et al. (2018) Natural history,
treatment (tx) patterns, and outcomes in MET dysregulated non-small cell lung
cancer (NSCLC) patients (pts). Presented at the EORTC-NCI-AACR Molecular
Targets and Cancer Therapeutics. Eur J Cancer 103: E131-E131.
102.
Wolf
J, Seto T, Han JY, Reguart N, Garon EB et al. (2019) Capmatinib (INC280) in
METΔex14-mutated advanced non-small cell lung cancer (NSCLC): Efficacy data
from the phase II GEOMETRY mono-1 study. J Clin Oncol 37: 9004-9004.
103.
Hyman
DM, Smyth LM, Donoghue M, Westin SN, Bedard PL et al. (2017) AKT Inhibition in
Solid Tumors With AKT1 Mutations. J Clin Oncol 35: 2251-2259. [Crossref]
104. Yang Z, Liang SQ, Yang H, Xu D, Bruggmann R et al. (2021) CRISPR-Mediated Kinome Editing Prioritizes a Synergistic Combination Therapy for FGFR1-Amplified Lung Cancer. Cancer Res 81: 3121-3133. [Crossref]
105. Canadian Task Force on Preventive Health Care (2016) Recommendations on screening for lung cancer. CMAJ 188: 425-432. [Crossref]
