HPA-Axis Functioning and Food Addiction Among Individuals Suffering from Severe Obesity and Awaiting Bariatric Surgery
A B S T R A C T
Similarities have been observed between substance dependence and overconsumption of food, leading to the development of the food addiction (FA) concept. While psychological markers of FA have often been documented, data on physiological markers remains scarce. This study aimed to investigate HPA-axis functioning through cortisol awakening response (CAR) in relation to FA among bariatric candidates. We hypothesized that participants presenting high FA symptomatology would present a blunted CAR when compared to participants presenting low FA symptomatology and that significant associations between CAR and eating behaviors would be observed within both groups. The final sample comprised 40 participants, who were invited to complete questionnaires and provide saliva samples upon awakening (T0, T15, and T30). Results from the two-way ANOVA with repeated measures showed a non-significant “time x group” interaction, indicating that CAR did not differ between groups. Moreover, results from correlational analyses showed different patterns of associations between CAR and eating behaviors within each group; further analyses showed that the relationship between CAR and food cravings triggered by cues in the environment was significantly moderated by FA symptomatology, as higher CAR was related to lower cue-triggered food cravings only in individuals presenting low FA symptomatology. While these findings do not support the presence of HPA-axis functioning differences in relation to FA, they suggest that the association of CAR with eating behaviors may depend on whether or not addictive tendencies are present. Further investigation of the association between CAR and eating behaviors in the context of FA will thus be essential.
Keywords
Food addiction, HPA-axis, cortisol awakening response, bariatric candidates, eating behaviors, food cravings
Introduction
The concept of food addiction (FA) has recently elicited growing interest, as it provides a useful way to gain insight into a specific set of problematic eating behaviors. In addition to anecdotal reports supporting the addictive qualities of food, FA was further described using parallels between the behavioral, psychological, and neurobiological components of substance dependence and overconsumption of food. In a comprehensive review, Davis & Carter (2009) highlighted the presence of cravings and relapse, the role of impulsivity and reward sensitivity, and the implication of the mesocorticolimbic and prefrontal systems in the compulsive use of both psychoactive substances and food [1]. Based on these parallels, the DSM-IV-TR substance dependence diagnostic criteria were applied to excessive food consumption and the Yale Food Addiction Scale (YFAS) was designed as a tool to operationalize FA [2, 3].
Using the YFAS, studies have documented the presence of FA among various samples. Among individuals suffering from severe obesity and awaiting bariatric surgery more specifically, prevalence rates up to 54% have been found, highlighting the importance of studying FA in this clinical sample [4]. Furthermore, many studies have investigated the psychological markers of FA. It was found that individuals with FA presented significantly more severe depression symptoms, childhood ADHD, impulsivity, and addictive personality traits than individuals without FA [5, 6]. While essential to consider psychological markers of addiction in relation to FA, it is also important to consider physiological markers. For instance, individuals with FA exhibit differences in neuronal circuitry (including dopamine signaling) and hormonal profiles (including gut hormones, pituitary polypeptide hormones, and adipokines) when compared to their counterparts without FA [7-9]. The functioning of the Hypothalamus-Pituitary-Adrenocortical (HPA) axis, often reflected by the stress hormone cortisol, is a physiological marker that has been associated with both addiction disorders and disordered eating behaviors. However, no study has specifically investigated HPA-axis functioning through cortisol awakening response (CAR) in relation to FA.
Indeed, CAR is known to be a reliable indicator of HPA-axis functioning [10]. While activation of the HPA axis following an acute stressor is crucial in promoting adaptive responses, prolonged activation of this system can become maladaptive, as reflected by an inability to adequately cope with various life events [11, 12]. Considering that addiction-related behaviors persistently stimulate functioning of the HPA axis, the compulsive nature of such behavior may be linked to its downregulation. In fact, blunted HPA-axis functioning has been observed among individuals suffering from alcohol dependence and pathological gambling, thus possibly representing a physiological marker of such disorders, including aspects of vulnerability and risk of relapse [13-16]. With regards to food and eating, it has been shown that stress, as reflected in HPA-axis functioning, constitutes a risk factor for obesity as it has been linked to various metabolic changes and problematic eating behaviors promoting obesity [17-21]. Lastly, HPA-axis dysfunctions have been associated with psychological variables closely related to FA, like impulsivity, sensation seeking, and poor decision-making highlighted by low punishment sensitivity and high reward dependence, as well as with eating variables closely related to FA, such as hedonic eating and other problematic eating behaviors such as disinhibited and binge eating [22-28].
Considering the multiple bridges established between substance dependence and overconsumption of food and the involvement of HPA-axis functioning in both fields, the present study aimed to explore HPA-axis functioning in relation to FA in a particularly at-risk clinical sample. More specifically, the difference in CAR as well as the associations between CAR and eating behaviors were examined among bariatric candidates presenting either high or low FA symptomatology. Based on the aforementioned literature, it was hypothesized that the high-FA group would exhibit a blunted CAR when compared to the low-FA group, reflecting a possible HPA-axis dysfunction in those with an addictive-like profile. It was also hypothesized that significant associations between CAR and eating behaviors would be observed in both the high-FA and the low-FA groups.
Methods & Materials
I Particpants and Procedure
This project was approved by the Research Ethics Committee of the Quebec Heart and Lung Institute, where participants were recruited. Eligible bariatric candidates were solicited at the time of their pre-operative visit if they were aged between 18 and 50 years old, as to only recruit pre-menopausal women considering the possible impact of sexual hormones on CAR, and if they were free of current psychiatric condition and/or severe cognitive disturbances that might interfere with data collection [29]. After providing informed consent, participants were given a booklet of questionnaires and nine centrifugation tubes for saliva sampling, each containing a small cotton swab (salivettes) (Starstedt; Rommelsdorf, Germany). They were also given oral and written instructions for saliva sampling. All research material was collected during the participants’ next hospital visit. Weight and height information were obtained from medical files.
The final sample included 40 individuals (30 women and 10 men), who presented a mean age of 39.9 years (SD = 7.3) and a mean BMI of 48.12 kg/m2 (SD = 6.05). All participants were White/Caucasian, except for one participant for whom information relative to ethnicity was missing. Most of the participants had completed a post-secondary degree (65%) and reported working full time (80%).
II Measures
FA
The YFAS is a self-report instrument designed to examine FA symptomatology over the last 12 months [3]. It comprises 25 items, among which 20 are based on the seven DSM-IV-TR diagnostic criteria for substance dependence [2]. Two more items are included to assess the presence of clinically significant distress/functional impairment and three more items provide clarification for subsequent items (primer items). Items contained in the YFAS are answered on a five-point Likert scale or on a yes-no basis. When scoring the instrument, a chart is used to determine when an item is endorsed and a criterion is considered fulfilled when at least one of its associated items is endorsed. A validation study of the French version of the YFAS by our research team showed that a 16-item version was best suitable for the present sample, showing good internal consistency (α = .85) [30].
Depression symptoms
The Beck Depression Inventory-II (BDI-II) is a self-report questionnaire composed of 21 items based on the DSM-IV diagnostic criteria for major depressive disorder and was used to evaluate the presence and severity of depression symptoms over the past six months [31, 32]. Each item is measured on a four-point Likert scale, representing various severity levels of depression symptoms, and is summed up to obtain a total score. In the present sample, good internal consistency was observed (α = .89).
Anxiety levels
In order to assess the general anxious disposition of participants, the 20-item trait subscale of the State and Trait Anxiety Inventory (STAI) was used [33]. Participants were asked to answer each item using a four-point Likert scale, indicating to which extent the item corresponds to their experience in general. After taking into account the reversed items, a total score can be obtained by adding up all items. In the present sample, excellent internal consistency was found (α = .90)
Eating pathology
The Eating Disorder Examination (EDE 12.0) is a semi-structured interview and consists of 62 items evaluating eating-related behaviors from the past three months [34]. In the context of the present study, only the “bulimic episodes and other episodes of overeating” section of the EDE was used in order to document the presence of binge eating episodes among participants. Items from this section were evaluated either on the basis of their frequency/severity (scored on a seven-point Likert scale) or using qualitative information.
Eating behaviors
The Three-Factor Eating Questionnaire (TFEQ) is a 51-item instrument used to measure three behavioral aspects of eating [35]: 1. cognitive dietary restraint (α = .76); 2. disinhibition toward food (α = .80); and 3. susceptibility to hunger (α = .84). The first section of the TFEQ includes 36 true-or-false items and the second section includes 15 four-point Likert-scale items. A score for each subscale can be computed by adding each item according to a point system. In the present sample, internal consistency of the three subscales was satisfying (shown above).
Food cravings
The Food Craving Questionnaire - Trait (FCQ-T) is a self-report instrument composed of 37 items and designed to evaluate the general experience of food cravings according to nine dimensions [36]: 1. having intentions and plans to consume food (α = .86); 2. anticipation of positive reinforcement that may result from eating (α = .90); 3. anticipation of relief from negative states and feelings as a result of eating (α = .87); 4. lack of control over eating (α = .91); 5. thoughts or preoccupation with food (α = .85); 6. craving as a physiological state (α = .81); 7. emotions that may be experienced before or during food cravings or eating (α = .95); 8. cues that may trigger food cravings (α = .88); and 9. guilt from cravings and/or for giving into them (α = .70). Respondents are asked to indicate how often each statement “would be true for them in general” using a six-point Likert scale. An overall score as well as subscale scores can be obtained by summing up all corresponding items. Internal consistency for the FCQ-T subscales (shown above) and total score (α = .97) was excellent in the present sample.
Hedonic hunger
The Power of Food Scale (PFS) is a self-report questionnaire used to measure three facets of hedonic hunger [37]: 1. participants’ response to food available in the environment (α = .87); 2. participants’ response to food present in the environment (α = .90); and 3. participants’ response to food in the environment that has been tasted (α = .80). It comprises 15 items, each rated on a five-point Likert scale. Answers can be added up in order to obtain each subscale score as well as a total score. Internal consistency for the overall PFS (α = .94) and its subscales (shown above) was excellent in the present sample.
HPA-axis functioning
In the present study, HPA-axis functioning was assessed through CAR, which represents the boost in cortisol occurring within 30 to 45 minutes of awakening [29]. Participants were instructed to complete three saliva samples on three different weekday mornings, in order to reduce intraindividual variability [29]. Upon awakening, they were asked to open the first salivette (identified “morning 1, sample 1”), put the cotton swab in their mouth for about 30 seconds, and put the cotton swab back in the salivette once it was filled with saliva (T0). They were then asked to repeat these steps 15 and 30 minutes after awakening (T15 and T30, respectively). They were instructed to store the salivettes in the freezer, until they could bring them back to the research facility. Participants were told to refrain from eating, drinking, smoking, and brushing their teeth before and throughout the complete procedure, as to not contaminate the samples. They were allowed to drink water, but not during the five minutes preceding each sample. Compliance with the procedure was verified through answers on a short questionnaire pertaining to the sampling procedure.
The salivary cortisol levels were determined using an immunoassay kit (expanded range high sensitivity salivary cortisol enzyme immunoassay kit from Salimetrics (Pennsylvania, United States)). As per the instructions provided with the immunoassay kit, the saliva samples were thawed completely, vortexed, and centrifuged at 1500 x g for 15 minutes. The saliva samples were then added to the assay plate. The average salivary cortisol levels for the three mornings were computed for each time point (T0, T15, and T30). Furthermore, CAR was measured by subtracting salivary cortisol levels at T0 from salivary cortisol levels at T30 (delta cortisol).
III Data analytic plan
Descriptive analyses were first conducted to draw up a general portrait of the sample and variables under investigation. According to the procedure described by Gearhardt et al. (2011), participants who endorsed three or more FA criteria were first classified in the high-FA group (N = 20) [38]. Those who endorsed one or less FA criteria and who represented the best possible match for the high-FA participants according to age, sex, BMI, depression symptoms, and anxiety levels were then classified in the low-FA group (N = 20). Those who endorsed two FA criteria were not considered as to create a more distinct separation between both groups. Chi-square tests and t-tests were used when appropriate to assess sociodemographic differences between the high- and the low-FA groups and to insure participants from both groups were adequately matched.
A two-way analysis of variance was performed in order to compare the high- and the low-FA groups on CAR, where the three time points at which saliva samples were obtained (T0, T15, and T30) were identified as the within-subject factor (time) and the group within which participants were classified (high-FA and low-FA) was identified as the between-subject factor (group). Correlational analyses were also conducted in order to document the associations between CAR (delta cortisol) and various eating behaviors (presence of binge eating, problematic eating behavior, food cravings, and hedonic hunger) among bariatric candidates with high and low FA symptomatology. Lastly, a multiple regression analysis was conducted using the first model of Hayes’ PROCESS macro for SPSS to test the moderating effect of FA (high or low) on the association between CAR (delta cortisol) and eating behaviors [39].
Results
Results from chi-square tests and t-tests revealed no significant difference between the high- and the low-FA groups. Participants from both groups did not differ in terms of education (χ2 = 1.077, p = .713) or employment (χ2 = 2.125, p = .713) and they did not differ in terms of sex (five men and 15 women in each group), age (t = .888, p = .380), BMI (t = -.580, p = .565), depression symptoms (BDI total score; t = -1.081, p = .287), or anxiety levels (STAI – trait subscale score; t = -1.413, p = .166).
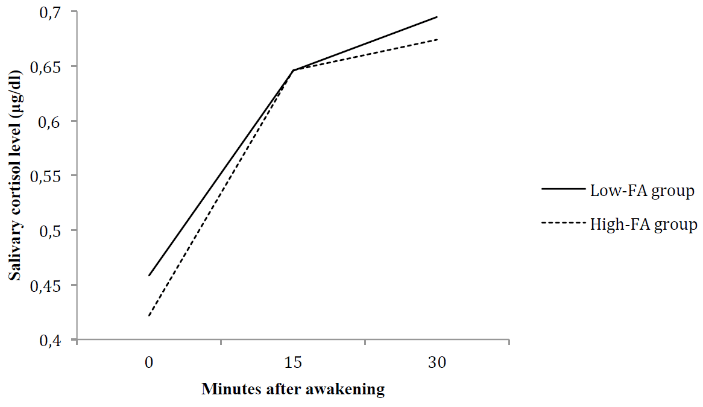
Figure 1: Cortisol awakening response in individuals presenting low and high food addiction symptomatology.
As seen in (Figure 1), results from the two-way ANOVA with repeated measures showed a significant effect of time on salivary cortisol levels (F(2,76) = 32.261, p < .001), indicating that participants overall exhibited higher salivary cortisol levels at T15 when compared to T0 (t = -.206, p < .001) and at T30 when compared to T0 (t = -.244, p < .001). However, the effect of group on salivary cortisol levels was non-significant (F(1,38) = 0.059, p = .810). The “time x group” interaction was also non-significant (F(2,76) = 0,159, p = .810), thus showing that the CAR of individuals presenting high FA symptomatology and low FA symptomatology did not differ throughout time.
Results from the correlational analyses (Table 1) showed that most associations between CAR and eating behaviors were not statistically significant. However, different patterns of association for the high- and the low-FA groups were observed. Although not significant, moderate negative associations were found between CAR and dietary restraint (p = .054) as well as CAR and hedonic hunger when food is tasted (p = .052) among individuals presenting high FA symptomatology, while weak positive associations were found among their counterparts. More importantly, a significant negative association was observed between CAR and the FCQT subscale related to food cravings triggered by cues in the environment (p = .014) among individuals presenting low FA symptomatology, while a weak and non-significant association was observed among individuals presenting high FA symptomatology.
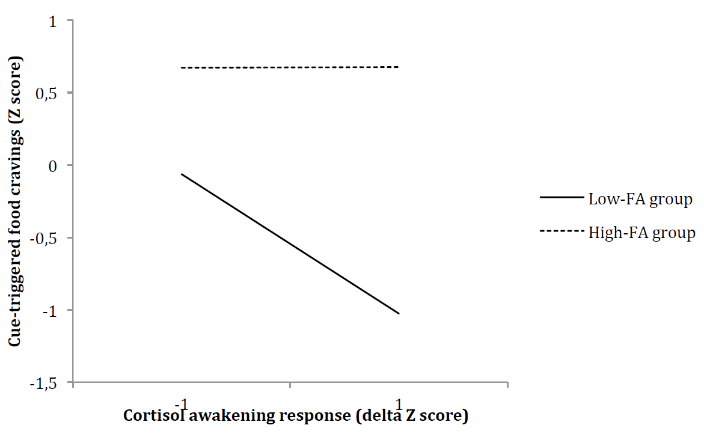
In light of this significant association, a multiple regression analysis was conducted to test the moderating effect of FA (high or low) on the correlation between CAR and cue-triggered food cravings. Results presented in (Table 2) showed that the overall model was significant (F(3,36) = 12.65, p < .001) and accounted for 51% of the explained variance in cue-triggered food cravings (R2 = .51). Furthermore, the “CAR x group” interaction was found to be a significant predictor of cue-triggered food cravings (F(1,36) = 4.6336, p = .04), accounting for a significant 6% increase in explained variance (R2 change = .0627). While increases in CAR were related to decreases in cue-triggered food cravings in individuals presenting low FA symptomatology, the association between CAR and cue-triggered food cravings was not significant among individuals presenting high FA symptomatology (Figure 2).
Table 1: Correlation coefficients of the association between cortisol awakening response and eating variables in individuals presenting low and high food addiction symptomatology.
|
Variables |
High-FA group (N = 20) |
Low-FA group (N = 20) |
|
PFS_food available |
-.357 |
-.081 |
|
PFS_food present |
.100 |
-.265 |
|
PFS_food tasted |
-.440† |
.055 |
|
PFS_total |
-.324 |
-.102 |
|
TFEQ_restraint |
-.437† |
.072 |
|
TFEQ_disinhibition |
-.058 |
-.204 |
|
TFEQ_hunger |
-.205 |
-.131 |
|
FCQT_intention |
-.147 |
-.151 |
|
FCQT_reinforcement |
-.101 |
.001 |
|
FCQT_relief |
.071 |
-.177 |
|
FCQT_control |
.122 |
-.296 |
|
FCQT_preoccupation |
-.227 |
.087 |
|
FCQT_physiological |
-.091 |
.057 |
|
FCQT_emotions |
-.146 |
-.371 |
|
FCQT_cues |
.010 |
-.541* |
|
FCQT_guilt |
-.165 |
-.118 |
|
FCQT_total |
-.100 |
-.222 |
|
EDE_binge eating |
-.103 |
-.022 |
Note. FA – food addiction; PFS – Power of Food Scale; TFEQ – Three-Factor Eating Questionnaire; FCQT – Food Craving Questionnaire-Trait
*p < .05; † marginally significant (p < .10)
Table 2: Multiple regression analysis predicting the effect of cortisol awakening response (delta) on food cravings triggered by cues in the environment.
|
Predictors |
β |
t value |
p |
|
Group |
1.1224 |
5.6604 |
.0000*** |
|
Cortisol awakening response |
-0.4819 |
-2.8362 |
.0074* |
|
Group x Cortisol awakening response |
0.4881 |
2.1526 |
.0381* |
*p < .05; **p < .01; ***p < .001.
Figure 2: Food addiction symptomatology as a moderator of the relationship between cortisol awakening response and food cravings triggered by cues in the environment.
Discussion
The present study aimed to explore HPA-axis functioning in relation to FA among bariatric candidates, by examining the difference in CAR between a high- and a low-FA group and by documenting the relationship between CAR and various eating behaviors within both groups. It was possible to conclude from the present findings that the high- and the low-FA groups were properly matched to control for the effect of potential confounding factors on CAR and that the procedure surrounding the collection of saliva samples was valid as the expected increase in salivary cortisol levels after awakening was observed.
Results also showed that the CAR of individuals in the high- and the low-FA groups did not differ. This finding did not correspond to our original hypothesis, namely that the CAR of participants from the former group would be blunted in light of their addictive tendencies toward food. It is important to consider that all participants involved in the present study presented severe obesity and that HPA-axis dysfunctions and obesity are interrelated [17]. A significant “time x group” interaction would have indicated an impact of FA on HPA-axis functioning above and beyond the impact of obesity on HPA-axis functioning. It is therefore plausible that the influence of FA on CAR may not have been potent enough to be detected among individuals presenting such severe obesity, especially since the sample size was small and may have hindered observation of a significant group difference (η2 = .004 for the interaction effect). It is also important to consider that disrupted sleep patterns can potentially influence CAR and that night eating syndrome, characterized by frequent awakening through the night, is common among bariatric candidates [40, 41]. The presence of such eating pathology may thus have acted as another confounding variable, for which the present experiment did not control. Lastly, it is important to consider that the collection of saliva samples included measurements at 15 and 30 minutes post-awakening. In a study on HPA-axis dysfunction in alcohol-dependent patients, significant group differences indicating an attenuated CAR in short-term abstinent patients when compared to long-term abstinent patients were found only 45 and 60 minutes after awakening [13]. Another study on salivary cortisol and binge eating disorder revealed that women suffering from this disorder showed lower total cortisol levels throughout the day (15 and 30 minutes after awakening in addition to 12:00PM, 3:00PM, and 8:00PM) when compared to women free of this disorder [28]. We cannot exclude that a longer saliva collection procedure could have allowed the observation of a group difference in HPA-axis functioning in the present study.
The correlational analyses showed few significant associations between CAR and eating behaviors. This finding does not support our hypothesis that CAR would be significantly associated with eating behaviors in both the high- and the low-FA groups. It is however possible to notice that many associations between CAR and eating behaviors were negative. This correlational pattern is similar to what was previously reported among men and women from the general population; according to the authors, these results suggest that lower HPA-axis reactivity may be associated with undesirable eating behaviors, such as disinhibition, susceptibility to hunger, and binge eating, among healthy individuals [26].
In the same line of thoughts, findings from the present study revealed that CAR was negatively related to cue-triggered food cravings among individuals presenting low FA symptomatology, but not among individuals presenting high FA symptomatology. Previous literature has highlighted the fact that certain problematic eating profiles modify the relationship between stress and eating patterns. For instance, restrained and emotional eaters as well as individuals suffering from binge eating disorder tend to overeat instead of lose appetite in times of stress [19]. Also noteworthy, findings from the correlational analyses revealed that some associations between CAR and eating behaviors tend to differ when individuals with or without FA are considered. FA could therefore represent another problematic eating profile that affects the relationship between stress and specific eating behaviors, where the influence of CAR on eating behaviors could depend on whether or not addictive tendencies are present.
The present results must be considered in light of some limitations. As previously stated, the sample size was small and may thus have influenced the detection of differences between the high- and low-FA groups in terms of HPA-axis functioning and its association with eating behaviors. Also, participants were recruited approximately three months prior to the bariatric surgery and may have already made changes in their eating habits with the help of a dietician. Future experiments would benefit from including a control group, inquiring about potential confounding variables specifically related to the nature of the addiction studied (for instance, the time and caloric content of the last meal and night eating syndrome), and testing participants earlier in the process leading to the bariatric surgery.
Conclusion
To our knowledge, this is the first study to explore HPA-axis functioning through CAR in relation to FA and eating behaviors in bariatric candidates. Findings from the present experiment certainly open the door to further examination of this physiological marker in the field of FA, which could help us gain insight into the reward pathway and its impact on eating behaviors in individuals presenting severe weight problems.
Conflicts of Interest
Dr. Tchernof as well as Dr. Biertho report grants from Johnson & Jonhson Medical Companies outside the submitted work. The other authors declare no conflict of interest.
Acknowledgements
We thank the Research Chair in Bariatric and Metabolic Surgery of the Quebec Heart and Lung Institute, more specifically Marc Lapointe and Mélanie Nadeau, as well as the whole surgery team for their assistance in data collection. We thank Hélène Paradis and Mélissa Pelletier for assisting with data analysis.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 10, Aug 2019Accepted: Wed 28, Aug 2019
Published: Mon 16, Sep 2019
Copyright
© 2023 Catherine Bégin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.PDR.2019.03.03
Author Info
Laurent Biertho Simone Lemieux André Tchernof Anne-Sophie Ouellette Catherine Bégin Christopher Rodrigue
Corresponding Author
Catherine BéginSchool of Psychology, Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada
Figures & Tables
Table 1: Correlation coefficients of the association between cortisol awakening response and eating variables in individuals presenting low and high food addiction symptomatology.
|
Variables |
High-FA group (N = 20) |
Low-FA group (N = 20) |
|
PFS_food available |
-.357 |
-.081 |
|
PFS_food present |
.100 |
-.265 |
|
PFS_food tasted |
-.440† |
.055 |
|
PFS_total |
-.324 |
-.102 |
|
TFEQ_restraint |
-.437† |
.072 |
|
TFEQ_disinhibition |
-.058 |
-.204 |
|
TFEQ_hunger |
-.205 |
-.131 |
|
FCQT_intention |
-.147 |
-.151 |
|
FCQT_reinforcement |
-.101 |
.001 |
|
FCQT_relief |
.071 |
-.177 |
|
FCQT_control |
.122 |
-.296 |
|
FCQT_preoccupation |
-.227 |
.087 |
|
FCQT_physiological |
-.091 |
.057 |
|
FCQT_emotions |
-.146 |
-.371 |
|
FCQT_cues |
.010 |
-.541* |
|
FCQT_guilt |
-.165 |
-.118 |
|
FCQT_total |
-.100 |
-.222 |
|
EDE_binge eating |
-.103 |
-.022 |
Note. FA – food addiction; PFS – Power of Food Scale; TFEQ – Three-Factor Eating Questionnaire; FCQT – Food Craving Questionnaire-Trait
*p < .05; † marginally significant (p < .10)
Table 2: Multiple regression analysis predicting the effect of cortisol awakening response (delta) on food cravings triggered by cues in the environment.
|
Predictors |
β |
t value |
p |
|
Group |
1.1224 |
5.6604 |
.0000*** |
|
Cortisol awakening response |
-0.4819 |
-2.8362 |
.0074* |
|
Group x Cortisol awakening response |
0.4881 |
2.1526 |
.0381* |
*p < .05; **p < .01; ***p < .001.
References
- Davis C, Carter JC (2009) Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite 53: 1-8. [Crossref]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Arlington, VA: American Psychiatric Association.
- Gearhardt AN, Corbin WR, Brownell KD (2009) Preliminary validation of the Yale Food Addiction Scale. Appetite 52: 430-436. [Crossref]
- Clark SM, Saules KK (2013) Validation of the Yale Food Addiction Scale among a weight-loss surgery population. Eat Behav 14: 216-219. [Crossref]
- Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS et al. (2011) Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 57: 711-717. [Crossref]
- Gearhardt AN, White MA, Masheb RM, Morgan PT, Crosby RD et al. (2012) An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord 45: 657-663. [Crossref]
- Davis C, Loxton NJ, Levitan RD, Kaplan AS, Carter JC et al. (2013) ‘Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiol Behav 118: 63-69. [Crossref]
- Davis C, Levitan RD, Kaplan AS, Kennedy JL, Carter JC (2014) Food cravings, appetite, and snack-food consumption in response to a psychomotor stimulant drug: the moderating effect of “food-addiction”. Front Psychol 5: 403. [Crossref]
- Pedram P, Sun G (2015) Hormonal and dietary characteristics in obese human subjects with and without food addiction. Nutrients 7: 223-238. [Crossref]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K et al. (1997) Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences 61: 2539-2549. [Crossref]
- de Kloet ER, Joëls M, Holsboer F (2005) Stress and the brain: From adaptation to disease. Nat Rev Neurosci 6: 463-475. [Crossref]
- Charmandari E, Tsigos C, Chrousos G (2005) Endocrinology of the stress response. Ann Rev Physiol 67: 259-284. [Crossref]
- Junghanns K, Horbach R, Ehrenthal D, Blank S, Backhaus J (2007) Cortisol awakening response in abstinent alcohol-dependent patients as a marker of HPA-axis dysfonction. Psychoneuroendocrinology 32: 1133-1137. [Crossref]
- Paris JJ, Franco C, Sodano R, Frye CA, Wulfert E (2009) Gambling pathology is associated with dampened cortisol response among men and women. Physiol Behav 99: 230-233. [Crossref]
- Lovallo WR (2006) Cortisol secretion patterns in addiction and addiction risks. Int J Psychophysiol 59: 195-202. [Crossref]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R et al. (2005) Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol 40: 80-85. [Crossref]
- Sinha R, Jastreboff AM (2013) Stress as a common factor for obesity and addiction. Biol Psychiatry 73: 827-835. [Crossref]
- Adam TC, Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91: 449-458. [Crossref]
- Gluck ME (2006) Stress response and binge eating disorder. Appetite 46: 26-30. [Crossref]
- Torres SJ, Nowson CA (2007) Relationship between stress, eating behavior, and obesity. Nutrition 23: 887-894. [Crossref]
- Rutters F, La Fleur S, Lemmens S, Born J, Martens M et al. (2012). The hypothalamic-pituitary-adrenal axis, obesity, and chronic stress exposure: Foods and HPA axis. Curr Obes Rep 1: 199-207.
- King RJ, Jones J, Scheuer JW, Curtis D, Zarcone VP (1990) Plasma cortisol correlates of impulsivity and substance abuse. Personal Individual Differ 11: 287-291.
- Rosenblitt JC, Soler H, Jonhson SE, Quadagno DM (2001) Sensation seeking and hormones in men and women: Exploring the link. Horm Behav 40: 396-402. [Crossref]
- van Honk J, Schutter DJ, Hermans EJ, Putman P (2003) Low cortisol levels and the balance between punishment sensitivity and reward dependency. NeuroReport 14: 1993-1996. [Crossref]
- Daubenmier J, Lustig RH, Hecht FM, Kristeller J, Woolley J et al. (2014) A new biomarker of hedonic eating? A preliminary investigation of cortisol and nausea responses to acute opioid blockade. Appetite 74: 92-100. [Crossref]
- Therrien F, Drapeau V, Lupien SJ, Beaulieu S, Doré J et al. (2008) Awakening cortisol response in relation to psychosocial profiles and eating behaviors. Physiol Behav 93: 282-288. [Crossref]
- Coutinho WF, Moreira RO, Spagnol C, Appolinario JC (2007) Does binge eating disorder alter cortisol secretion in obese women? Eat Behav 8: 59-64. [Crossref]
- Larsen JK, van Ramshorst B, van Doornen LJP, Geenen R (2009) Salivary cortisol and binge eating disorder in obese women after surgery for morbid obesity. Int J Behav Med 16: 311-315. [Crossref]
- Clow A, Thorn L, Evans P, Hucklebridge F (2004) The awakening cortisol response: Methodological issues and significance. Stress 7: 29-37. [Crossref]
- Ouellette AS, Rodrigue C, Lemieux S, Tchernof A, Biertho L et al. (2017) Yale Food Addiction Scale: examining the psychometric properties of the French version among Individuals with severe obesity awaiting bariatric surgery. Psychology 8: 2547-2561.
- Beck AT, Steer RA, Brown GK (1996) BDI-II, Beck depression inventory: manual, 2nd ed. Boston, MA: Harcourt Brace.
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Arlington, VA: American Psychiatric Association.
- Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory STAI (form Y) (" self-evaluation questionnaire"). Palo Alto, CA: Consulting Psychologist Press.
- Cooper Z, Fairburn C (1987) The Eating Disorder Examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disorder 6: 1-8.
- Stunkard AJ, Messick S (1985) The Three Factor Eating Questionnaire to measure dietary restraint, disinhibition, and hunger. J Psychosom Res 29: 71-83. [Crossref]
- Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA (2000) The development and validation of the State and Trait Food-Cravings Questionnaires. Behav Res Ther 31: 151-173.
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG et al. (2009) The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 53: 114-118. [Crossref]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR et al. (2011) Neural correlates of food addiction. Arch Gen Psychiatry 68: 808-816. [Crossref]
- Hayes A (2013) Introduction to mediation, moderation, and conditional process analysis. New York, NY: The Guilford Press.
- Chida Y, Steptoe A (2009) Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol Psychol 80: 265-278. [Crossref]
- De Zwaan M, Burgard MA, Schenck CH, Mitchell JE (2003) Nighttime eating: A review of the literature. Eur Eat Disorder Rev 11: 7-24.
