How Phytocomponents May be Valuable Against Oxidative Stress in Brain Tissue?
A B S T R A C T
This short commentary is intended to briefly discuss the benefits of plant secondary metabolites against brain tissue injury promoted by oxidative stress. Henceforth, the physicochemical features and biological activities of phytocomponents were assessed based on literature reports, which showcased their thermodynamic feasibility to reduce reactive oxygen species. It was strongly hinted that secondary metabolites possessing antioxidant potential may be of use in the therapeutics against neurodegenerative disorders owing to free radical scavenging, what hinders further damage to brain tissue.
Keywords
Antioxidants, polyphenols, brain tissue injury, herbal, folk medicine
Antioxidant Plant Secondary Metabolites and Their Biosynthesis">Antioxidant Plant Secondary Metabolites and Their Biosynthesis
The richness of phytochemicals provided by plant secondary metabolism is widely known in health sciences. These compounds may be obtained in purer forms using several extraction methods, providing thence distinct preparations with standard concentrations of selected phytochemical markers [1, 2]. Considering that most of these preparations are the first contact to healthcare in regions of low income or where plant-based medicine plays a bigger role in therapeutics, the investigation of the components of these preparations as well as their biological potential is of upmost importance [3, 4].
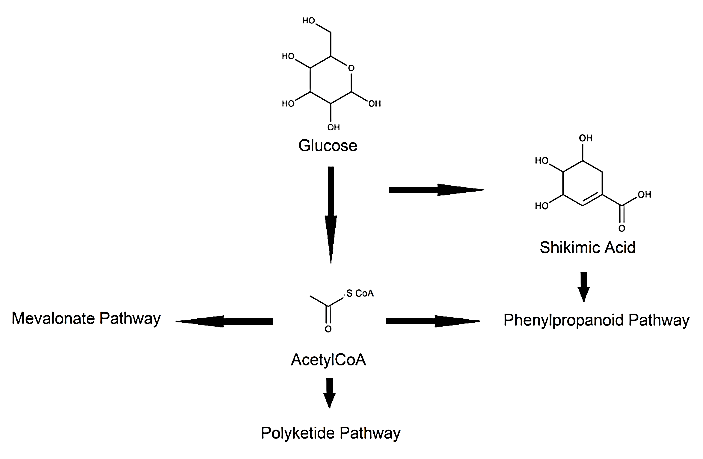
Regarding the therapeutic potential of phytochemicals, there are several biosynthetic pathway products which could be exploited in healthcare. Amongst these pathways are: i. Phenylpropanoid; ii. Polyketide; and iii. Mevalonate pathways [5, 6]. Although diverse in the sense of biological activities, many products from these biosynthetic routes showcase phenolic moieties, which are known to confer thermodynamic feasibility of reactive oxygen species (ROS) scavenging [7-9]. Figure 1 showcases a simplified sketch of these biosynthetic pathways.
The Physicochemical Aspects of Phytochemical Antioxidants
The antioxidant capacity of phytochemicals is a well-known feature, and its mechanisms are nonetheless explored in many cosmetical and nutraceutical products [9, 10]. Given the relationship between the chemical structure and physicochemical features such as antioxidant capacity, many compounds bearing similar structural characteristics also showcase similar thermodynamic feasibility to undergo oxidation [11-13]. Amongst these compounds are polyphenols, whose oxidation mechanism often contemplates reversibility, as showcased with o-catechol as instance in (Figure 2).
As showcased in (Figure 2), polyphenols are thermodynamically feasible to undergo oxidation, therefore donating two electrons and two protons to an endogenous antioxidant (peroxidases, superoxide dismutase and others) or even directly to ROS [13, 14]. This process results in the restitution of the endogenous antioxidant or the stabilization of the free radical and considering that the end-product of o-catechol oxidation (i.e. o-quinone) may undergo reduction due to the reversibility of this reaction, the antioxidant capacity of polyphenols may be even higher than expected [14, 15].
Figure 1: Simplified sketch of the Phenylpropanoid; Polyketide; and Mevalonate pathways.
Figure 2: Oxidation mechanism of o-catechol contemplating reversibility following the equivalence of protons and electrons.
How Can Secondary Metabolites Hinder Brain Tissue Damage Upon Oxidative Stress?
Brain tissue is highly susceptible to oxidative damage hence the high aerobic metabolism of these cells. Literature states that higher cellular activity leads to enhanced production of ROS as byproducts of regular metabolic processes, and the buildup of these reactive components may lead to tissue damage. In this sense, it can be implied that the endogenous antioxidant arsenal plays a large role in controlling ROS-related damage as well as preserving neurons [16, 17]. Considering that exogenous antioxidants such as plant secondary metabolites may restitute and preserve the activity of the endogenous antioxidant arsenal, as well as scavenge ROS, it can be suggested that these phytochemicals may play a part in aiding brain tissue to keep its integrity. Notwithstanding, several reports showcased the effectiveness of plant-based antioxidants such as polyphenols in mopping up free radicals and hindering lipid peroxidation, as well as preserving nervous functions such as memory, mobility, balance and other cognitive features [11, 12].
The benefits of polyphenolic phytochemicals consumption may be related to both their favorable thermodynamic to undergo redox reactions, as well as their proneness to bind to several bodily receptors, thence limiting physicochemical changes in the environment which could enhance ROS build up. For instance, it is widely known that inflammation increases the genesis of free radicals, being anti-inflammatory agents also valuable in keeping tissue integrity. Moreover, polyphenols are acknowledged to bind to many inflammation-related targets and nucleic acids, as well as enzymes whose role is to regulate neuron communication, such as acetylcholinesterase [11, 12, 18].
However, although many reports support this assumption, polyphenols are highly polar compounds, what suggests that they might not easily cross blood-brain barrier. In this sense, some researchers obtained remarkable enhancement of antioxidant-related neuroprotection by encapsulating polyphenols and other polar antioxidant compounds into lipidic and other less hydrophilic carriers [17-19]. Notwithstanding, this kinetic aspect of plant-based antioxidants is of upmost importance when their biological activity is concerned.
Conclusion
This short commentary was intended to briefly discuss the benefits of plant secondary metabolites against brain tissue injury promoted by oxidative stress. It was strongly hinted that secondary metabolites possessing antioxidant potential may be of use in the therapeutics against neurodegenerative disorders owing to free radical scavenging, what hinders further damage to brain tissue. However, more studies are needed to evaluate the kinetics of these compounds regarding the crossing of the blood-brain barrier and how the use of lipidic carriers may improve their access to the brain.
Acknowledgements
Author would like to thank CAPES, Brazil for the financial support.
Conflicts of Interest
None.
Article Info
Article Type
Review ArticlePublication history
Received: Thu 27, Feb 2020Accepted: Sat 14, Mar 2020
Published: Fri 20, Mar 2020
Copyright
© 2023 Douglas Vieira Thomaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GDT.2020.01.04
Author Info
Corresponding Author
Douglas Vieira ThomazFaculty of Pharmacy, Federal University of Goiás, Setor Leste Universitário, Goiânia – Goias State, Brazi
Figures & Tables
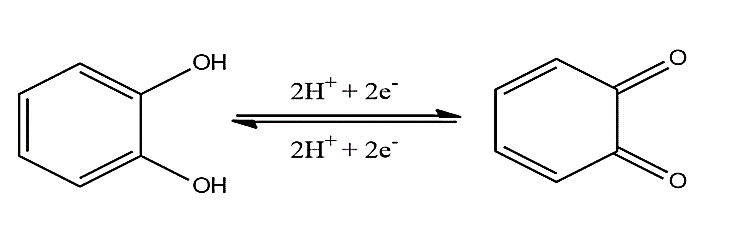
References
- Cordell GA (2014) Phytochemistry and traditional medicine-The revolution continues, Phytochemistry Letters, 10: viii-xl.
- Jamwal K, Bhattacharya S, Puri S (2018) Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J Applied Research on Medicinal and Aromatic Plants 9: 26-38.
- Jivad N, Rabiei Z (2015) Review on herbal medicine on brain ischemia and reperfusion. Asian Pacific J Trop Biomed 5: 789-795.
- Gao C, Shen J (2017) Chapter Six - Metabolic Factors and Adult Neurogenesis: Impacts of Chinese Herbal Medicine on Brain Repair in Neurological Diseases. Int Rev Neurobiol 135: 117-147. [Crossref]
- Xu J, Wei K, Zhang G, Lei L, Yang D et al. (2018) Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J Ethnopharmacol 225: 18-30. [Crossref]
- Irani S, Todd CD, Wei Y, Bonham-Smith PC (2019) Changes in phenylpropanoid pathway gene expression in roots and leaves of susceptible and resistant Brassica napus lines in response to Plasmodiophora brassicae inoculation. Physiological Mol Plant Pathol 106: 196-203.
- Göbel A, Rauner M, Hofbauer LC, Rachner TD (2020) Cholesterol and beyond - The role of the mevalonate pathway in cancer biology. Biochim Biophys Acta Rev Cancer 1873: 188351. [Crossref]
- Moreno EKG, Thomaz DV, Machado FB, Leite KCS, Rodrigues ESB et al. (2019) Antioxidant Study and Electroanalytical Investigation of Selected Herbal Samples Used in Folk Medicine. Int J Electrochem Sci 14: 838-847.
- Chen Q, Man H, Zhu L, Guo Z, Wang X et al. (2020) Enhanced plant antioxidant capacity and biodegradation of phenol by immobilizing peroxidase on amphoteric nitrogen-doped carbon dots. Catalysis Communications 134: 105847.
- Leite KCS, Garcia LF, Lobon GS, Thomaz DV, Moreno EKG et al. (2018) Antioxidant activity evaluation of dried herbal extracts: an electroanalytical approach. Revista Brasileira de Farmacognosia-Brazilian J Pharmacognosy 28: 325-332. [Crossref]
- Thomaz DV, Peixoto LF, Oliveira TS, Fajemiore JO, Neri HFS et al. (2018) Antioxidant and Neuroprotective Properties of Eugenia dysenterica Leaves. Oxid Med Cell Longev 2018: 3250908. [Crossref]
- Oliveira TS, Thomaz DV, Neri HFS, Cerqueira LB, Garcia LF et al. (2018) Neuroprotective Effect of Camb. Leaves Is Associated with Anticholinesterase and Antioxidant Properties. Oxidative Med Cellular Longevity 2018: 9842908. [Crossref]
- Thomaz DV, Leite KCS, Moreno EKG, Garcia LF, Alecrim MF et al. (2018) Electrochemical Study of Commercial Black Tea Samples. Int J Electrochemical Sci 13: 5433-5439.
- Thomaz DV, Couto RO, Roberth AO, Oliveira LAR, Leite KCS et al. (2018) Assessment of Noni (Morinda citrifolia L.) Products Authenticity by Solid State Voltammetry. Int J Electrochem Sci 13: 8983-8994.
- Thomaz DV, Machado FB, Rodrigues ESB, Cunha CEP, Carvalho MF et al. (2018) Assessment of Antioxidant Quality in Comercial Carqueja (Baccaris trimera) Samples by Electrochemical Tools. Res J Life Sci, Bioinformatics, Pharmaceutical Chemical Sci 4: 239-246.
- Freeman LR, Keller JN (2012) Oxidative stress and cerebral endothelial cells: Regulation of the blood–brain-barrier and antioxidant-based interventions. Biochim Biophys Acta Molecular Basis of Disease 1822: 822-829. [Crossref]
- Petro M, Jaffer H, Yang J, Kabu S, Morris VB et al. (2016) Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 81: 169-180. [Crossref]
- Thomaz DV, Oliveira MG, Rodrigues ESB, Silva VB, Santos PA (2019) Physicochemical investigation of Psoralen binding to double stranded DNA through electroanalytical and cheminformatic approaches. In: 5th International Electronic Conference on Medicinal Chemistry, 2019, Online. ECMC-5.
- Agrawal M, Saraf S, Saraf S, Dubey SK, Puri A et al. (2020) Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J Controlled Release 321: 372-415. [Crossref]
- Sohrabi MJ, Dehpour AR, Attar F, Hasan A, Sadeghi NM et al. (2019) Silymarin-albumin nanoplex: Preparation and its potential application as an antioxidant in nervous system in vitro and in vivo. Int J Pharm 572: 118824. [Crossref]
