High-Percentage of Early Resectable Pancreatic Ductal Adenocarcinoma is Unidentified on Abdominal CT Obtained for Unrelated Diagnosis
A B S T R A C T
Objective: Pancreatic ductal adenocarcinoma (PDAC) has the best survival when detected early with 5-year survival near 40% for small, resectable PDAC. We evaluate the undiagnosed PDAC imaging features on routine CT and their impact on resectability.
Methods: 76 of the screened 134 CTs from 1/1/2012 to 12/31/2018 using our tumor registry were obtained prior to PDAC diagnosis for other indications at least one month before presentation. Each cross-sectional study was reviewed for features of early PDAC: pancreatic mass, pancreatic ductal dilatation, perivascular/peripancreatic soft-tissue infiltration, omental lesions/ascites, and lymphadenopathy. When such features were detectible by the reviewing radiologists, the original CT readings were classified as concordant/discrepant. Descriptive statistics are reported for discrepant reads, tumor resectability, and tumor size.
Results: Of the 76 cases from 46 unique subjects (30 male/16 female), 25 CTs (33%) had undetected PDAC imaging features: masses (15/19 unreported), ductal dilatation (16/20 unreported), and peripancreatic/perivascular soft-tissue infiltration (20/36 unreported). 63% of early PDAC features were not identified initially. One year before clinical diagnosis, 75-80% of the PDAC cases were resectable; at < 6 months before clinical diagnosis, only 29% were resectable.
Conclusion: Improving early detection of key PDAC features on routine CT examinations can potentially improve patient outcomes.
Keywords
Pancreatic cancer, early detection, staging
Introduction
The American Cancer Society estimates the diagnosis of 56,770 new cases of pancreatic cancer in 2019 with an estimated 45,750 total deaths [1]. The 5-year survival rate for pancreatic ductal adenocarcinoma (PDAC) continues to be the lowest (9%) of all cancer types [1, 2]. Unlike most cancer types, advances that improve the survival of pancreatic cancer have been slow. This is partly because PDAC is commonly diagnosed during relatively late disease stages. Surgical resection with negative margins remains the only treatment to potentially cure the disease. However, resection is only possible in 15–20% of patients at the time of initial diagnosis [3]. Survival is directly related to tumor size at the time of resection; lesions < 1 cm have near 100% survival at 5 years [4]. This makes early diagnosis of PDAC key to patient survival. Late-stage PDAC can cause a wide range of symptoms, including jaundice, abdominal pain, back pain, decreased appetite, and weight loss. Early-stage pancreatic cancers do not typically cause specific signs or symptoms. However, patients can present with vague abdominal pain and other symptoms that overlap with late-stage PDAC, such as weight loss, jaundice, and loss of appetite [4, 5]. In cases of small PDAC, jaundice may be a strong indicator of death within 5 years [4, 5]. The most frequently reported symptom is vague abdominal pain, which usually is further evaluated with CT of the abdomen and pelvis (CTAP) [4, 5]. The correct interpretation of CTAP for vague abdominal pain is crucial for identifying early PDAC and altering patient survival [6].
Prior reports have demonstrated several features that are suggestive of early PDAC. These include hypoattenuating mass, upstream pancreatic ductal dilatation, and subtle bulge of the pancreatic border [7]. In these studies, ductal dilatation greater than 2 mm was found to significantly increase the odds of having PDAC [8]. Based on studies by Gangi et al. and Ahn et al., hypoattenuating mass, pancreatic ductal dilatation, and cut-off have the highest sensitivity for detecting small PDAC [7, 9]. The small masses of early PDAC tend to be better detected with MRI, as some of the early, well-differentiated, small PDAC enhance similar to the adjacent pancreatic parenchyma preventing their detection [10, 11]. Although much of the literature discusses the features of early PDAC, very little has been reported regarding what proportion of these features tend to be undetected on imaging and the potential effects this may have on patient outcomes. Our goal in this report is to retrospectively evaluate pre-diagnostic images, identify the rate of under-detection for features of early PDAC, and estimate the change in tumor resectability with delayed diagnoses.
Materials and Methods
I Patient Selection
This retrospective study is Health Insurance Portability and Accountability Act (HIPPA) compliant. The institutional review board approved the study and waived the requirement for informed consent for patient data review. Patients with PDAC were identified in our tumor registry from 01/01/2012 to 12/31/2018 and selected based on the presence of abdominal imaging scans performed prior to radiographic diagnosis of PDAC. Patient inclusion criteria for the study were as follows:
i. Histologically confirmed pancreatic ductal carcinoma.
ii. CT, MR, PET or MRCP imaging performed prior to diagnosis of PDAC.
iii. Imaging performed at least 1 month (30 days) before the clinical diagnosis date.
II CT Imaging Acquisition and Data Analysis
Externally generated images and those performed on-site were reviewed via the Picture Archiving and Communications System (PACS, Fuji Synapse v. 4.7, FujiFilms Holdings America). The CT protocol used by outside institutions varied in terms of slice thickness and contrast administration. Studies performed at the Banner Gateway Medical Center were obtained using an upgraded 64-slice Toshiba CT scanner (Aquilion) with a slice thickness of 3 mm using 75 mL of Isovue 370 at a rate of 3 mL/s or without IV contrast. Studies were obtained 70 s after initiation of contrast injection. The images were re-interpreted by 2 radiologists: JC, a body fellowship-trained diagnostic radiologist with nine years of independent practice experience, and PK, a nuclear fellowship-trained radiologist with 8 years of independent practice experience. The images were assessed for early features of PDAC, including the presence of a mass, main pancreatic ductal dilatation, lymph node involvement, perivascular and peripancreatic soft tissue infiltration/hazy appearance, omental lesions or ascites, and liver metastasis.
The findings seen by at least one radiologist were included in the analysis as positive. When comparing the original read with the re-read, the findings were classified as either a discrepancy or an agreement. Findings were classified as discrepant when the original read was normal and the re-read identified visible signs of PDAC. The CT study was considered discrepant if the original read had one or more discrepant early features. Findings were classified as in agreement when the feature was identified by radiologists on the original and later readings or if neither reading identified any features suggestive of PDAC. In cases where the original (early read) diagnosis was pancreatitis without mentioning peripancreatic infiltration, perivascular infiltration, or omental infiltration/ascites, the reads were considered concordant if no mass or pancreatic ductal dilatation was present.
Masses were further classified as either being hypodense (cystic) or solid. The mass size was calculated by multiplying the length of the mass (cm) by the width of the mass (cm) from axial CT images with the largest cross-section of the mass. Cystic masses were not considered in this analysis. Solid masses were classified as resectable vs. unresectable. Lesions were classified as unresectable if the images had one or more of these additional findings: perivascular soft tissue involvement, liver metastasis, or omental lesions. The remaining lesions were classified as resectable. These findings were then categorized by time of imaging (in months) prior to diagnosis.
III Statistical Analysis
Descriptive statistics are provided as means (standard deviations) or counts (percentages), as appropriate. Chi-square and Fishers’ Exact tests were used to evaluate differences in categorical variables between groups. Spearman’s correlation was used to evaluate the strength of the relationship between time and tumor mass, and Mann-Whitney was employed to evaluate resectability and time since diagnosis. Kappa statistics were used to evaluate the agreement between raters. Alpha was set at 0.05 (two-tailed) as the criterion for statistical significance. SPSS version 27 (IBM Corp., Armonk, NY) was used for the analyses.
Results
I Study Population
One hundred and thirty-four independent imaging studies were initially screened for this study, including cases within one month of diagnosis. After this exclusion criterion was applied, 76 imaging studies on patients with PDAC prior to their diagnosis were eligible for the study. These included 46 unique subjects, 30 of whom were male (median age at diagnosis 75 years, range 48 - 85) and 16 were female (median age at diagnosis 71.5 years, range 21 - 83). Overall, 25 imaging studies were considered discrepant with 14 unique subjects. At the time of discrepant imaging, there were 14 males and 11 females with median age, at the imaging of 73 years (range 54-84). For the 51 concordant imaging studies, there were 34 males and 17 females with median age, at imaging, of 62 years (range 19 - 84). Table 1 lists the demographics of the subjects.
Table 1: Patient demographics.
|
Gender |
Count
(%) |
Mean
Age at Diagnosis (SD) |
|
Male |
30
(65.2) |
69.4
(11.2) |
|
Female |
16
(34.8) |
66.8
(14.9) |
|
Case
Types |
Number |
Mean
Age at Imaging (SD) |
|
Concordant |
51
(67.1) |
63.4
(13.2) |
|
Discrepant |
25
(32.9) |
69.1
(9.1) |
SD: Standard Deviation.
II Imaging Discrepancy and Discrepant Features
The 76 studies were a combination of non-contrast CT, IV contrast-enhanced CT, and MRI. 31 of the studies were non-contrast CT images (including PET/CT), while 42 studies were contrast-enhanced CT studies (contrast-enhanced CT abdomen and pelvis and 1 CT angiography). 3 studies were MRI, including MR abdomen (2, on the same patient) and MRCP (1). 6 of the 31 non-contrast CT images were re-evaluated and considered to have discrepant findings relevant to pancreatic cancer. 18 of the contrast-enhanced CT studies were re-evaluated and considered to have discrepant findings. 1 of 3 MR included discrepant features. Table 2 lists the types of studies and the number of discrepant studies in that modality. For CT studies, 48 of these studies were normal or had simple pancreatitis with their time from diagnosis ranging from 1.3 to 133.5 months (47.0 ± 34.8 months). For simplicity of analysis, the remainder of the manuscript will focus strictly on CT studies, given the small number of MRI studies in this cohort.
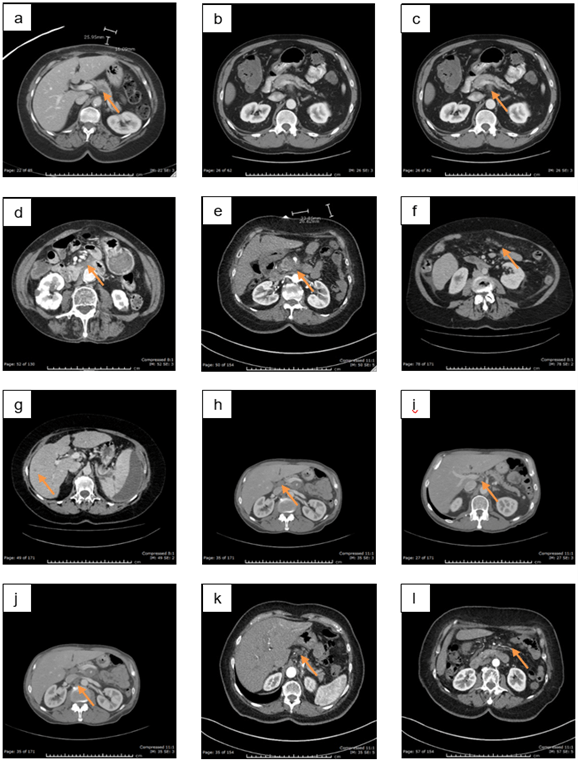
The studies were analysed at both the imaging study and imaging feature level to identify differences between non-contrast and contrast-enhanced CT studies. Table 2 shows fewer discrepancies between the original read and the re-read when the study did not receive IV contrast (17.9%) than when contrast was used (41.4%), p=0.045. Among the 73 CT studies, 85 imaging features were suggestive of PDAC. Many of these features were not identified in the pre-diagnostic studies. These features included pancreatic mass, pancreatic ductal dilation, perivascular soft-tissue infiltration, peripancreatic soft-tissue infiltration, omental lesions/ascites, liver metastasis and retroperitoneal lymphadenopathy. An example of the features is shown in (Figure 1). These and the discrepant features are tabulated in (Table 3). 75% of main features suggestive of PDAC were not identified and included pancreatic mass, pancreatic ductal dilatation, and perivascular soft tissue. 40% of the peripancreatic soft tissue was not identified, while 25% of the omental metastasis and peripancreatic/retroperitoneal lymph nodes were not identified. The difference in discrepancies between non-contrast CT and contrast CT is not statistically significant for pancreatic mass (100% discordant for non-contrast CT and 71.4% for contrast CT, p > 0.05) or pancreatic ductal dilatation (75% discordant for non-contrast CT and 81.4% discordant for contrast CT, p > 0.05). The difference is significant for peripancreatic (0% for non-contrast CT and 66.7% for contrast CT, p = 0.007) and perivascular (0% for non-contrast CT and 92.3% for contrast CT, p = 0.005) soft tissue infiltration. Overall, the number of discrepancies is also statistically different between non-contrast and contrast CT studies (34.6% for non-contrast CT and 74.6% for contrast CT, p = 0.001).
Table 2: Number of imaging modalities and discrepancies per
modality.
|
Modality |
Images/Discrepancies
(%) |
|
Contrast
CT Abd/Pelvis |
41/17
(41.4) |
|
Non-Contrast-CT
Abd/Pelvis |
28/5
(17.9) |
|
PET |
3/1
(33.3) |
|
MRI |
3/1
(33.3) |
|
CTA
Chest/Abd/ Pelvis |
1/1
(100) |
Note:
Numbers within cells denote the total number of features/discrepancies (%
discrepant).
Table 3: Features of PDAC identified on imaging studies and
their discrepancies.
|
Modality |
Pancreatic mass |
Pancreatic ductal dilation |
Perivascular soft tissue involvement1 |
Peripancreatic soft tissue involvement2 |
Omental lesions/ascites |
Liver metastasis |
Retroperitoneal/Peripancreatic LN |
Total3 |
|
Non-contrast CT |
5/5 (100) |
4/3 (75) |
3/0 (0) |
8/0 (0) |
3/0 (0 |
1/0 (0) |
2/1 (50) |
26/9 (34.6) |
|
Contrast CT |
14/10(71.4) |
16/13 (81.3) |
13/12 (92.3) |
12/8 (66.7) |
1/1 (100) |
1/0 (0) |
2/0 (0) |
59/44(74.6) |
Numbers within cells denote the
total number of features/discrepancies (% discrepant). P-values reflect
significant differences between non-contrast and contrast CT for the column. 1
- p=0.005, 2 - p=0.007, 3 - p=0.001.
Figure 1: Examples of features of PDAC a) Pancreatic body mass, b) Pancreatic ductal dilatation and cut-off, c) Perivascular ST infiltration, d) Peripancreatic ST infiltration, e) Pancreatic mass with perivascular and peripancreatic ST infiltration, f) Omental implants, g) Liver metastasis, h) Hepatoduodenal LN, i) Right celiac LN, j) Aortocaval LN, k) Gastrohepatic LN, l) Mesenteric LN.
III Resectability of Masses
The originally unidentified pancreatic masses were analysed for their size and resectability in relationship to the time prior to the actual diagnosis. These data are provided in (Table 4). On average, PDAC mass sizes were relatively stable in time when imaged one year or more prior to diagnosis (1.7 cm2) but became larger when image times approached diagnosis (4.09 cm2 at 1-3 months from diagnosis). The correlation between the size of the lesion and time of observation was rs= 0.56, p=0.024. Similarly, the average resectability of these masses was also greater with earlier diagnosis (75-85% 12+ months from diagnosis, 20% at 1-3 months from diagnosis), p=0.031. These findings stress the need to identify the early features of PDAC.
IV Indications for CT Studies
The indications of the CT studies evaluated for the present study are provided in (Table 5). Among the reasons noted for these studies, none specifically indicates PDAC. The predominant indication for both non-contrast and contrast-enhanced CT was abdominal pain, which is a nonspecific symptom. The second most common indication was “no indication listed on requisition” which is commonly encountered in routine clinical practice. The third most common indication was renal stone evaluation. These indications show that early PDAC is insidious and requires high clinical suspicion for identification because the most commonly provided histories have no direct relevance to PDAC.
Table 4: Unidentified solid PDAC masses prior to diagnosis.
|
Time from Diagnosis (mo) |
Total Number Solid Masses |
Mean Size Solid Mass (cm2 ± SD) |
Number Resectable Masses |
Number of Nonresectable Masses |
|
1-3 |
5 |
4.09 ± 1.89 |
1 |
4 |
|
4-6 |
2 |
2.28 ± 2.37 |
1 |
1 |
|
7-12 |
0 |
0.00 |
0 |
0 |
|
13-24 |
5 |
1.70 ± 1.02 |
4 |
1 |
|
> 24 |
4 |
1.66 ± 0.72 |
3 |
1 |
Table 5: Study indications for the imaging studies.
|
Indications
for Studies |
Non-contrast
CT: Number
(%) |
Contrast
CT: Number
(%) |
|
Abdominal
Pain/Discomfort |
10
(32) |
25
(59) |
|
No
Indication Listed |
6
(20) |
8
(19) |
|
Renal
Stones |
8
(26) |
0 |
|
Abscess
Evaluation |
3
(10) |
0 |
|
Cancer
Follow-Up |
2
(6) |
5
(12) |
|
Bleed |
1
(3) |
2
(5) |
|
Others |
1
(3) |
2
(5) |
|
Total |
31 |
42 |
V Concordance Between Radiologists
Kappa analysis was conducted on the early features of PDAC to assess how well both radiologists agreed on the findings. The features with sufficient numbers for analysis included: pancreatic ductal dilatation, perivascular soft tissue infiltration, and peripancreatic soft tissue infiltration. There was good agreement for ductal dilatation (kappa 0.708), while there was a poor agreement for perivascular and peripancreatic soft tissue infiltration (kappa 0.144 and 0.047, respectively).
Discussion
Our data show the importance of identifying the potential features of PDAC as early as possible, particularly in studies where pancreatic cancer is not suspected. When identified early (approximately one year before diagnosis), the vast majority of PDAC is resectable, but this trend reverses around 6 months prior to diagnosis (Table 4). This emphasizes the need for radiologists to check for the critical features of PDAC: pancreatic mass, ductal dilatation, and perivascular/peripancreatic infiltration. Identifying these early features is key to the early diagnosis of PDAC. Detecting small, resectable PDAC is the only known cure for PDAC. Studies have shown that the majority of PDACs at the time of diagnosis have progressed past the point of resectability, resulting in a poor prognosis for the patient [7, 12-14]. This fact is supported by the report from the American Cancer Society that the 5-year survival rate for PDAC is 39% if localized, but only 13% in the presence of regional spread [15]. A tumor is unresectable if >180 degrees of solid tumor contact with SMA or celiac artery, solid tumor contact with the aorta, or unreconstructable SMV/portal vein due to tumor involvement [14]. Although our definition of unresectable is slightly more liberal and would clinically be classified as either locally advanced or borderline resectable, this does not detract from the fact that these lesions are classified as regional involvement with worse outcomes than the resectable lesions. In all, our data strongly support the need for earlier diagnosis for PDAC to improve outcomes.
One of the defining aspects of the severity of this disease is its growth rate. Although a clear timeline for disease progression is not known, it has been suggested that it takes 11.7 years from the time of tumor initiation to become clinically diagnosed cancer [12]. Most patients do not show malignant mass at two or more years before diagnosis, as demonstrated in our data. Other cystic and benign lesions may have been noted more than two years before diagnosis, but these were without malignant features. This supports the theory of PDAC having a rapidly progressive growth rate after disease onset. A report by Furukawa found the tumor doubling time to be between 64 and 255 days [16]. In the initial stages of disease development, growth trends follow the slower rate. But, once the tumor is detectable by imaging, whether CT or MRI, the growth shifts to trend along the faster rate with a decreased doubling time [16, 17]. Our data from (Table 4) show a similar trend where the average size of pancreatic mass across the study sample is relatively steady but becomes significantly larger as the time to diagnosis comes closer. This point emphasizes the potential benefits derived from screening while also shedding light on the associated challenge.
Screening for PDAC continues to be a complex challenge, and for that reason, no guidelines for screening currently exist for the general population. Scans are often ordered on patients for simple complaints such as abdominal pain or other vague and nondescript reasons. While these complaints are quite broad, critical subtle findings can be seen on imaging by the radiologist to improve early detection of PDAC. The most common abnormalities seen on imaging in association with PDAC are pancreatic duct dilation, duct interruption, upstream atrophy, contour abnormality, and CBD dilation [18]. The challenge for cancer diagnosis is that these early changes in imaging are often mistaken for more common pancreatic complications, such as pancreatitis or benign cysts. One study found that 76% of small PDACs have secondary changes, with 63% involving the main pancreatic duct or common bile duct, 63% showing an abrupt pancreatic duct cut-off, 21% showing parenchymal atrophy, and 14% showing contour abnormalities [13]. Our findings are similar; of the 24 CT cases with discrepant reads for pancreatic cancer, 66% of the cases showed dilated pancreatic duct.
Biomarkers hold potential for screening as they have been useful for other cancers such as prostate and breast cancer. The two markers commonly associated with PDAC are KRAS and carbohydrate antigen 19-9 (CA 19-9), but neither is specific for PDAC. While a KRAS mutation is seen in almost all pancreatic cancerous lesions, it is also seen in other cancers such as colon cancer [18]. CA 19-9 faces the same challenge. The serum levels of CA 19-9 are significantly elevated in PDAC and other gastrointestinal malignancies [18]. CA 19-9 is classically used in follow-up to monitor treatment response. However, CA19-9 can also be positive in nonmalignant diseases such as chronic pancreatitis, cholangitis, biliary stasis, cirrhosis, and other gastrointestinal cancers and is therefore not a reliable marker prior to PDAC diagnosis [12]. While there are novel biomarkers currently being studied, nothing has yet been identified as sensitive and specific for early disease detection.
While there are several options for imaging, the most commonly used first-line modality is computed tomography (CT) [14]. CT offers a lower cost than other techniques with wider availability compared to modalities such as MRI and EUS [14]. Additionally, CT is more accurate in determining tumor resectability (73-87%) than MRI (70-79%) [13]. The classic CT finding suggestive of PDAC is hypoattenuating pancreatic mass in the setting of pancreatic duct dilation and atrophy of the upstream pancreas [14]. However, there is no agreement on whether the CT should be contrast-enhanced or non-contrast. A recent study noted that contrast-enhanced CT was significantly more effective at identifying distant metastasis, scalene node metastasis, and peritoneal disseminated disease [19]. This study supports what was found in our data in two ways. Not only were more features identified on contrast CT over non-contrast CT, more imaging features were also undiagnosed initially. It is hypothesized that contrast CT provides better visualization of abdominal anatomy but may also present more opportunities to overlook these imaging features. For example, better visualization simultaneously increases both the “set size” and visual information (clutter) in the image, factors known to impair visual scanning performance [20, 21]. CT is the best imaging modality for invasion of disease into surrounding vascular structures, including the celiac artery, superior mesenteric artery, and common hepatic artery, a key factor in determining resectability of the tumor [19]. It has been reported that overall contrast-enhanced CT has a sensitivity of 83.3% and specificity of 90.3% compared to 72.7% and 90.2%, respectively, for non-contrast CT [19]. While there is limited research supporting contrast-enhanced CT over non-contrast, data previously reported and those of our study suggest that contrast-enhanced imaging is superior for diagnostic purposes by identifying more features.
Our findings are clinically relevant as these imaging studies were acquired from community hospitals. The research has studied multiple methods for improving lesion detection. Of the many solutions proposed, only a real-time second observer is the most effective at improving detection but also the least cost-effective [22-24]. With the development of technology, the 2nd-observer may be a more practical solution than all others as the 2nd-observer role can be implemented through computer-aided detection (CADe). This can have a significant impact as CADe improved breast cancer detection on screening mammography [25, 26]. Likewise, retinal hemorrhage detection also improved with the assistance of artificial intelligence (AI)-based 2nd-observer algorithm [27-29]. Early development of AI-based second observer is still in rudimentary phases, able to detect large but not smaller PDAC [30]. These results are encouraging for computer-aided detection of PDAC.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 09, Dec 2021Accepted: Mon 27, Dec 2021
Published: Fri 31, Dec 2021
Copyright
© 2023 John C. Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2021.02.03
Author Info
Talayna Leonard Robert Lemme Cati Kral Briana Santiago Chris Elberts Stephanie Dewald Patrick McGonagill Paul Waclawski Curt Bay Phillip J Koo Megan Papesh Stephen Goldinger Vikram Kodibagkar Tomislov Dragovich Michael Choti Hongzhi Wang Madappa Kundranda John C. Chang
Corresponding Author
John C. ChangDepartment of Radiology, Banner MD Anderson Cancer Center, Gilbert, Arizona, USA
Figures & Tables
Table 1: Patient demographics.
|
Gender |
Count
(%) |
Mean
Age at Diagnosis (SD) |
|
Male |
30
(65.2) |
69.4
(11.2) |
|
Female |
16
(34.8) |
66.8
(14.9) |
|
Case
Types |
Number |
Mean
Age at Imaging (SD) |
|
Concordant |
51
(67.1) |
63.4
(13.2) |
|
Discrepant |
25
(32.9) |
69.1
(9.1) |
SD: Standard Deviation.
Table 2: Number of imaging modalities and discrepancies per
modality.
|
Modality |
Images/Discrepancies
(%) |
|
Contrast
CT Abd/Pelvis |
41/17
(41.4) |
|
Non-Contrast-CT
Abd/Pelvis |
28/5
(17.9) |
|
PET |
3/1
(33.3) |
|
MRI |
3/1
(33.3) |
|
CTA
Chest/Abd/ Pelvis |
1/1
(100) |
Note:
Numbers within cells denote the total number of features/discrepancies (%
discrepant).
Table 3: Features of PDAC identified on imaging studies and
their discrepancies.
|
Modality |
Pancreatic mass |
Pancreatic ductal dilation |
Perivascular soft tissue involvement1 |
Peripancreatic soft tissue involvement2 |
Omental lesions/ascites |
Liver metastasis |
Retroperitoneal/Peripancreatic LN |
Total3 |
|
Non-contrast CT |
5/5 (100) |
4/3 (75) |
3/0 (0) |
8/0 (0) |
3/0 (0 |
1/0 (0) |
2/1 (50) |
26/9 (34.6) |
|
Contrast CT |
14/10(71.4) |
16/13 (81.3) |
13/12 (92.3) |
12/8 (66.7) |
1/1 (100) |
1/0 (0) |
2/0 (0) |
59/44(74.6) |
Numbers within cells denote the
total number of features/discrepancies (% discrepant). P-values reflect
significant differences between non-contrast and contrast CT for the column. 1
- p=0.005, 2 - p=0.007, 3 - p=0.001.
Table 4: Unidentified solid PDAC masses prior to diagnosis.
|
Time from Diagnosis (mo) |
Total Number Solid Masses |
Mean Size Solid Mass (cm2 ± SD) |
Number Resectable Masses |
Number of Nonresectable Masses |
|
1-3 |
5 |
4.09 ± 1.89 |
1 |
4 |
|
4-6 |
2 |
2.28 ± 2.37 |
1 |
1 |
|
7-12 |
0 |
0.00 |
0 |
0 |
|
13-24 |
5 |
1.70 ± 1.02 |
4 |
1 |
|
> 24 |
4 |
1.66 ± 0.72 |
3 |
1 |
Table 5: Study indications for the imaging studies.
|
Indications
for Studies |
Non-contrast
CT: Number
(%) |
Contrast
CT: Number
(%) |
|
Abdominal
Pain/Discomfort |
10
(32) |
25
(59) |
|
No
Indication Listed |
6
(20) |
8
(19) |
|
Renal
Stones |
8
(26) |
0 |
|
Abscess
Evaluation |
3
(10) |
0 |
|
Cancer
Follow-Up |
2
(6) |
5
(12) |
|
Bleed |
1
(3) |
2
(5) |
|
Others |
1
(3) |
2
(5) |
|
Total |
31 |
42 |
References
1. Siegel RL, Miller
KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69: 7-34. [Crossref]
2. Chang J, Schomer D,
Dragovich T (2015) Anatomical, Physiological, and Molecular Imaging for
Pancreatic Cancer: Current Clinical Use and Future Implications. Biomed Res
Int 2015: 269641. [Crossref]
3. Kommalapati A,
Tella SH, Goyal G, Ma WW, Mahipal A (2018) Contemporary Management of Localized
Resectable Pancreatic Cancer. Cancers (Basel) 10: 24. [Crossref]
4. Ariyama J, Suyama
M, Satoh K, Sai J (1998) Imaging of small pancreatic ductal adenocarcinoma. Pancreas
16: 396-401. [Crossref]
5. Ishikawa O,
Ohigashi H, Imaoka S, Nakaizumi A, Uehara H et al. (1999) Minute carcinoma of
the pancreas measuring 1 cm or less in diameter--collective review of Japanese
case reports. Hepatogastroenterology 46: 8-15. [Crossref]
6. Irie H, Honda H,
Kaneko K, Kuroiwa T, Yoshimitsu K et al. (1997) Comparison of helical CT and MR
imaging in detecting and staging small pancreatic adenocarcinoma. Abdom
Imaging 22: 429-433. [Crossref]
7. Gangi S, Fletcher
JG, Christensen JA, Harmsen WS, Crownhart BS et al. (2004) Time Interval
Between Abnormalities Seen on CT and the Clinical Diagnosis of Pancreatic
Cancer: Retrospective Review of CT Scans Obtained Before Diagnosis. AJR Am J
Roentgenol 182: 897-903. [Crossref]
8. Tanaka S, Nakaizumi A, Ioka T, Oshikawa O, Uehara H et al. (2002) Main
pancreatic duct dilatation: A sign of high risk for pancreatic cancer. Jpn J
Clin Oncol 32: 407-411. [Crossref]
9. Ahn SS, Kim MJ,
Choi JY, Hong HS, Chung YE et al. (2009) Indicative findings of pancreatic
cancer in prediagnostic CT. Eur Radiol 19: 2448-2455. [Crossref]
10. Jang KM, Kim SH,
Kim YK, Song KD, Lee SJ et al. (2015) Missed pancreatic ductal adenocarcinoma:
Assessment of early imaging findings on prediagnostic magnetic resonance
imaging. Eur J Radiol 84: 1473-1479. [Crossref]
11. Yoon SH, Lee JM,
Cho JY, Lee KB, Kim JE et al. (2011) Small (≤ 20 mm) pancreatic
adenocarcinomas: Analysis of enhancement patterns and secondary signs with
multiphasic multidetector CT. Radiology 259: 442-452. [Crossref]
12. Chari ST, Kelly K,
Hollingsworth MA, Thayer SP, Ahlquist DA et al. (2015) Early detection of
sporadic pancreatic cancer: summative review. Pancreas 44: 693-712. [Crossref]
13. Haj Mirzaian A,
Kawamoto S, Zaheer A, Hruban RH, Fishman EK et al. (2020) Pitfalls in the MDCT
of pancreatic cancer: strategies for minimizing errors. Abdom Radiol 45:
457-478. [Crossref]
14. Singhi AD, Koay EJ,
Chari ST, Maitra A (2019) Early Detection of Pancreatic Cancer: Opportunities
and Challenges. Gastroenterology 156: 2024-2040. [Crossref]
15. Noone A (2019)
Survival Rates for Pancreatic Cancer. Cancer Facts Figures.
16. Furukawa H, Iwata
R, Moriyama N (2001) Growth rate of pancreatic adenocarcinoma: Initial clinical
experience. Pancreas 22: 366-369. [Crossref]
17. Nakamura T, Masuda
K, Harada S, Akioka K, Sako H (2013) Pancreatic cancer: Slow progression in the
early stages. Int J Surg Case Rep 4: 693-696. [Crossref]
18. Chang JC, Kundranda
M (2017) Novel Diagnostic and Predictive Biomarkers in Pancreatic
Adenocarcinoma. Int J Mol Sci 18: 667. [Crossref]
19. Yoneyama T,
Tateishi U, Endo I, Inoue T (2014) Staging accuracy of pancreatic cancer :
Comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur J
Radiol 83: 1734-1739. [Crossref]
20. Beck MR, Lohrenz
MC, Trafton JG (2010) Measuring search efficiency in complex visual search
tasks: Global and local clutter. J Exp Psychol Appl 16: 238-250. [Crossref]
21. Palmer J (1994)
Set-size effects in visual search: The effect of attention is independent of
the stimulus for simple tasks. Vision Res 34: 1703-1721. [Crossref]
22. Itri JN, Tappouni
RR, McEachern RO, Pesch AJ, Patel SH (2018) Fundamentals of Diagnostic Error in
Imaging. Radiographics 38: 1845-1865. [Crossref]
23. Waite S, Scott J,
Gale B, Fuchs T, Kolla S et al. (2017) Interpretive Error in Radiology. AJR
Am J Roentgenol 208: 739-749. [Crossref]
24. Posso M, Puig T,
Carles M, Rue M, Canelo Aybar C et al. (2017) Effectiveness and
cost-effectiveness of double reading in digital mammography screening: a
systematic review and meta-anslysis. Eur J Radiol 96: 40-49. [Crossref]
25. Cole EB, Zhang Z,
Marques HS, Nishikawa RM, Hendrick RE et al. (2012) Assessing the stand-alone
sensitivity of computer-aided detection with cancer cases from the Digital
Mammographic Imaging Screening Trial. AJR Am J Roentgenol 199: W392-W401.
[Crossref]
26. Fenton JJ, Xing G,
Elmore JG, Bang H, Chen SL et al. (2013) Short-term outcomes of screening
mammography using computer-aided detection: A population-based study of
medicare enrollees. Ann Intern Med 158: 580-587. [Crossref]
27. Nielsen KB, Lautrup
ML, Andersen JKH, Savarimuthu TR, Grauslund J (2019) Deep Learning-Based
Algorithms in Screening of Diabetic Retinopathy: A Systematic Review of
Diagnostic Performance. Ophthalmol Retina 3: 294-304. [Crossref]
28. Sayres R, Taly A,
Rahimy E, Blumer K, Coz D et al. (2019) Using a Deep Learning Algorithm and
Integrated Gradients Explanation to Assist Grading for Diabetic Retinopathy. Ophthalmology
126: 552-564. [Crossref]
29. Voets M, Mollersen K, Bongo LA (2019) Reproduction study using public data of: Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. PloS One 14: e0217541. [Crossref]
30. Chu LC, Park S, Kawamoto S, Wang Y, Zhou Y et al. (2019) Application of Deep Learning to Pancreatic Cancer Detection: Lessons Learned From Our Initial Experience. J Am Coll Radiol 16: 1338-1342. [Crossref]
