Heart and Neural Crest Derivatives Expressed Transcript 2 (HAND2) is Reduced in Women with Breast Cancer. A Case-Control Study
A B S T R A C T
Background: The search for new markers for breast cancer (BC) has been sought in order to better understand this type of cancer. The Heart and Neural Crest Derivatives Expressed Transcript 2 (HAND2) has been related to endometrial cancer but there are scant data related to BC. The aim of this study is to compare the immunohistochemical expression of HßAND2 in normal breast tissue vs. BC and to correlate with the estrogen receptor (ERα).
Patients and Methods: In this case-control study, 19 formalin–fixed, paraffin-embedded tissues were obtained from pathological archives. Benign (n=9; control) and cancer (n=10) breast tissue were analyzed with immunohistochemistry for HAND2 (Ab60037), at dilution 1:50 at pH 9 and ERα (SP1). ImageJ software with "color deconvolution" was used for analysis of the expression of these proteins. The sample size was calculated (power=95%, α error= 1%) to identify an increase from mean 15 DAB units (control) to 40 DAB units in cancer.
Results: HAND2 expression (mean ± SD) was 15.5 ± 6.1 (cancer) versus 44.8 ± 21.1 (control) (P=0.002, Student t-test). Its expression was mainly present in the cytosol of the cells. No correlation was observed between ERα and HAND2 (Pearson r = -0.28 (95%CI=-0.6 to 0.22; P=0.2).
Conclusions: The protein expression of HAND2, using Ab60037 antibody, is reduced in breast cancer, compared with normal breast tissue. The expression of HAND2 is not correlated with ERα expression.
Keywords
Breast cancer, HAND2, immunohistochemistry, ERα
Introduction
Breast cancer is the most common cancer in women in both developed and developing countries and the second most common cancer in the world [1]. Breast cancer is definitely not a single disease, but rather a group of diseases characterized by clinical, morphological, and molecular heterogeneity; the description of the subtypes of breast tumors has become more and more sophisticated [2]. Four molecular subtypes are commonly recognized in the current practice: luminal A, luminal B, HER2-enriched, and basal-like (also known as triple-negative breast cancer) to which the categories of claudin-low and normal-like can also be added. Also, integrated analysis of copy number alterations with gene expression analysis further extended the number of subtypes to ten [3]. Nevertheless, new markers for breast cancer have been sought in order to have a better characterization of the tumor and to choose the best possible treatment [4]. Among the new tumor markers in general, the Heart and Neural Crest Derivatives Expressed Transcript 2 (HAND2) called our attention. This protein is encoded by 3727 base pairs located in the long arm of chromosome 4 [5]. HAND2 protein expression is located mainly in the nuclear compartment, but it is also found in the cytosol [6]. Li et al. found that the inhibitory effects of progesterone under endometrial proliferation were mediated by HAND2. These authors verified that in a knockout mice model for hand2, an increased endometrial proliferation was observed [7]. In women, Buell-Gutbrod et al. found that biopsies derived from endometrial cancer had a lower expression of HAND2, compared to normal controls. The immunohistochemical expression of HAND2 is accentuated in benign endometrial diseases but is significantly reduced or absent in cases of endometrial hyperplasia or carcinoma [8-10]. HAND2 expression in endometrial cancer is absent, which may be used as a biomarker in the cases of hyperplasia and endometrial cancer [9]. In addition, HAND2 inhibits the ligand‑dependent transcriptional activation function of estrogen receptor alpha (ERα). This has been confirmed by mRNA expression of vascular endothelial growth factor, an ERα‑downstream factor, that was decreased by the overexpression of HAND2 in ERα human breast cancer cell lines [11]. However, a literature review on HAND2 expression in breast cancer tissue yielded no results (PUBMED search HAND2 AND breast AND cancer, on October 5th, 2019). Furthermore, most of the data in formalin-fixed paraffin embedded (FFPE) tissue related to HAND2 used a polyclonal antibody from Santa Cruz SC-9409 [8–10], which has been discontinued, or a polyclonal antibody (AF3876, R&D Systems, Minneapolis, MN 55413) that has been recommended for frozen sections in immunohistochemistry [12, 13]. Differences in gene expression, between frozen and FFPE tissue samples, has been demonstrated by others [14]. According to the Human Protein Atlas, the AF3876 was classified as "approved" in a four tier scale (uncertain, approved, supported, enhanced). The discontinuation of an antibody, the lack of data on HAND2 expression in breast cancer, allied to the proliferative effects of estrogen on the endometrium, the absence of inhibitory action of progesterone due to low expression of HAND2 and the presence of estrogen as risk factor for breast cancer led us to verify whether the expression of HAND2 would be reduced in breast cancer.
The objective of this study is to verify the immunohistochemical expression of HAND2 using a monoclonal antibody in breast cancer biopsies compared to normal breast tissue. The secondary objective is to correlate the expression of HAND2 with estrogen and progesterone receptors. We hypothesized that HAND2 will be reduced in breast cancer tissue.
Material and methods
I Study design -Setting
This is a case-control study. Paraffin blocks, dated between January 1st, 2015 and December 30th, 2016, were obtained from the pathological archive of Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil, for analysis. Slides were reviewed by a certified board pathologist to confirm the diagnosis.
II Participants
Women aged between 30- and 70-year-old, with a diagnosis of invasive ductal carcinoma, with or without ductal carcinoma in situ, and those submitted to breast surgery for benign conditions (e.g., breast reduction mammoplasty) were included in the sample. Those with lobular carcinoma or with intraductal papilloma were excluded.
III Variables
The main outcome analyzed was the protein expression of HAND2 and ERalpha measured by the intensity of the chromogen (3,3'-diaminobenzidine - DAB). Age is a potential confounder since breast cancer has a higher incidence in older women. Student t-test will be used to verify the difference between the means. In case of a significant difference, ANCOVA analysis was used to overcome this possible confounder.
Data sources/measurement
I Immunohistochemistry
Sections 4 µm thick were submitted to standard immunohistochemistry technique [15]. Briefly, paraffin sections were deparaffinized, rehydrated, and rinsed with phosphate-buffered saline solution (PBS). Slides were incubated in sodium citrate solution, pH=9 at 95ºC for 20 minutes for antigen retrieval. Endogenous peroxidase was blocked with hydrogen peroxide 5% in methanol for 20 min. After blocking nonspecific sites with skimmed powder milk 5% diluted in PBS, the slides were rinsed with distilled water 2 x 5 min in PBS. Primary antibodies were incubated for 12 hours at 4ºC in a humid dark chamber: HAND2 (Ab60037- Anti-HAND2 antibody - Carboxyterminal end; Abcam Cambridge, MA 02139-1517, USA), at dilution 1:50 at pH 9. After 2 x 5 min in rinse PBS, secondary antibody (AP132P - Goat anti-rabbit IgG; Merck KGaA, Darmstadt, Germany) was incubated for 90 minutes at 22ºC in the same chamber using a dilution of 1:200. Detection of the primary antibody was obtained using the Liquid DAB+Substrate Chromogen System (K3468, Dako, DK-2600 Glostrup, Denmark) according to the manufacturer's instructions using 3,3'-diaminobenzidine (DAB) as chromogen. The slides were counterstained with hematoxylin, mounted and analyzed with an optical microscope. Negative controls were obtained by replacing the primary antibody with mouse IgG2a, kappa monoclonal - Isotype Control (ab170191 - Abcam). Estrogen and Progesterone Receptor were analyzed with CONFIRM anti-ER (SP1) and anti-progesterone receptor (1E2) antibodies (Roche Diagnostics GmbH, Mannheim, Germany, D-68305) using BenchMark ULTRA Instrument (Roche Diagnostics, Indianapolis, IN, USA) according to manufacturer's instructions. Hepatocarcinoma and known breast cancer estrogen/progesterone receptor positive were used as a positive external control for HAND2 and ERα/PR, respectively.
Stained sections were analyzed under an optical microscope (Olympus BX51 microscope; Olympus Optical Co., Tokyo, Japan) connected to a digital color camera/Q-Color 5 (Olympus). Images were obtained with a 20x objective UPLanFI (resolution: 2.75 mm), at a size of 2560 x 1920 pixels (resolution: 1 mm = 3000 pixels), under standard conditions. Pictures were taken from the whole slide to perform image analysis.
II Image Analysis with ImageJ
In order to reduce bias, photomicrographs were coded and blindly analyzed with specific software (ImageJ - v1.43j; National Institutes of Health, Bethesda, MD, available at http://rsbweb.nih.gov/ij/) using a built-in "color deconvolution plugin" for hematoxylin and DAB built-in vector [16]. The analysis was performed as previously described using Digital HSCORE (D-HSCORE) [17, 18]. Briefly, only the glandular and tumoral sites of the tissue sections were selected as the region of interest (ROI). After selecting the ROI, images were submitted to "color deconvolution" analysis. The image with DAB staining was used for analysis.
III Study size
The sample size was calculated according to the literature, using the formula n=f(α/2, β) × 2 × σ2 / (μ1 − μ2)2, where μ1 and μ2 are the expected mean outcome in the control and experimental group respectively, σ is the standard deviation, and f(α, β) = [Φ-1(α) + Φ-1(β)], where Φ-1 is the cumulative distribution function of a standardised normal deviate [19]. In order to have a power of 95%, an alpha error of 1% to identify an increase in the primary mean outcome measure from 15 in the control group to 40 in the experimental group, having a standard deviation of 10, a minimum of 6 cases in each group would be necessary. The standard deviation was obtained from a pilot study with 5 normal samples.
IV Quantitative variables
From the 3 images obtained from “color deconvolution, the average DAB intensity, from image 2, was calculated according to the formula: ƒ= 255- i, where ƒ= final DAB intensity, i= mean DAB intensity obtained from the software. The final DAB intensity varied from 0 (white, no expression) to 255 (dark brown, highest expression). Groups were divided into those with and without breast cancer.
V Statistical Analysis
GraphPad Prism version 8 for Macintosh (GraphPad Software Inc., San Diego, CA) was used for statistical analysis, using Fisher’s exact test for categorical data, Pearson correlation between ERα and HAND2 expression and the unpaired Student t-test with Welch correction to compare the expression of HAND2 (final DAB intensity) between the 2 groups, if data had a Gaussian distribution and different SDs. Gaussian distribution was calculated with the D’Agostino & Pearson omnibus normality test. ANCOVA analysis was conducted to identify whether age between groups had an influence on HAND2 expression. HAND2 expression was the dependent variable; fixed factors were the groups, and age was the concomitant variable.
VI Reduction of bias
In order to verify the accuracy of the HAND2 protein expression location, a set of samples (breast tissue with and without cancer) were stained following the same procedures described above, but using another monoclonal antibody against HAND2, i.e., Rabbit anti-HAND2 antibody - ab200040 - amino acid 1 to the carboxyterminal end (abcam), at different dilutions (1:1000 to 1:50) at pH 9. Meduloblastoma was used as external positive control; negative controls were obtained by replacing the primary antibody with rabbit IgG, monoclonal isotype Control (ab172730 - abcam).
Results
I Participants
From 40 potentially eligible cases, 20 samples were obtained for the study. Later, one sample was removed for analysis (no breast tissue was found in the paraffin block), yielding 19 breast samples (benign=9; cancer=10) for analysis.
II Descriptive data
Details of the sampled population are depicted in Table 1. All DAB values passed the D'Agostino & Pearson omnibus normality test. No difference was observed between the mean histological area analyzed in both groups (P=0.2; Student t-test; data not shown).
III Outcome data
No difference in DAB expression [mean(SD)] was observed between groups when ERα expression was analyzed [cases=74.6(46.2) vs. controls=98.4(54); P=0.3. Student-t test with Welch's correction]. No correlation was found between ERα and HAND2 expression (r=-0.28; 95%CI -0.6 to 0.22; P=0.3; Pearson correlation test - data not shown).
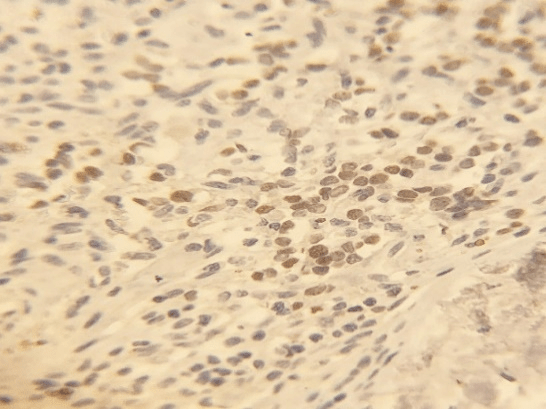
The comparison between HAND2 protein expression (mean ± SD) in tissue section with breast cancer (15.5 ± 6.1) and controls (44.8 ± 21.1) was significantly reduced in those with breast cancer (P=0.002. Student-t test with Welch's correction, Figure 1). ANCOVA analysis was conducted to identify whether age between groups had an influence on HAND2 expression. After running ANCOVA analysis, HAND2 expression was adjusted for age, and the P value between groups was 0.004, confirming that HAND2 expression was significantly different, despite the age difference between groups. The expression of HAND2 protein was mainly in the cytosol of the cells (Figure 2D).
The location of HAND2 protein expression using different antibodies showed similar results. Of note, the same antibody (ab200040), using the conditions in the same slide, but with different tissues, revealed that different tissues have different location expression of HAND2 (supplement).
Figure 1: Immunohistochemical expression of HAND2 protein stained with DAB (3,3'-diaminobenzidine, in arbitrary units - AU) in breast tissue with invasive ductal carcinoma (cancer) and in normal breast tissue (control). Statistical analysis with Student t-test. Bars represent mean values, each dot represents the mean value of individual cases.
Figure 2: Photomicrographs of HAND2 protein expression in (A) in situ component of invasive ductal carcinoma, (B) in normal breast tissue and in (C) invasive ductal carcinoma. Protein localization is mainly in the cytosol compartment (D). Hepatocarcinoma was used as an external negative (E) and positive (F) controls. Bars represent 0.1 mm
Table 1: Characteristics of the studied population.
|
Characteristics |
Breast Cancer (n=10) |
Controls (n=9) |
P |
|
Age (years) mean(SD) |
49.7(12.5) |
37.1(10.5) |
0.02a |
|
Ethnicity caucasian/non-caucasian |
8/2 |
7/2 |
1b |
|
Pathology Report Invasive ductal carcinoma (n) Normal breast tissue (n) Fibroadenoma (n) Fibromicrocystic changes |
10 |
6 1 2 |
|
|
Estrogen Receptor (positive/negative) |
10/0 |
|
|
|
Progesterone Receptor (positive/negative) |
7/3 |
|
|
|
HER2 0, +1 +2 +3 |
6 1 3 |
|
|
|
Nottingham Grading System (n)% |
|
|
|
|
I |
1 (10) |
|
|
|
II |
4 (40) |
|
|
|
III |
5 (50) |
|
|
a Unpaired Student t-test b Fisher's exact test
Discussion
HAND2 protein expression is decreased in breast cancer, compared to normal breast tissue and no correlation was found between HAND2 and ERα protein expression. For the best of our knowledge, these findings are novel and no direct comparison can be made with breast tissue; similar results, however, were reported in endometrial hyperplasia and adenocarcinoma showing a lower expression of HAND2 [8]. It is known that breast and endometrial cancer, among other factors, has a direct relationship with estrogen exposure [20, 21]. In vitro, using breast cancer cell line, Fukuda et al. demonstrated that HAND2 represses the transcriptional activation function of ERα, and not the ER itself [11]. This is in accordance with our findings: no difference was found when ERα protein nuclear expression was compared between cases and controls. HAND2 protein expression location, using ab60037 and ab200040, was in the cytosol of the cells. Previous reports, using an antibody that has been discontinued (sc-9409, Santa Cruz Biotechnology), revealed that HAND2 expression was mainly nuclear [8, 22]. This discrepancy could be related to the differences between the antibodies. This study has a few limitations. Despite all the efforts, we not able to use the fully automated immunohistochemical staining systems (BenchMark ULTRA), and manual staining was used. We did not make subgroup analysis for different types of breast cancer, i.e., luminal A, luminal B, triple-negative. However, the small range of distribution of the expression of HAND2 (Figure 1) allied to the no correlation to ERα suggest that HAND2 has an independent expression. However, further studies are necessary to confirm this hypothesis.
However, there are many strengths. The sample size was properly calculated. A post hoc analysis yielded a power of 92.3%, with an alpha error of 1%. The use of ImageJ software reduced the subjective bias for DAB expression and the correlation between ERα and HAND2 used the same method of quantification. Furthermore, we used proper immunohistochemical staining using a non-specific primary antibody, instead of omitting it. Proper external positive and negative controls were also used. It is likely that these results have external validity, as long as the same methodology is applied. Our findings may lead to investigate if HAND2 may be used as a biomarker or as a prognostic factor in breast cancer.
Conclusion
The immunoexpression of HAND2 is reduced in breast cancer, compared with normal breast tissue and HAND2/ERα expressions are not correlated.
Contribution
R.F.S., J.L.P., E.P.G.N. and Q.L.A. designed the study. R.F.. and Q.L.A. wrote the main manuscript text; QLA LAG carried out he experimental work, all authors analysed the data, reviewed and approved the manuscript.
Conflicts of interest
All authors declare they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from Hospital de Clínicas de Porto Alegre Ethical Review Board (CAAE 47158115.5.0000.5327). This article does not contain any direct study with human participants or animals. The use of informed consent was waived by our local ethics committee. Only R.F.S., L.A.G. and Q.L.S. had access granted to the identify some information from the patient's electronic records. These authors signed a confidentiality form approved by local ethical committee as stated above.
Funding
This study was funded by FIPE (Fundo de Incentivo à Pesquisa e a Eventos) of Hospital de Clínicas de Porto Alegre [grant 15-0327]. Support was given to buy laboratory reagents.
Supplementary Figure
Figure 1: Immunostaining of hepatocarcinoma using antibody ab200040
Figure 2(A&B): Immunostaining using ab200040 antibody in A) in breast cancer (note the faint immunostaining in the cytosol) and B) meduloblastoma (note the nuclear staining).
Article Info
Article Type
Case StudyPublication history
Received: Mon 07, Oct 2019Accepted: Mon 28, Oct 2019
Published: Thu 31, Oct 2019
Copyright
© 2023 Ricardo F. Savaris. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2019.5.12
Author Info
Ernesto de Paula Guedes neto José Luiz Pedrini Luiza Azevedo Gross Quiti dos Anjos Lopes Ricardo F. Savaris
Corresponding Author
Ricardo F. SavarisPostgraduate Program in Health Science: Gynecology and Obstetrics, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
Figures & Tables
Table 1: Characteristics of the studied population.
|
Characteristics |
Breast Cancer (n=10) |
Controls (n=9) |
P |
|
Age (years) mean(SD) |
49.7(12.5) |
37.1(10.5) |
0.02a |
|
Ethnicity caucasian/non-caucasian |
8/2 |
7/2 |
1b |
|
Pathology Report Invasive ductal carcinoma (n) Normal breast tissue (n) Fibroadenoma (n) Fibromicrocystic changes |
10 |
6 1 2 |
|
|
Estrogen Receptor (positive/negative) |
10/0 |
|
|
|
Progesterone Receptor (positive/negative) |
7/3 |
|
|
|
HER2 0, +1 +2 +3 |
6 1 3 |
|
|
|
Nottingham Grading System (n)% |
|
|
|
|
I |
1 (10) |
|
|
|
II |
4 (40) |
|
|
|
III |
5 (50) |
|
|
a Unpaired Student t-test b Fisher's exact test




References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424. [Crossref]
- Cappelletti V, Iorio E, Miodini P, Silvestri M, Dugo M et al. (2017) Metabolic Footprints and Molecular Subtypes in Breast. Cancer Dis Markers : 7687851. [Crossref]
- Curtis C, Shah SP, Chin S-F, Turashvili G, Rueda OM et al. (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486: 346-52. [Crossref]
- Kim RN, Moon H-G, Han W, Noh D-Y (2018) Perspective Insight into Future Potential Fusion Gene Transcript Biomarker Candidates in Breast Cancer. Int J Mol Sci 19: . [Crossref]
- Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T et al. (2004) Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet 36: 40-45. [Crossref]
- Database GHG. HAND2 Gene - GeneCards | HAND2 Protein | HAND2 Antibody.
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H et al. (2011) The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331: 912-916. [Crossref]
- Buell-Gutbrod R, Cavallo A, Lee N, Montag A, Gwin K. (2015) Heart and Neural Crest Derivatives Expressed Transcript 2 (HAND2): a novel biomarker for the identification of atypical hyperplasia and Type I endometrial carcinoma. Int J Gynecol Pathol 34: 65-73. [Crossref]
- Kannan A, Bhurke A, Sitruk-Ware R, Lalitkumar PG, Gemzell-Danielsson K et al. (2018) Characterization of Molecular Changes in Endometrium Associated with Chronic Use of Progesterone Receptor Modulators: Ulipristal Acetate Versus Mifepristone. Reprod Sci 25: 320-328. [Crossref]
- Whitaker LHR, Murray AA, Matthews R, Shaw G, Williams ARW, Saunders PTK et al. (2017) Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum Reprod 32: 531-543. [Crossref]
- Fukuda T, Shirane A, Wada-Hiraike O, Oda K, Tanikawa M et al. (2015) HAND2-mediated proteolysis negatively regulates the function of estrogen receptor α. Mol Med Rep 12: 5538-5544. [Crossref]
- Santa Cruz Biotechnology has discontinued SC-9409.
- Human/Mouse HAND2 Antibody.
- Lüder Ripoli F, Mohr A, Conradine Hammer S, Willenbrock S, Hewicker-Trautwein M et al. (2016) A Comparison of Fresh Frozen vs. Formalin-Fixed, Paraffin-Embedded Specimens of Canine Mammary Tumors via Branched-DNA. Assay Int J Mol Sci 17. [Crossref]
- Rasmussen OF, Rudbeck L (2015) Immunohistochemistry: A Dako Perspective. Handbook of Practical Immunohistochemistry. 57-67.
- Ruifrok AC, Johnston DA (2001) Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291-299. [Crossref]
- Grudzinski M, Fuhrich DG, Savaris RF (2015) Expression of elafin in fallopian tubes of ectopic pregnancies is reduced. Appl Immunohistochem Mol Morphol 23: 349-354. [Crossref]
- Fuhrich DG, Lessey BA, Savaris RF (2013) Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytopathol Histpathol 35: 210-6. [Crossref]
- Julious SA (2009) Sample Sizes for Clinical Trials. CRC Press.
- Weiderpass E, Adami H-O, Baron JA, Magnusson C, Bergstrom R et al. (1999) Risk of Endometrial Cancer Following Estrogen Replacement with and Without Progestins. J Natl Cancer Inst 91: 1131-1137. [Crossref]
- Travis RC, Key TJ (2003) Oestrogen exposure and breast cancer risk. Breast Cancer Res 5: 239-247. [Crossref]
- Flannery CA, Fleming AG, Choe GH, Naqvi H, Zhang M et al. (2016) Endometrial Cancer-Associated FGF18 Expression Is Reduced by Bazedoxifene in Human Endometrial Stromal Cells In Vitro and in Murine Endometrium. Endocrinology 157: 3699-3708. [Crossref]
