Journals
Hand and Foot Syndrome Associated with Capecitabine
A B S T R A C T
Having cancer impacts the patient both psychologically and physically. However, if cancer is accompanied with HFS, the patient’s condition gets even worse. Capecitabine is a prodrug approved to treat several types of cancer such as breast and colorectal cancer. It is associated with several adverse effects such as hand and foot syndrome (HFS) which mostly affects the palms and legs. The provoked mechanisms of HFS are not clear yet. They require further validation of HFS pathogenesis. Up to now, prevention and treatment of HFS are not clearly solidified. Evidence supports the use of celecoxib, urea-based creams, and vitamin E to reduce the severity and the incidence of HFS. This review aims to provide the gaps and ways to further clinical evaluations.
Keywords
Capecitabine, hand and foot syndrome, palmar-plantar erythrodysesthesia, celecoxib, pyridoxine
What is Already Known About This Subject
i. The review outlines one of the main chemotherapeutic agents namely, capecitabine which causes hand and foot syndrome.
ii. It shows the incidence and the clinical manifestation of HFS.
iii. Some studies proposed different hypothesis to explain the mechanisms of HFS but provided limited clarifications.
iv. Other studies provided information on how to manage and control HFS.
What This Study Adds
i. HFS adverse event has to be prevented or at least managed to the extreme level. Novel research should concentrate on certain pathways to explain the main provoked mechanism.
ii. Dose modification or discontinuation will not be the solid solution; therefore, further validations are needed.
iii. This review will hopefully bridge the gap and open gates for further research to prevent the HFS serious reaction of capecitabine.
Introduction
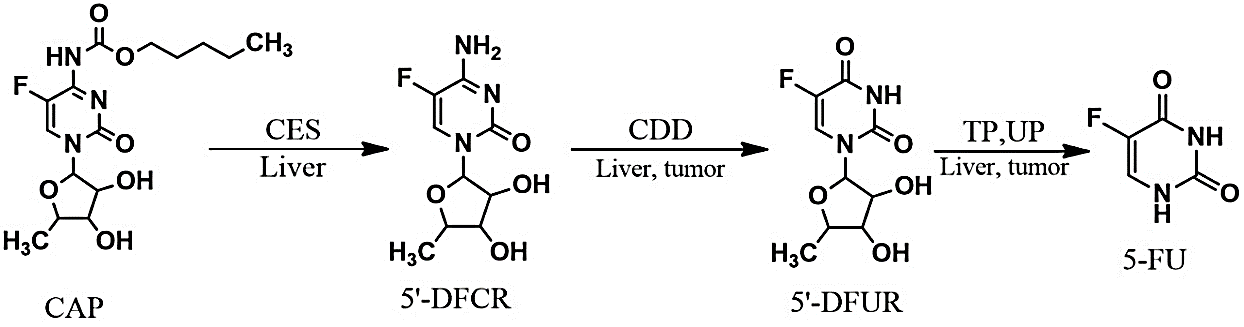
Capecitabine (Xeloda) is an orally administered prodrug, well-tolerated compared to IV 5-fluorouracil/Leucovorin, and cell cycle-specific (S phase). It functions as an antimetabolic fluoropyrimidine deoxynucleoside carbamate novel drug. With the aid of thymidine phosphorylase (TP), capecitabine will be converted in vivo into 5-fluorouracil (5-FU) which is concentrated mainly in tumor tissues (Figure 1) [1]. Capecitabine pharmacokinetic profile exhibits a high and rapid absorption in the gastrointestinal tract which is then converted after several steps into 5-fluorouracil (5-FU) [2]. However, 5-FU shows an unpredictable gastrointestinal absorption and rapid degradation, and as such it should be given intravenously. Besides, it shows a saturable pharmacokinetics because its plasma concentrations depend on rate of administration and drug dosage [3]. Capecitabine has been approved for the treatment of several kinds of cancers including advanced breast cancer which is unresponsive to anthracycline and paclitaxel- containing regimen [4]. It has also been approved for the treatment of metastatic colorectal cancer as first-line therapy [5].
Figure 1: Capecitabine metabolic pathway.
CAP: Capecitabine; CES: Carboxylesterase; CDD: Cytidine Deaminase; TP: Thymidine Phosphorylase; UP: Uridine Phosphorylase; 5’-DFCR: 5’Deoxyfluorocytidine; 5’-DFUR: 5’Deoxyfluorouridine; 5-FU: 5-Fluorouridine.
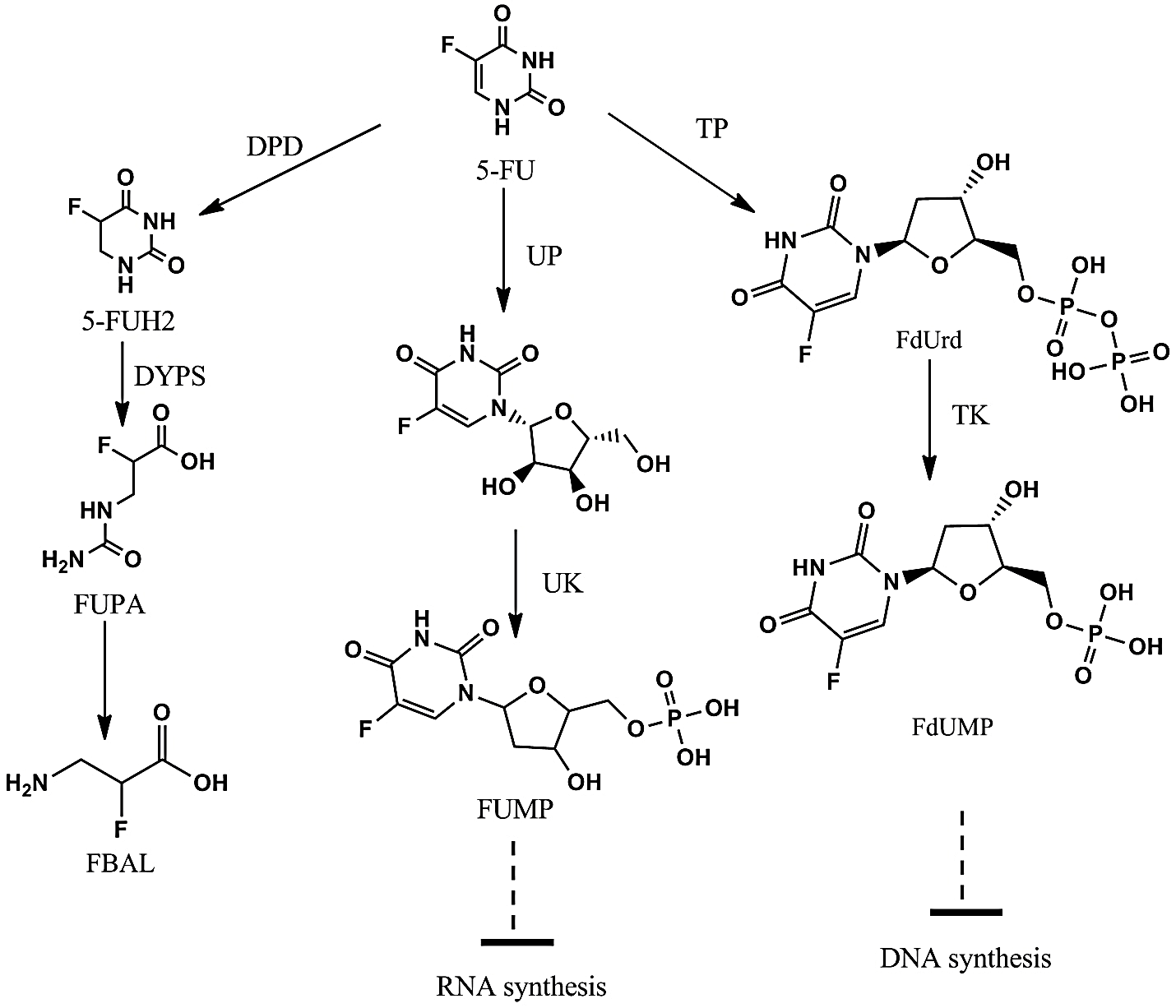
Figure 2: 5-Flourouracil metabolism pathway.
5’FU: 5-Flourouracil; FUH2: Dihydrofluorouracil; FUPA: a-Fluoro-b-ureidopropionate; FUrd: Fluorouridine; FUMP: Fluorouridine monophosphate; FdUMP: Fluorodeoxyuridine Monophosphate; FBAL: a-Fluoro-b-alanine; FdUrd: Fluorodeoxyuridine; DPD: Dihydropyrimidine Dehydrogenase; DPYS: Dihydropyrimidine; UPB1: β-Ureidopropionase; TP: Thymidine Phosphorylase; TK: Thymidine Kinase; UP: Uridine Phosphorylase; UK: Uridine Kinase.
In 2016 meta-analysis, the effect of capecitabine-based regimen as a first-line therapy was compared to free-capecitabine regimen in advanced-stage breast cancer. The researchers found that progression free survival and overall response rate with capecitabine-based chemotherapy was significantly higher than treatment with capecitabine-free chemotherapy [3]. Capecitabine is a 5'-deoxy-S-fluorouridine (5'-DFUR) carbonate derivative which is absorbed in prodrug form through the intestine. It needs three subsequent activating steps induced by enzyme catalysis. Through intestine, capecitabine is absorbed and then it is converted by carboxylesterase (CES) into 5'-deoxy-S-fluorocytidine (5'-DFCR), and then by cytidine deaminase (CDD) into 5'-deoxy-S-fluorouridine (5'-DFUR), with both conversions occurring in the liver. Lastly, the S'-DFUR is converted into the active form by thymidine phosphorylase(TP) which occurs in tumor and normal cells. On the other hand, TP enzyme is found in higher concentration (3-10x) in tumor cells (Figure 1) [3, 6]. This permits a selective activation of the drug and low systemic toxicity [6-8].
Thymidine phosphorylase (TP) metabolizes 5-FU into 5-fluorodeoxyuridine (5FdUrd). 5-fluorodeoxyuridine (5FdUrd) binds to thymidylate synthase and to tetrahydrofolate, which forms a stabilized complex hindering formation of thymidine from thymine. Thus, DNA synthesis is inhibited, resulting in cell death. Furthermore, the 5FdUrd can be metabolized into fluorouridine mono- and triphosphate (FdUMP and FdUTP), which are immediately incorporated into the DNA, resulting in deficiency in DNA structures. The mRNA polymerase will even utilize FdUTP for formation of mRNA, resulting in inhibition of the mRNA translation [3]. However, Dihydropyrimidine dehydrogenase (DPD) catalyzes the conversion of fluorouracil to the non-cytotoxic compounds (Figure 2) [9, 10].
The primary toxicities of capecitabine include Hand-foot syndrome (HFS) whereas administration of 5-FU was more probably to result in neutropenia, diarrhea, and mucositis. Side effects associated with capecitabine use were also delayed compared with those associated with 5-FU use [11].
Hand and Foot Syndrome (HFS), Pathogenesis, Prophylaxis and Treatment
I Overview and Incidence of HFS
Hand and foot syndrome (HFS) is a skin reaction (also known as palmar-plantar erythrodysesthesia (PPE)), which refers to a condition where the palms of the hands and soles of the feet turn dry, crimped, red, numb, tingling and swelling [7, 12-15]. HFS can be caused by several drugs other than capecitabine such as fluorouracil (5-FU), liposomal doxorubicin, doxorubicin, cytarabine, sunitinib and sorafenib [12-14]. Evidence supports that the rate of developed HFS due to capecitabine is higher than with other drugs such as 5-FU, docetaxel, gemcitabine, and imatinib [14]. Almost 50% of capecitabine-treated patients experience HFS with 17% of grade 3 HFS [14].
II Clinical Manifestation
Clinical manifestation occasionally occurs during the early cycles of capecitabine, or in later cycles. There are different grading systems for the HFS severity, and these were classified by the WHO into four different grades from I to IV with Grade IV being the most severe. The U.S. National Cancer Institute (NCI) has a different grading system for HFS that consists of three grades [8]. In this study we shall adopt the WHO Grading system (Table 1).
Table 1: Summary of the HFS WHO Grading System.
|
Grades |
Clinical domain |
Functional domain |
|
1 |
Dysesthesia/paresthesia, tingling in hands and feet. |
Inconvenience without disturbing daily activities. |
|
2 |
Difficulty in walking and holding objects, painless swelling and erythema. |
Difficulty in doing daily activities. |
|
3 |
Swelling in palms and soles with painful erythema, periungual erythema and swelling. |
Severe discomfort, inability to work or perform diurnal activities. |
|
4 |
Desquamation, ulceration, blistering, severe pain. |
Severe discomfort, inability to work or perform diurnal activities. |
In Grade I, several skin changes start to appear such as numbness, dysesthesia, paresthesia, tingling and erythema. These changes do not usually affect the patient’s daily activity, but dosage adjustments of capecitabine are needed based on the severity of HFS. In Grade II, skin changes (e.g., erythema, swelling) with pain impact diurnal efficiency, a condition which necessitates changes in dose events of capecitabine. As for grade III, there are severe skin changes such as ulceration, moist desquamation, and blistering accompanied by pain, severe annoyance and difficulty to perform daily activities. This grade accounts for 10 to 70% of all cases. Grade IV is accompanied by severe epidermal necrosis [14, 16]. However, when HFS reaches Grade ≥ 2, capecitabine therapy should be stopped instantly and restarted at a reduced dose after the toxicity settles down to Grade 1.
Although HFS symptoms usually subside within 1-2 weeks of stopping the treatment, perpetual complications might still occur [6, 13, 14]. A previous report showed that with the use of capecitabine, epidermal destruction could occur leading to loss of fingerprints [17]. In addition, frequent incidents of HFS can lead to thickening of palmoplantar area turning it into a cornified layer that resembles a keratoderma [6].
III Pathogenesis of Capecitabine-Induced HFS
Hitherto the mechanisms of HFS are still unknown, and there are limited data available on how to prevent them or manage them [6, 15]. However, different hypotheses of capecitabine-induced HFS pathogenesis have been suggested [6]. One of the hypotheses stated that capecitabine causes keratinocytes vascular degeneration, apoptosis, perivascular lymphocytic filtration, and edema. Its excretion from eccrine sweat glands, mostly abundant in palms and plantar, leads to the accumulation of its metabolites and explains why hand/foot areas more affected than others. Moreover, the elevated levels of thymidylate phosphorylase (TP) and uridine phosphorylase (UP), and the decreased level of DPD expression in the keratocytes, can contribute to the increased capecitabine metabolite level [6]. As a result, capecitabine-induced HFS may occur because of over expression of TP in the skin mainly in the hands and feet. The rapid cell proliferation in these areas and the increase of TP activity are due to active epidermal regeneration. To elaborate further, palm cells have increased sensitivity to cytotoxic drugs because basal keratinocytes proliferation rate of the back cells is significantly lower than that of palm cells [14].
Furthermore, it is believed that increased vascularization, temperature, and pressure in the hands and feet may lead to HFS [6]. TP is considered an angiogenic marker tightly related to the increased blood flow in the palm. For this reason, HFS might be associated with increased blood flow in the area. It seems that further studies are required to see whether TP or DPD has a key main role in the pathogenesis of capecitabine induced HFS [6]. Oxidative stress is generated by metabolite accumulation in hand/foot areas, which contributes to the pathogenesis of HFS, mediated by chemokines as interleukin (IL-8, IL-1b, IL-1a, IL-6), GRO, fractalkine [12, 16]. Thermoreceptor (TRPM2), which appears on the keratinocyte surface, will be sensed by Reactive Oxygen Species (ROS) in the environment. This will lead to chemokine productions and the creation of a hole on skin surface through Ca+2 influx. Chemokines will induce death factors productions as Tumor Necrotic Factor (TNF) and Fas ligand which cause apoptosis [16]. These observations highlight the connection between the concentration of anticancer medications in eccrine sweat and subsequent clinical and histological changes in the skin [17].
Another hypothesis propounds that cyclooxygenase’s (COX-2) over expression in palm and feet by capecitabine and its metabolites. COX-2 enzyme plays a main role in inflammation and pain. Therefore, celecoxib which is selective (COX-2) inhibitor may have a key role in the HFS treatment plan [6, 18, 19]. ATP-binding cassette (ABC) as a carrier system-might have a key role in fluoropyrimidine based response in which it determines the drug concentration within the cell and lead to skin reactions on hands and feet. ABC is one of the membrane transport systems containing proteins that transfer miscellaneous drugs and endogenous compounds such as capecitabine metabolites to the cell. It also removes chemotherapeutic agents from cancerous cells and prohibits drug accumulation in the tumor cells, which lead to drug failure [6, 20]. For example, three ABCB1 SNPs showed a significant association with moderate-sever HFS [6].
HFS and Pharmacogenomics
Inter-individual and inter-regional heterogeneity subsists with regard to toxicity and efficacy profiles and may be partially elucidated by genetic variation [21]. Gene polymorphisms can describe a vicinity of patient pharmacodynamic variability of anticancer drugs, especially fluoropyrimidines. Genes polymorphisms may notably affect pharmacodynamics of fluoropyrimidines, including capecitabine, are thymidylate synthase (TS), methylene tetrahydrofolate reductase (MTHFR), and dihydropyrimidine dehydrogenase (DPD). DPD deficiency in breast cancer patients receiving capecitabine, has a role in efficacy and toxicity, which require further investigations [22]. The DPYD gene encodes DPD, which catalyzes the rate-limiting step in the breakdown of fluoropyrimidines. The nonfunctional DPYD variants which have been associated with low DPD activity and an increased risk of toxicity with fluoropyrimidines [23]. One of the toxicities is HFS, was strongly associated with higher levels of DPD due to elevation of Capecitabine catabolites [24].
The Clinical Pharmacogenetics Implementation Consortium (CPIC) has published dosing recommendations for fluoropyrimidines (capecitabine, fluorouracil) based on DPYD genotype. CPIC recommends using an alternative drug for patients who are “poor metabolizers”. These individuals bear two copies of non-functional DPYD variants and typically have complete DPD deficiency. Moreover, they have the increased risk for severe or even fatal drug toxicity when treated with fluoropyrimidine drugs. CPIC also suggests a 50% reduction in initial dose for “intermediate metabolizers”. These individuals carry a combination of a normal-function and a non-functional variant and typically have reduced DPD activity [25].
Overall, the prevalence of individuals who are heterozygous for nonfunctional variant DPYD alleles (partially DPD deficient) that place them at risk of severe drug reactions is estimated to be as high as 3-5%, but this varies in different populations. For example, in the Dutch population, the DPYD*2A had an allele frequency of 0.91% in Caucasians [23]. Moreover, DPD polymorphisms rs12132152 and rs76387818 were strongly associated with HFS [6]. Thus, Both the Clinical Pharmacogenetics Implementation Consortium and Royal Dutch Association for the Advancement of Pharmacy-Pharmacogenetics Working Group have recommended fluoropyrimidine dosing guidelines based on DPYD genotypes [25].
Besides, thymidylate synthase (TS), encoded by TYMS gene, it’s inhibition by the active metabolite fluorodeoxyuridine monophosphate prevents the formation of thymidylate (dTMP), which is a precursor for DNA synthesis, leading to cell-cycle arrest and apoptosis. TYMS is an enzyme which catalyzes the conversion of dUMP to dTMP and is the main intracellular target of the active 5-FU metabolite, 5-FdUMP, which compose a ternary complex with TYMS and 5-10 MTHF. However, mechanism of resistance to 5-FU is due to mainly raised TYMS expression [21]. Ooyama et al. observed that the copy number of TYMS (18p11.32) exhibited a strong association with drug resistance, which may lead to the use of TYMS copy number as a predictive marker for fluoropyrimidines drug sensitivity [26]. Moreover, TYMS polymorphisms rs2612091 and rs2741171 were strongly associated with HFS [6]. Thus, as it may seem, the clinical relevance of pharmacogenetics in capecitabine-containing regimens, should be investigated [21].
Management and Prophylaxis of HFS
Up to date, HFS management is achieved through treatment interruption, or dose modification which may also affect the treatment efficacy [6, 12, 27]. Randomized controlled trial recommended effective prevention of HFS associated with pegylated liposomal doxorubicin (PLD), docetaxel, 5-FU, and capecitabine [19, 20]. As stated, the prevention of HFS-exacerbation is warranted. It seems that avoidance of prolonged heat exposure, hand tools and knives, keeping the pressure off, and taking a break from exercise will reduce the HFS exacerbation [6]. A few tips are required to reduce the pain through patting patting the skin rather than rubbing it, keeping it moist, trying to wear slippers or ventilated shoes and avoiding the unbreathable ones in addition to staying away from harsh chemicals, and elevating the hand and feet when sitting or lying down [6]. On the other hand, cooling is not practical for drugs given orally or by continuous intravenous infusion [6].
Preventative measures against HFS are considered the cornerstone. They are targeted toward symptoms control which usually shows improvement after 1-2 weeks and involve pain control, frequent emollient use, wound care, high-potency topical corticosteroids, and topical keratolytics [6]. The discontinuation of chemotherapeutic drugs or dose reduction of the involved medicine is usually the mainstay of HFS management [14]. In penultimate, pharmacologic interventions such as dexamethasone, celecoxib, and vitamin E, which exhibited an eminent reduction in symptoms of HFS, are extremely important [12]. Since HFS is an inflammatory reaction, celecoxib showed to be effective in its management [28]. Urea-based creams may also be effective in increasing the median time for the first episode and reducing the severity of the HFS [27]. Other studies showed that using vitamin E was effective in minimizing the incidence of capecitabine-induced HFS without the need to reduce the dose or to influence its efficacy [29, 30]. There are contradictory results regarding the use of pyridoxine in capecitabine-induced HFS prevention. Zhou et al. noticed that pyridoxine was not able to reduce capecitabine-induced HFS [31]. Therefore, further trials are needed to prevent episodes of HFS.
Conclusion
Hand-foot syndrome (HSF) is a remarkable side effect closely related to some chemotherapeutic agents such as capecitabine. So, when capecitabine is administered, HFS is a most likely reaction. Dose modification or discontinuation might help but it is not the only solution. Previous studies suggested different pathways with no solid proofs. Therefore, further evaluations are needed to control and prevent this event.
Article Info
Article Type
Review ArticlePublication history
Received: Sat 01, Aug 2020Accepted: Mon 17, Aug 2020
Published: Tue 25, Aug 2020
Copyright
© 2023 Louai Alsaloumi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2020.02.03
Author Info
Louai Alsaloumi Shaima Shawagfeh Bilgen Başgut
Corresponding Author
Louai AlsaloumiDepartment of Clinical Pharmacy, Faculty of Pharmacy, Near East University, Nicosia, Northern Cyprus, Turkey
Figures & Tables
Table 1: Summary of the HFS WHO Grading System.
|
Grades |
Clinical domain |
Functional domain |
|
1 |
Dysesthesia/paresthesia, tingling in hands and feet. |
Inconvenience without disturbing daily activities. |
|
2 |
Difficulty in walking and holding objects, painless swelling and erythema. |
Difficulty in doing daily activities. |
|
3 |
Swelling in palms and soles with painful erythema, periungual erythema and swelling. |
Severe discomfort, inability to work or perform diurnal activities. |
|
4 |
Desquamation, ulceration, blistering, severe pain. |
Severe discomfort, inability to work or perform diurnal activities. |
CAP: Capecitabine; CES: Carboxylesterase; CDD: Cytidine Deaminase; TP: Thymidine Phosphorylase; UP: Uridine Phosphorylase; 5’-DFCR: 5’Deoxyfluorocytidine; 5’-DFUR: 5’Deoxyfluorouridine; 5-FU: 5-Fluorouridine.
5’FU: 5-Flourouracil; FUH2: Dihydrofluorouracil; FUPA: a-Fluoro-b-ureidopropionate; FUrd: Fluorouridine; FUMP: Fluorouridine monophosphate; FdUMP: Fluorodeoxyuridine Monophosphate; FBAL: a-Fluoro-b-alanine; FdUrd: Fluorodeoxyuridine; DPD: Dihydropyrimidine Dehydrogenase; DPYS: Dihydropyrimidine; UPB1: β-Ureidopropionase; TP: Thymidine Phosphorylase; TK: Thymidine Kinase; UP: Uridine Phosphorylase; UK: Uridine Kinase.
References
- Di Xu, Xiu Chen, Xingjiang Li, Zhixiang Mao, Wenjuan Tang et al. (2019) Addition of capecitabine in breast cancer first-line chemotherapy improves survival of breast cancer patients. J Cancer 10: 418-429. [Crossref]
- Zuo L, Li G, Xu Y, Zhang F (2016) Meta-analysis of capecitabine-based chemotherapy versus capecitabine-free chemotherapy in patients with metastatic breast cancer. Int J Clin Experiment Med 9: 18808-18815.
- Georgios V Koukourakis, Vassilios Kouloulias, Michael J Koukourakis, Georgios A Zacharias et al. (2008) Efficacy of the oral fluorouracil pro-drug capecitabine in cancer treatment: a review. Molecules 13: 1897-1922. [Crossref]
- F Cardoso, E Senkus, A Costa, E Papadopoulos, M Aapro et al. (2018) 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 29: 1634-1657. [Crossref]
- Aras E, Yucel KT, Ekincioglu AB, Gullu I (2019) Capecitabine Induced Hand-Foot Syndrome: A Systematic Review of Case Reports. Clin Experiment Health Sci 9: 178-191.
- Yan Lou, Qian Wang, Jinqi Zheng, Haihong Hu, Lin Liu et al. (2016) Possible pathways of capecitabine-induced hand-foot syndrome. Chem Res Toxicol 29: 1591-1601. [Crossref]
- Katherina Podlekareva Farr, Akmal Safwat (2011) Palmar-plantar erythrodysesthesia associated with chemotherapy and its treatment. Case Reports Oncol 4: 229-235. [Crossref]
- Sehe Dong Lee, Hye Jeong Kim, Seung Jae Hwang, Yoon Jung Kim, Seung Hyun Nam (2007) Hand-foot syndrome with scleroderma-like change induced by the oral capecitabine: a case report. Korean J Intern Med 22: 109-112. [Crossref]
- Vinay Sharma, Sonu Kumar Gupta, Malkhey Verma (2019) Dihydropyrimidine dehydrogenase in the metabolism of the anticancer drugs. Cancer Chemother Pharmacol 84: 1157-1166. [Crossref]
- Bonnie Mae Gerbrecht (2003) Current Canadian experience with capecitabine: partnering with patients to optimize therapy. Cancer Nurs 26: 161-167. [Crossref]
- Chris Twelves, Alfred Wong, Marek P Nowacki, Markus Abt, Howard Burris 3rd et al. (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352: 2696-2704. [Crossref]
- Kristen K Miller, Loren Gorcey, Beth N McLellan (2014) Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol 71: 787-794. [Crossref]
- Joan D Webster Gandy, Chris How, Karen Harrold (2007) Palmar–plantar erythrodysesthesia (PPE): a literature review with commentary on experience in a cancer centre. Eur J Oncol Nur 11: 238-246. [Crossref]
- Palaniappan M, Srinivasamurthy S, Dubashi B, Chandrasekaran A (2014) Anticancer drug induced palmar plantar erythrodysesthesia. J Clin Diagn Res 8: HC01- HC03. [Crossref]
- Sarah M Gressett, Brad L Stanford, Fred Hardwicke (2006) Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract 12: 131-141. [Crossref]
- Noriyuki Yokomichi, Teruaki Nagasawa, Ariella Coler Reilly, Hiroyuki Suzuki, Yoshiki Kubota et al. (2013) Pathogenesis of Hand-Foot Syndrome induced by PEG-modified liposomal Doxorubicin. Human Cell 26: 8-18. [Crossref]
- Kamil N, Kamil S, Ahmed SP, Ashraf R, Khurram M, Ali MO (2010) Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci 23: 7-14. [Crossref]
- Zhang R-X, Wu X-J, Lu S-X, Pan Z-Z, Wan D-S, Chen G (2011) The effect of COX-2 inhibitor on capecitabine-induced hand–foot syndrome in patients with stage II/III colorectal cancer: a phase II randomized prospective study. J Cancer Res Clin Oncol 137: 953-957. [Crossref]
- P G Corrie, R Bulusu, C B Wilson, G Armstrong, S Bond et al. (2012) A randomised study evaluating the use of pyridoxine to avoid capecitabine dose modifications. Br J Cancer 107: 585-587. [Crossref]
- Ankit Jain, Biswajit Dubashi (2012) Docetaxel-induced hand foot syndrome:" No dose is a safe dose". J Pharmacol Pharmacother 3: 200 201. [Crossref]
- Nicholas Syn, Soo Chin Lee, Boon Cher Goh, Wei Peng Yong (2016) Capecitabine pharmacogenetics: historical milestones and progress toward clinical implementation. Pharmacogenomics. [Crossref]
- Rémy Largillier, Marie Christine Etienne Grimaldi, Jean Louis Formento, Joseph Ciccolini, Jean François Nebbia et al. (2006) Pharmacogenetics of capecitabine in advanced breast cancer patients. Clin Cancer Res 12: 5496-5502. [Crossref]
- Dean L (2016) Capecitabine Therapy and DPYD Genotype. Medical Genetics Summaries [Internet]: National Center for Biotechnology Information (US). [Crossref]
- Gérard Milano, Marie Christine Etienne Grimaldi, Mireille Mari, Sandra Lassalle, Jean Louis Formento et al. (2008) Candidate mechanisms for capecitabine‐related hand–foot syndrome. Br J Clin Pharmacol 66: 88-95. [Crossref]
- Caudle K, Thorn C, Klein T, et al. (2013) Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacology Therapeutics 94: 640-645. [Crossref]
- Akio Ooyama, Yoshihiro Okayama, Teiji Takechi, Yoshikazu Sugimoto, Toshinori Oka et al. (2007) Genome‐wide screening of loci associated with drug resistance to 5‐fluorouracil‐based drugs. Cancer Sci 98: 577-583. [Crossref]
- ZhengGang Ren, KangShun Zhu, HaiYan Kang, MinQiang Lu, ZengQiang Qu et al. (2012) A randomized controlled phase II study of the prophylactic effect of urea-based cream on the hand-foot skin reaction associated with sorafenib in advanced hepatocellular carcinoma. J Clin Oncol 33: 894-900. [Crossref]
- R X Zhang, X J Wu, D S Wan, Z H Lu, L H Kong et al. (2012) Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial. Ann Oncol 23: 1348-1353. [Crossref]
- Yamamoto D, Yamamoto C, Tanaka K (2008) Novel and effective management of capecitabine induced hand foot syndrome. J Clin Oncol 26: 20615-20615.
- V Nikolaou, K Syrigos, M W Saif (2016) Incidence and implications of chemotherapy related hand-foot syndrome. Expert Opin Drug Saf 15: 1625-1633. [Crossref]
- Yun Zhou, Ling Peng, Yingjie Li, Lixun Chen (2013) Prophylactic pyridoxine was not able to reduce the incidence of capecitabine-induced hand‑foot syndrome: A meta‑analysis. Biomed Rep 1: 873-878. [Crossref]
