Fire and Explosion Hazards Due to Medical Oxygen Handling During Coronavirus Pandemic
A B S T R A C T
With the continued outbreak of the coronavirus and the increase in the need for medical oxygen, it became necessary to take all measures for the safe handling of gas. Oxygen is very reactive and behaves differently to air, compressed air, nitrogen and other inert gases. Medical oxygen, at high pressure, from a cylinder, can react violently with hydrocarbons such as oil and grease which may be used mistakenly in cylinder valve or regulator. The oxidation products are a potentially explosive hydroperoxide. Nearly all materials including rubber, textiles, and metals will burn vigorously in the presence of oxygen. Atmospheric air contains nitrogen 78%, oxygen about 21% and with 1% remaining including a variety of other gases like carbon dioxide and argon. Even a small increase in the oxygen level in the air to about 24% can create a dangerous situation. It becomes easier to start a fire, which will then burn hotter and more fiercely than in atmospheric air and may be impossible to put the fire out. Increase the concentration of oxygen due to leaking valve or hose in a poorly ventilated room or in confined space can quickly create a dangerous level.
Keywords
Medical oxygen, coronavirus, fire, oxidation
Introduction
A fire requires three elements before it can burn. The first is heat; even though fire produces heat, it needs a source of heat as an ignition source to start burning. Fuel is the second element and the third is oxygen or any oxidizer. The most important chemical reaction of a hydrocarbon, for lubrication is oxidation. Oxidation of hydrocarbons is referred commonly to as combustion. When one burns wood, paper, fuel oil or natural gas, for example, the hydrocarbons are oxidized. Hydrocarbons as oils consider as fuels that readily combust at high and enough temperatures and that combustion can begin in the absence of a spark under certain conditions [1]. Atmospheric air contains oxygen about 21%, nitrogen 78%, and with 1% remaining gases like argon and carbon dioxide. Along with other gases, oxygen gas can become very dangerous if not handled properly. High concentration of oxygen can make objects burn and in some extreme cases explosion can occur [2]. Even a small increase of oxygen to about 24% level in the air can create a very dangerous situation from fire standpoint. It becomes easier to start a fire and burns hotter and may be almost impossible to put the fire out. A leaking in valves or hoses in a poorly ventilated place or in confined space can increase the oxygen concentration to a very dangerous level [3].
Fire, heat, explosion, and pressure generation considers as undesired reactions. Reactive chemicals and materials, resins, monomers, peroxides and explosives all have the potential to produce heat by exothermic reactions, these are typically unwanted. If the heat is not removed the potential for a runaway reaction can occur [4]. There are many questions on the minds of the general public: Why does oil make oxygen explode? Can oil and oxygen ignite without a spark? The scientifically answer for these questions requires a simple description of the oxidation process. Oils and greases can spontaneously ignite in oxygen enriched atmospheres. In principle, most metals, metal alloys and all organic materials burn in oxygen. Oil and grease are extremely dangerous when contrasts to oxygen because they can easily be ignited and burn explosively due to a chain reaction occurs in oxygen equipment. It is very important that oil and grease must never be used to lubricate a valve spindle if it comes into contact with oxygen.
Oxidation of Hydrocarbons
Oxidation process is an unwanted in lubricants (containing hydrocarbons C20-C70). Lubricating oils are composed of 80-90% petroleum hydrocarbon distillate with 10-20% additives to impart specific properties to the oil. The petroleum hydrocarbon distillate generally consists of naphthenic or paraffinic compounds [5]. Oxidation process can start with the presence of oxidative agents like oxygen to form a wide range of oxidation products with lower or higher molecular weight relative to the original oil. Iron and copper in metal parts, harsh conditions such high pressure, high temperature; high friction and high metal concentration consider as factors that enhance lubricant oxidation [6, 7]. Why do all organic chemicals just sitting around the laboratory at room temperature have the potential to explode spontaneously? The answer is that they can react with oxygen, in the air at room temperature in a process of chain reactions to form organic peroxides, which can spontaneously react explosively in case of shaking or opening the cap. Organic peroxides are examples of compounds that have fuel (C and H atoms) in the same compound with the oxidant. These compounds are solid and liquid explosives similar to dynamite or TNT. Consider an organic molecule; called R-H, where (R) could be an alkyl or any fragment containing (C, H and O) atoms. In the presence of (O2) this molecule can undergo the auto oxidation reaction.
R-H + O2 → ROOH (1)
This reaction never happens significantly as a single step at any temperature because the R-H bond strength is > 330 kJ/mol for any C-H bond. However, consider the chain reaction sequence:
R-H → R. + H. (2)
R. + O2 → ROO . (3)
ROO . + R-H → ROOH + R. (4)
The net reaction is:
R –H +O2- → ROOH (5)
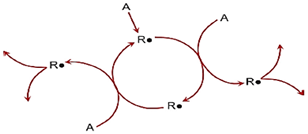
Equations 2, 3 & 4 show how hydrocarbon is converted into peroxide as mid products. The chain reaction shows that the reactants are an organic molecule and oxygen. The product is a potentially explosive hydroperoxide. It can be sketched as a chain in (Figure 1). Note that this reaction can produce two reactive free radicals from one. In case of chain branching reactions in free radical reactions. This propagation steps produce more radical species than they start with. The propagation steps can also increase the concentration of free radical species due to free radicals’ growth exponentially, leading to a rapid acceleration of the reaction and the possibility of chain-branching explosion as shown in (Figure 2) [8]. Most oils and greases consist of chains of molecules consist of carbon and hydrogen. The bonds holding them together are weak enough that, when exposed to the air, they can form more stable compounds with oxygen. The process is called oxidation, and it releases energy in the form of heat. Oxidation process usually doesn’t produce enough heat to start a fire, but heat can build, however, when the surface area of exposed oil increases and air circulation is reduced. In case of temperature reaches the ignition temperature of the oil, they burst into flames [1]. Oils and greases can spontaneously ignite in atmospheres consider as are oxygen enriched environments. Oil and grease often cause a chain reaction in oxygen equipments which may even cause metal to burn or melt which greatly increases the potential for fire or explosion [9, 10]. The auto-ignition of lubricating oil droplet as an example of hydrocarbon oxidation process mainly includes two steps: first the liquid vaporization and second oxidation occur. Figure 3 illustrates the mechanism of the auto-ignition process of the lubricating oil droplet [11].
Figure 1: Chain reaction for hydrocarbon oxidation.
Figure 2: A schematic of chain branching reactions where the propagation steps produce more than one radical.
Figure 3: Mechanism of the auto-ignition process of the lubricating oil droplet.
Avoid Rapid Opening of the Oxygen Cylinder Valve
Adiabatic pressure can occur when oxygen is injected in case of sudden high pressure into a system with low pressure. In this condition, the gas can flow at the speed of sound. This case often occurs with valves and fittings in gas operation systems. When a gas very quickly collides with a resistance, the temperature will rise very quickly due to the adiabatic pressure. This is always the case when the gases are compressed very quickly so that no heat energy is wasted. The general rule is the higher the initial pressure and higher temperature can cause ignition. This chuck is used in the diesel engine. Figure 4 shows the temperature condition in case of high and low gas speed in the valves [12].
Figure 4: Temperature condition in case of high and low gas speed in the valves.
In case of opening the valve of the oxygen cylinder too quickly, this leads to a rapid flow of gas, carrying with it some iron particles from the inner walls of the cylinder that result from corrosion as shown in (Figure 5). These particles lead to heat up the gas inside regulator (Figure 6) causes to ignite oxygen due to friction of fast iron particles as shown in (Figure 7). The accumulation of heat inside regulator lead to valve or cylinder explosion. In addition, ignition temperature of heavy hydrocarbons such as greases highly depends mainly on oxygen pressure. When oxygen pressure increasing, grease increased mass percentages are higher, because the grease surface molecules are oxidized to form peroxide bridge (-O-O-). In other words, grease surface oxidation rate is related to the oxygen pressure. The greater the pressure of oxygen, grease is easier to spontaneous combustion releasing heat and explosion as shown in (Figure 8). Increasing oxygen pressure also reduces the burning rate of grease samples as shown in (Figure 9) [13].
Figure 5: Iron particles rises from the inner walls of the cylinder.
Figure 6: Iron particles heat up the gas inside the regulator.
Figure 7: The accumulation of heat inside regulator lead to valve or cylinder explosion.
Figure 8: Ignition temperature of grease samples decreases with increasing oxygen pressure.
Figure 9: Burning rate of grease samples decreases with increasing oxygen pressure.
Oxygen Enrichment Increases the Possibility of Fire and Explosion
Increase the O2 levels to about 24% can increases fire risk. Not only combustible materials can ignite more easily, but also, they can burn more fiercely and hotter. Flame extinguishing could become almost impossible. In case of oxygen under pressure, medical oxygen can react violently with grease and oil which would normally be unreactive. Most materials routinely used in other circumstances are incompatible with oxygen-rich environments, such as grease and oil may spontaneously ignited and create fire or react explosively. Oxygen enriched atmosphere is very dangerous, the main danger to people is that clothing or hair can easily ignited, causing serious or even fatal burns. A forbidden case where oxygen is being used is smoking [3]. For more information for those interested in this matter it is possible to view a number of relevant research papers published by researchers and are part of multiple researches concerned with safety issues for the individual and society [14-16].
Safety Handling of Medical Oxygen to Avoid Fire and Explosion
Several safety procedures must be taken in consideration to avoid fire and explosion includes:
i. Never use oil or grease to lubricate oxygen equipment.
ii. Prevent oxygen enrichment; a well-ventilated space is required.
iii. Smoking should be forbidden where oxygen is being used.
iv. Avoid rapid opening the oxygen cylinder valve.
v. Use oxygen equipment which is designed for oxygen service.
Employers are required by law to assess risks in the workplace and to take all reasonable precautions in practice to ensure the safety of workers and members of the public. Careful examination of the risks of using oxygen should be included in the risk assessment.
Conclusion
Oxygen is very reactive gas and behaves differently to air. Medical oxygen, at high pressure can react violently with hydrocarbons such as oil and grease. Even a small increase in the oxygen level in the air to about 24% can create a dangerous situation as it is easier to start a fire, which will then burn hotter. Increase the oxygen concentration due to leaking valve or hose in a poorly ventilated room or confined space can create a dangerous situation from fire and explosion standpoint. All safety procedures are required to be followed for safe gas handling and the prevention of accidents.
Recommendations
With the continuing outbreak of the coronavirus and the increase in the need for medical oxygen, this was not only used in medical centers, but went beyond that to household uses to treat patients infected with the coronavirus. The matter that makes it imperative for everyone to use the safe gas by increasing awareness and education about gas dangers and how to handle the safe way to prevent fire and explosion accidents of gas cylinders.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 09, Aug 2021Accepted: Sat 11, Sep 2021
Published: Thu 11, Nov 2021
Copyright
© 2023 Wedad H. Al Dahhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2021.02.01
Author Info
Wedad H. Al Dahhan Emad Yousif
Corresponding Author
Wedad H. Al DahhanDepartment of Chemistry, College of Science, Al-Nahrain University, Baghdad, Iraq
Figures & Tables








References
1. Deziel C (2017) Oil
& Oxygen Ignite without a Spark? Scienc.
2. Metro W (2020) The
Uses of Oxygen Gas for Medical Clinics. Weil Supp Med Oxy.
3. Take Care with
Oxygen, Fire and Explosion Hazards in the use of Oxygen. (2008) Heal Safe
Exec.
4. Liu S (2020)
Combustion, reactive hazard, and bioprocess safety. Bioprocess Engineering
2020: 719-772. [Crossref]
5. Michael J (2005)
Encyclopedia of Toxicology. Elsev 2.
6. Mortier RM, Fox MF,
Orszulik S (2010) Chemistry and Technology of Lubricants. Sprin 3.
7. Rudnick L (2009)
Lubricant Additives: Chemistry and applications. CRC Pres.
8.
Liu S (2020) Bioprocess Engineering, Combustion, Reactive Hazard and Bioprocess Safety. Elsev
3.
9. Cryo Air Gases,
Herose Group (2021) Why the absence of oil and grease is so important. Valv
Comm.
10. Specialty
Lubricants. IKV Gro.
11. Ping Y, Wuqiang L,
Liyan F, Ming J, Jiangping T (2017) A Numerical Investigation of the
Vaporization Process of Lubricating Oil Droplets under Gas Engine Conditions. Dalia
Uni Techno.
12. Serto Oxygen
Testing (2003) Why is oil and grease-free so important in oxygen systems?
13. Guohui1 Z, Lijing
Z, Gang T, Liang Z (2013) Experimental Study on Combustion Characteristics of
Grease Attached on Oxygen Cylinder in High-pressure Oxygen. App Mechan Mater
331: 591-594.
14. Al Dahhan W, Al
Mashhadani M, Raheem R, Yousif E (2020) Iraq Faces the COVID-19 with Limited
Health Capabilities and Major Medical Challenges. Lat Am J Biotec Lif Sci
5: 3.
15. Wedad H, Al Dahhan W, Fadhil Z, Bufaroosha M, Mohammed S et al. (2020) A Case Report and Review: Be Aware to Avoid Accidents at Home. Ope J Safe Sci Techn 10: 33-41.
16. Al Dahhan W, Yousif E (2018) Hydrogen Balloons: Bright Colors but Hidden Fire Hazard. Int J Pub Heal Saf 3: 1-6.
