Fatigue and Associated Symptoms in Patients with a Primary Brain Tumor
A B S T R A C T
Purpose: To determine the prevalence and predictors of fatigue in clinically stable primary brain tumor patients, we used objective and subjective measures and a cross-sectional design.
Methods: Eighty-five brain tumor patients completed self-report measures of fatigue, sleep disturbance, mood and growth hormone deficiency. Objective measures of sleep, cognition and neurological function were carried out. Comparisons were made between patients with no-mild, moderate and severe fatigue.
Results: Sixty-seven per cent of patients (n=57) were experiencing moderate or severe fatigue at the time of assessment. Statistically significant differences between no-mild, moderate and severe fatigue groups were found when examining percentage daytime activity (p=0.035), processing speed (p = 0.0006), anxiety (p=0.008), depression (p<0.0001), neurological function (p<0.0001), growth hormone deficiency (p<0.0001) and epileptic drug type (p=0.011). Memory, executive function and verbal fluency were not found to significantly differ across groups. Sleep duration and efficiency were not correlated with fatigue. Using regression analysis, anxiety and neurological function predictors were independently found to be associated with fatigue (p=0.017 and p=0.0003 respectively).
Conclusions: Findings suggest neurological function and anxiety independently contribute to fatigue in stable brain tumor patients. A ‘neurological model’ may offer a better understanding of fatigue in the brain tumor population than a ‘cancer model’. This study supports the recommendation of a core data set for assessing fatigue that includes a measure of neurological function, alongside patient perceptions of causation (physical and mental fatigue). This may potentially be helpful in selecting treatment options or in interpretation of drug trials of fatigue.
Keywords
Fatigue, anxiety, neurological function, primary brain tumor, quality of life, glioma
Introduction
Fatigue is the most common symptom reported in patients living with a brain tumor [1-5]. It can be associated with primary factors related to the disease process and treatment (e.g. surgery, radiotherapy) and secondary factors including sleep disturbance, depression, endocrine changes, and medication side-effects [2-3, 6-11]. Researchers have proposed a core set of assessments for all brain tumor patients to identify symptoms of fatigue, distress (anxiety and depression), drowsiness, difficulty remembering, and difficulty with sleeping [12]. These are often co-occurring and related symptoms that may be contributing to poor quality of life, and thus offer avenues for treatment.
Attempts to understand fatigue in brain tumor patients might consider a cancer-related fatigue model. Cancer-related fatigue is “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” [13]. However, cancer-related fatigue often relates to anorexia, cachexia and systemic diffuse cancer, which are uncommon with CNS tumors, and generally does not incorporate the neurological impairment and disability commonly experienced following brain or spinal cord tumors. Further, theories of primary fatigue in neurological disorders include structural damage to the nervous system with reduced glucose metabolism, reduced inhibition in the cerebral cortex and abnormal cytokine profiles [14]. Therefore, a neurological-related fatigue model, common in patients with multiple sclerosis (MS), stroke, traumatic brain injury and epilepsy, may offer a better understanding of fatigue in brain tumor patients, that incorporates impairment and disability associated with the nervous system [15-19].
Material and methods
I Aims
This exploratory study aimed to determine the prevalence and predictors of fatigue in clinically stable primary brain tumor patients who were not under active treatment with radiotherapy or chemotherapy. The study also aimed to determine whether primary or secondary factors were more closely associated with the presence of fatigue.
II Setting
Patients were recruited from a neuro-oncology outpatient clinic at the Western General Hospital in Edinburgh, Scotland. This centre covers a large catchment area (1.83million people). Potential patients were identified and received a letter about the study along with a patient information sheet. Assessments were divided across two appointments to avoid patients becoming too fatigued, as per ethical approval recommendations, and due to the potential impact on cognitive test performance. These appointments were two weeks apart in order to collect sufficient sleep data. Patients were also offered the second appointment via telephone to reduce any bias associated with patients unable to travel due to mobility or financial circumstances and, for these patients, all measures with a visual component were completed at the first appointment. Demographic and clinical data were obtained from patients’ medical records.
III Patients
We included adults diagnosed with a primary brain tumor, aged ≥ 18 years, English speaking, with imaging stable disease. We also included those who had not received any treatment (surgery, radiotherapy or chemotherapy) within the 3 months prior to participation in the study, to reduce possible acute treatment effects. We excluded those who were unable to understand the consent process due to language or cognitive problems; this was assessed via the treating team that identified those eligible to participate.
IV Measures
Fatigue
• The Brief Fatigue Inventory (BFI) is a short self-report questionnaire containing three items relating to fatigue “severity” and six items relating to the “interference” of fatigue on daily functioning in the last 24 hours. Patients respond on a scale from 0 to 10; fatigue scores are categorized as none (0), mild (1-3), moderate (4-6) and severe (7-10) [20].
• Individuals experiencing at least some level of fatigue also completed the Semi-structured clinical interview for fatigue (SCIF), a questionnaire developed for this study. The SCIF contains items regarding the severity of any mental or physical fatigue separately on a Likert scale (0-10) and open-ended questions asking patients to identify when their fatigue first began, what patients think was the primary cause(s), and what alleviates or aggravates their fatigue.
Neurological Impairment
•The Kurtzke Expanded Disability Status Scale (EDSS) assesses neurological function across eight systems (including pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, cerebral or mental, and other) and scores overall disability between 0 (normal neurological exam) and 10 (death) [21].
•The Nine-hole peg test (NHPT) assesses upper limb function dexterity [22].
•The Timed 10 metre walk (TMWT) assesses lower limb function/gait speed [22].
Cognition
The following measures were used to assess cognition:
|
Measure |
Domain |
|
Hopkins Verbal Learning Test-Revised (HVLT-R) [23] |
Verbal memory |
|
Williams Delayed Recall Test (WDRT) [22] |
Visual memory |
|
Controlled Oral Word Association Test (COWAT) [24] |
Verbal fluency |
|
Trail Making Test Part A(TMT-A) [25] |
Information processing speed |
|
Trail Making Test Part B(TMT-B) [25] |
Executive function |
|
Test of Premorbid Functioning (TOPF) [26] |
Estimated premorbid intelligence |
|
Cognitive Failures Questionnaire (CFQ) [27] |
Subjective cognitive impairment (completed by relatives) |
Mood
The Hospital Anxiety and Depression Scale (HAD) is a self-report measure of anxiety and depression and has been partially validated in brain tumor patients [28, 29].
Sleep
• Patients wore an Actiwatch on their non-dominant wrist for 7 nights and 7 days, which provides an objective measure of daytime and nighttime sleep and wake activity [30-32].
• A Sleep Diary was used to assess total nighttime sleep, total day-time naps, percent daytime activity.
• The Epworth Sleepiness Scale (ESS) screens for subjective daytime sleepiness [33].
• The Patient Sleep Questionnaire provides details regarding self and informant-reported night-time sleep difficulties (e.g. snoring; breathing pauses; choking or gasping; etc.).
Growth Hormone
The Adult Growth Hormone Deficiency Questionnaire (AGHDQ) is a clinical tool used to screen for growth hormone deficiency (i.e. pituitary failure). Patients who scored ≥10 were considered to positively screen for further investigation of pituitary function [34].
Medication
The type and dose of medication of drugs (e.g. steroids; anti-epileptic drugs; anti-depressants) were recorded at the time of the assessment.
V Standard Protocol Approvals, Registrations and Patient Consents
The NHS Research Ethics Committee gave ethical approval for the study. Written informed consent was obtained from patients prior to participation. Patients were not blinded to the nature of the study.
VI Statistical Methods
Data analysis was performed using SAS for Windows version 9.4. In order to assess fatigue, patients were grouped into ‘none/mild’, ‘moderate’ and ‘severe’ fatigue groups based on their scores from BFI questionnaire. Patient demographics (i.e. sex; age; years in education; and marital status), tumor (i.e. grade; location) and treatment information (radiotherapy, chemotherapy; years since diagnosis; years since treatment) were analyzed to assess the relationship with fatigue groups using Chi-square tests or one-way ANOVA (non-parametric methods used where appropriate). The relationship between BFI Interference and BFI Severity scores were examined using Pearson’s correlation. A descriptive analysis of SCIF results was included regarding information on type of fatigue, subjective alleviators and triggers of patient fatigue.
Physical and cognitive assessments whose outcomes were continuous (i.e. EDSS) were analyzed using Kruskal-Wallis across BFI fatigue groups and those whose outcomes were categorical (i.e. NHPT; HVLT-R; TMWT; TMT-A; TMT-B; COWAT, medication, ESS) were assessed using Chi-square tests (where expected counts were small, the p-value from the Fisher’s test were presented instead). Normative data was used to create age and education-adjusted z-scores for the HVLT-R, COWAT and TMT. Patients were categorized as impaired or unimpaired on each cognitive task using a cut-off of 1.5SD. Results from the CFQ (completed by relatives or partners) were analyzed using a one-way ANOVA. HADS scores for anxiety (HADS-A) and depression (HADS-D) were analyzed as both a continuous measure, using one-way ANOVA, and as a categorical measure (<8 =normal; ≥8 = abnormal), using Chi-square test. Growth hormone deficiency was split according to a clinical cut-off of ≥10 representing a positive screen and compared across fatigue severity groups using a Chi-square test.
Logistic regression analysis was used to examine whether selected predictor variables could be associated with fatigue. Following a series of univariate analyses, any statistically significant variables (p<0.1) were fitted initially into a full multivariate logistic model followed by a forwards and backwards stepwise logistic regression models. Due to the exploratory nature of this study, and its small sample size, no adjustment was made for multiple comparisons.
Results
I Patient Recruitment
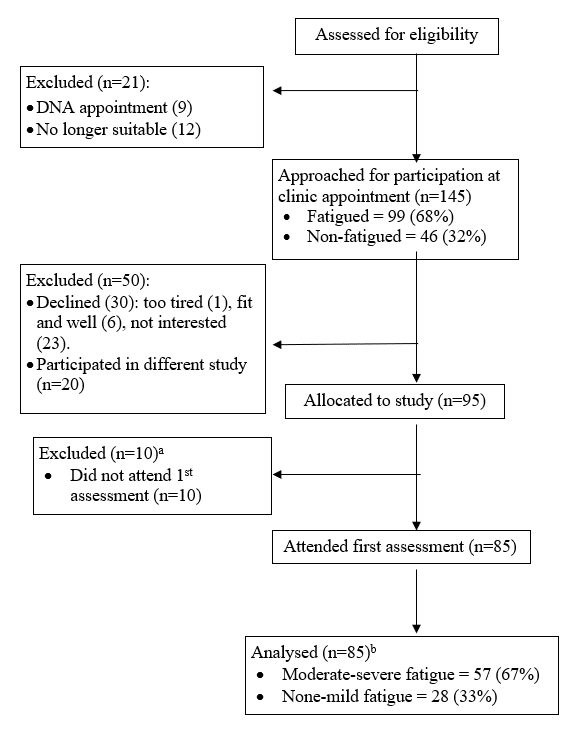
Recruitment was carried out over a 12-month period. After screening for eligibility, 166 patients were identified, 95 (57%) agreed to take part in the study and 85 (51%) gave informed consent and attended at least one assessment (Figure 1). All 85 patients attended the first visit, but ten patients did not attend their second appointment, resulting in missing data. Reasons for not attending included work commitments (N=3), transport difficulties (N=2), symptom severity (N=4) and no longer interested in participating (N=1).
Figure 1: Study Recruitment Flow Diagram.
a10 patients did not complete a 1st appointment: two did not attend the appointment and did not want to reschedule; two cancelled the appointment due to health (n=2) or travel (n=1) reasons; five patients did not know their availability at the time of recruitment and requested a phone call to arrange an appointment time, but could not be reached via the contact details provided.
b10 patients missed their second appointment due to a work commitment (n=3), were unable to make travel arrangements (n=2), their symptoms were too bad (n=4) or were no longer interested (n=1).
II Clinical and Socio-demographic Characteristics
Twenty-eight patients self-reported no or mild fatigue, 41 reported moderate fatigue and 16 reported severe fatigue using the BFI. There was no evidence of any significant differences in demographic or tumor characteristics across fatigue groups (Table 1). Fatigue was equally prevalent in high-grade and low-grade brain tumor patients (p=0.151). There was evidence of a significant correlation (r=0.78, p<0.0001) between fatigue severity (Mean=4.9, SD=2.9) and interference (Mean=3.8, SD=2.8) in daily life. Therefore, no further subgroup analyses were completed.
Data collected from the SCIF (N=75) identified that nine patients (12%) felt predominantly mentally fatigued, four (5%) felt mainly physically fatigued, and 43 (57%), felt equivalent levels of physical and mental fatigue; with 19 (25%) feeling none-mild physical and mental fatigue. There was no evidence of a relationship between physical (p=0.554) or mental (p=0.742) fatigue and glioma grade therefore a subgroup analysis was not completed.
Table 1: Demographic Characteristics and Clinical Characteristics of the Patients (N (%))
|
|
Fatigue Group |
p |
||
|
None/Mild (N=28) |
Moderate (N=41) |
Severe (N=16) |
||
|
Demographic Characteristics |
||||
|
Age (years)* |
46.9 (12.1) |
48.3 (9.6) |
49.8 (13.3) |
0.718 |
|
Female |
14 (50) |
21 (51) |
8 (50) |
0.994 |
|
Education (years)* |
13.8 (3.2) |
13.3 (3.4) |
12.9 (2.4) |
0.623 |
|
FSIQ |
105.3 (13.4) |
103.6 (12.9) (N=39) |
101.4 (13.5) (N=15) |
0.647 |
|
Married |
20 (71) |
23 (56) |
12 (75) |
0.269 |
|
Clinical Characteristics |
||||
|
WHO Grade 1 |
1 (4) |
3 (7) |
5 (31) |
0.151 |
|
WHO Grade 2 |
11 (39) |
24 (59) |
3 (19) |
|
|
WHO Grade 3 |
8 (29) |
8 (20) |
4 (25) |
|
|
WHO Grade 4 |
8 (29) |
6 (15) |
4 (25) |
|
|
Tumor Side – Left |
10 (36) |
15 (37) |
4 (25) |
0.840 |
|
Tumor Side – Right |
13 (46) |
18 (44) |
10 (63) |
|
|
Tumor Side - Both |
5 (18) |
8 (20) |
2 (13) |
|
|
Radiotherapy |
23 (82) |
29 (71) |
14 (88) |
0.309 |
|
Chemotherapy |
8 (29) |
13 (32) |
8 (50) |
0.319 |
|
Years since diagnosis** |
5 (2.0-10.5) |
6 (2.0-14.0) |
8 (5.5-16.5) |
0.410 |
|
Years since treatment** |
4.1 (1.5-6.0) |
4.5 (2.0-11.9) |
5.7 (2.0-11.0) |
0.653 |
|
Anti-epileptic drug use |
||||
|
Current use |
16 (57) |
28 (68) |
9 (56) |
0.551 |
|
Other medications |
||||
|
Steroid |
1 (4) |
5 (12) |
3 (9) |
0.265 |
|
Anti-depressant |
2 (7) |
3 (7) |
5 (31) |
0.052 |
|
Statin |
3 (11) |
5 (12) |
2 (13) |
0.999 |
|
Diabetes |
1 (4) |
5 (12) |
2 (13) |
0.540 |
|
Anti-anxiety |
2 (7) |
3 (7) |
1 (6) |
0.999 |
|
Blood pressure |
2 (7) |
4 (10) |
2 (13) |
0.890 |
Abbreviations: FSIQ Full Scale IQ estimated from the Test of Premorbid Functioning
*mean (SD), **median (interquartile range)
III Neurological Impairment
Fatigue was associated with neurological disability, assessed via the EDSS (N=84, p<0.0001; Table 2). Patients in the more severe fatigue groups were more likely to be impaired on upper limb function scores using the NHPT (N=79, p=0.026) and lower limb function scores using the TMWT (N=81, p=0.009).
Table 2: Percentage of patients classified as impaired from physical tests
|
Test |
|
BFI Group N/Total N (%) |
p-value |
||
|
N |
None-Mild |
Moderate |
Severe |
||
|
EDSSa* |
84 |
1.8 (0.0-2.0) N=28 |
2.0 (2.0-3.0) N=40 |
3.5 (2.5-6.3) N=16 |
<0.0001 |
|
NHPTb |
79 |
4/28 (14%) |
14/38 (37%) |
7/13 (54%) |
0.026 |
|
TMWTb |
81 |
15/28 (54%) |
27/39 (69%) |
14 (100%) |
0.009 |
aHigher score = better performance bHigher score = poorer performance
Abbreviations: EDSSKurtzke Expanded Disability Status Scale; NHPT Nine Hole Peg Test; TMWT Ten Metre Walk Test.
*Continuous – median (interquartile range); Kruskal-Wallis Test
Table 3: Percentage of patients classified as impaired from cognitive tests
|
Test |
|
Fatigue Group N/Total N (%) |
p-value |
||
|
N |
None-Mild |
Moderate |
Severe |
||
|
HVLT-R |
81 |
|
|
|
|
|
Immediate Recalla |
|
11/26 (42%) |
25/39 (64%) |
9/16 (56%) |
0.223 |
|
Delayed Recalla |
|
9/26 (35%) |
18/39 (46%) |
7/16 (44%) |
0.645 |
|
Retention %a |
|
6/25 (24%) |
20/39 (51%) |
6/16 (38%) |
0.092 |
|
Delayed Recognitiona |
|
2/25 (8%) |
9/39 (23%) |
5/16 (31%) |
0.154 |
|
MAE COWATa |
80 |
7/26 (27%) |
13/39 (33%) |
9/16 (56%) |
0.142 |
|
TMT Ab |
81 |
3/28 (11%) |
11/38 (29%) |
10/15 (67%) |
0.0006 |
|
TMT Bb |
81 |
9/28 (32%) |
17/38 (45%) |
8/15 (53%) |
0.363 |
|
WDRTb |
79 |
13/27 (48%) |
13/37 (35%) |
4/15 (27%) |
0.345 |
aHigher score = better performance bHigher score = poorer performance
Abbreviations: HVLT-R Hopkins Verbal Learning Test-Revised; MAE COWAT Multilingual Aphasia Examination-Controlled Oral Word Association Test, TMT Trail making test, WDRT William’s Delayed Recall Test
IV Cognition
There was a significant relationship across fatigue groups in information processing speed scores assessed using the Trails Making Test A (N=81, p=0.0006). No other significant differences were found (Table 3). Fifty-eight relatives or partners completed the CFQ. There was no evidence of a significant relationship between average CFQ results and fatigue groups (p=0.186).
V Mood
Severely fatigued patients reported significantly higher mean anxiety (p<0.0001) and depression (N=83, p<0.0001) compared with no-mild or moderate fatigue groups. Once categorized as normal or abnormal mood levels, there was evidence of a statistically significant difference in abnormal levels of anxiety across none/mild (39%), moderate (59%) and severe (88%) fatigue groups (p=0.008). Similarly, supra-threshold depressive symptoms increased across none/mild (11%), moderate (28%) and severe (75%) fatigue groups (p <0.0001).
VI Growth Hormone Deficiency
Seventy-six patients completed the AGHDQ. Evidence of a significant relationship was found between fatigue groups and scoring above the clinical cut-off (i.e. positive screen ≥10) for growth hormone deficiency investigation (p<0.0001). Medical file review at the point of recruitment end revealed twelve patients were further investigated for growth hormone deficiency during the recruitment period. Of these, seven (assessed via IGF1 level) were concluded, notably, to be growth hormone deficient. This may have highlighted a potentially reversible cause of fatigue in this subgroup of participants, however further research is needed to follow-up whether fatigue symptoms resolve with treatment.
VII Medication
There was no evidence of a relationship between prescription of steroids, anxiolytics, anti-epileptic drugs (AEDs), statins, diabetes or high-blood pressure medication, and fatigue groups (Table 1) however the relationship between prescription of anti-depressants and fatigue groups was verging on statistical significance. Based on evidence from a previous study, we carried out a post-hoc analysis comparing patients taking enzyme-inducing and those taking non-enzyme inducing AEDs; 17 patients taking multiple AEDs were excluded from the analysis [35]. Patients taking non-enzyme inducing AEDs (e.g. Levetircetam) were more likely to be in the higher fatigue groups (Fisher’s p =0.036). We further explored patients taking Levetiracetam (N=24) and those taking other AEDs (N=12); a relationship approaching statistical significance was found between monotherapy AED and Levetircetam across fatigue groups (p=0.053). It is noted however, that these findings are underpowered and require further investigation.
VIII Sleep
Thirteen patients were excluded from the sleep analysis because no Actigraphy data was recorded on watches, leaving data for 72 patients. Actigraphy data obtained indicated fatigue was greater in patients with lower average percentage day-time activity (p = 0.035), although this was not reflected in percentage night-time sleep. Subjective reports of rest and activity using the sleep diary showed no evidence of a significant relationship with fatigue. No relationships were found with informant-reported night-time sleep difficulties. Investigating reports of daytime sleepiness using the ESS, patients with greater fatigue were more likely to self-report an abnormal frequency of falling asleep during the day (N=82, p=0.013). This relationship was not found in informant-reported scores of daytime sleepiness (N=49, p=0.734).
IX Predictors of fatigue
Exploratory univariate results provided evidence to suggest that depressive symptoms (OR=5.990; 95% CI (1.613, 22.242); p=0.008), anxiety (OR=3.177; 95% CI (1.235, 8.171); p=0.017) and neurological impairment using the EDSS (OR=2.333; 95% CI (1.465, 3.714); p=0.0004) scores were good predictors of fatigue severity. Full forward stepwise and backward stepwise multivariate logistic models were fitted using these three significant predictors to check the stability of the model. Using all three procedures the final regression model included neurological functioning, as assessed using the EDSS (OR=2.657; 95% CI (1.567, 4.506); p=0.0003) and anxiety, as assessed using the anxiety subscale of the HADS (OR=4.017; 95% CI (1.286, 12.543); p=0.017).
Discussion
Cancer fatigue is common in patients with systemic cancer (i.e. anorexia; cachexia and systemic diffuse cancer) [13]. Damage to the central nervous system also causes fatigue [7-10]. In this study, all patients had brain tumor without systemic spread, and most had surgical resection and radiotherapy. We aimed to determine the prevalence and predictors of fatigue in a clinically stable primary brain tumor population. Any identified predictors of fatigue would then help to ascertain the core assessments necessary in someone with fatigue, and subsequently potential treatment options, and could help understand whether a neurological model is more appropriate for understanding fatigue in this population [19].
I Neurological and Functional Impairment
We found patients with greater fatigue were more likely to have neurological impairments. Using regression analysis, neurological impairment remained a significant predictor of fatigue when accounting for other significant symptoms. Neurological impairment has been found to be associated with fatigue in MS [15, 17, 18, 36]. For example, in a survey of 507 consecutive patients affected by clinically definite MS, patients with severe disability were at a significantly higher risk of fatigue. Others have identified single items of EDSS that found fatigue to be associated with the occurrence of cerebellar, sphincter, pyramidal and sensory signs, but not cognitive impairment [36]. This highlights the need to report neurological impairment in this group of patients, which is not assessed in a cancer model of fatigue. This may also highlight patients that may benefit from interventions such as physiotherapy or exercise programs or anti-spasticity agents.
II Cognition
Interestingly, although many patients with fatigue complain of cognitive problems, we did not find any evidence of an association between fatigue and cognitive performance on most tasks, including visual and verbal memory, and executive function. This is consistent with previous research in the cancer population [37]. The only positive association was with a test of information processing speed. This might suggest that patients with greater fatigue are slower at processing information and perhaps this is why increased fatigue has been associated with increased self-reported cognitive impairment [2]. Our cognitive findings are consistent with previous research in patients with MS and patients with traumatic brain injury, which supports the evidence for a relationship between fatigue and processing speed, whilst other neuropsychological test performance was similar across all patients [38, 39].
III Mood
There is a consistent relationship between fatigue and mood disturbance in cancer, including brain tumor [2, 40, 41]. In keeping with this we found that fatigued patients were significantly more likely to have higher levels of both anxiety and depressive symptoms. Anxiety remained a significant predictor of fatigue in a regression analysis. It is possible that patients experiencing fatigue may develop feelings of worry and anxiety around their inability to function normally and carry out everyday activities. Alternatively, evidence from longitudinal research has found baseline anxiety to be associated with later cancer-related fatigue [29, 42]. These findings suggest the importance of anxiety assessment in all patients, with preliminary findings to suggest the investigation of psychological (e.g. CBT) or medical (e.g. anti-depressants) therapeutic options in patients reporting high levels of fatigue and mood difficulties.
IV Sleep
Patients with severe fatigue often perceive problems with lower subjective sleep quality and un-refreshed sleep [43, 44]. This was also found in this study. Many of these patients also reported symptoms of anxiety, which may have further influenced symptom reporting.
V Growth Hormone Deficiency
We found 63% of patients scored above the clinical cut-off score for growth hormone deficiency screening, and this was significantly related to fatigue. During the recruitment period 12 patients were reviewed by endocrinology and, notably, seven were identified as growth hormone deficient. Whilst offering preliminary findings, these results highlight the potential need to screen all highly fatigued brain tumor patients for growth hormone deficiency. Presently there is limited evidence regarding the prevalence of growth hormone deficiency in adults with a brain tumor. One study has found growth hormone deficiency in 31% of patients with tumors located outside the hypothalamic-pituitary axis, and all patients were also experiencing moderate to severe fatigue [10].
VI Anti-Epileptic Drug Use
We found a univariate relationship between fatigue and non-enzyme inducing AEDs. As most patients were taking Levetiracetam and given that many patients on Levetiracetam may experience fatigue, we carried out a post-hoc analysis exploring Levetiracetam compared with other AEDs [45-47]. We found a relationship between higher fatigue and Levetiracetam use. Further research exploring fatigue as a side effect of AEDs would help identify whether fatigue symptoms should be taken into account when selecting or managing medications.
VII Limitations
This study was built as an exploratory study, and by its nature (and small sample size) has low power. As such, no adjustment for multiple comparisons has been made and findings need to be confirmed in any future study. Selection bias is also a possibility. One reason patient gave for not participating in the study was because they were not experiencing fatigue. However, we attempted to reduce hospital attender bias by offering both home visits and telephone interviews.
The cross-sectional design and presence of symptom clustering make causal directions difficult to interpret. Our results are best viewed as tentative and should be prospectively confirmed. The prevalence of missing data is also noted in relation to the possible impact on statistical analyses. Future research may help to prevent this by reducing the numbers of measures in order to complete data collection at one time.
Conclusions
This exploratory study highlights the role of physical impairment and symptoms of depression as significant predictors of fatigue severity. Multiple regression revealed that both depression and disability were significant predictors of fatigue, together accounting for approximately 23% of the variance in patients' self-reported fatigue measure. These predictors have also been consistently found in other neurological disorders, specifically in MS literature. Kroencke found that fatigue and disability were correlated in patients with different severities of MS (r=0.33) [17]. Bol found fatigue was associated with depression and physical disability and that physical disability is associated with disease severity and fatigue-related fear and avoidance behaviour in MS [15]. Schreurs found relationships between physical and mental fatigue, and reduced activity and motivation with depression and physical disabilities that were established cross-sectionally by regression analyses and longitudinally by structural equation modelling [18]. Patient-reported “physical fatigue” was related with physical disabilities, and “mental fatigue” was associated with depression [19]. This may suggest that patients with neurological impairment/disability may be more, or less, responsive to treatments that involve physiotherapy/exercise than those without neurological impairment/disability and further work is needed to explore this. Those with mood difficulties may derive more benefit from talking therapy or antidepressants. A “neurological model” of fatigue that evaluates neurological disability may be more appropriate than a cancer model in future studies of fatigue in glioma.
The complexity in assessing contributing factors of fatigue may provide some explanation as to the lack of evidence for the single modality treatments of fatigue, such as modafinil in the brain tumor population in a recent Cochrane review. This study emphasises the need for a holistic assessment and targeted but multifaceted management of anxiety, depression and physical disability may be more effective. Overall, the use of neurological physical outcomes in addition to subjective patient measures and relative reports, adds to our understanding of potential interventions. Future research will further define the accuracy and reliability of this approach.
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the NHS Scotland Research Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Acknowledgements
With thanks to Stevie Williams for his technical assistance in the set-up and processing of the Actiwatch data, and to the Edinburgh Lothian Health Foundation for helping fund the project.
Funding
The study was funded in part by a grant from the Edinburgh Lothian Health Foundation.
Disclosures
There are no disclosures relevant to this manuscript.
Statistical analysis completed by: Tuck S (academic affiliation).
Article Info
Article Type
Research ArticlePublication history
Received: Thu 01, Aug 2019Accepted: Mon 26, Aug 2019
Published: Wed 20, Nov 2019
Copyright
© 2023 Julia Day. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2019.03.04
Author Info
Rooney A Berntzen B Erridge S Gillespie D Grant R Julia Day Peoples S Tuck S
Corresponding Author
Julia DayDepartment of Clinical Neurosciences, Western General Hospital, Edinburgh
Figures & Tables
Table 1: Demographic Characteristics and Clinical Characteristics of the Patients (N (%))
|
|
Fatigue Group |
p |
||
|
None/Mild (N=28) |
Moderate (N=41) |
Severe (N=16) |
||
|
Demographic Characteristics |
||||
|
Age (years)* |
46.9 (12.1) |
48.3 (9.6) |
49.8 (13.3) |
0.718 |
|
Female |
14 (50) |
21 (51) |
8 (50) |
0.994 |
|
Education (years)* |
13.8 (3.2) |
13.3 (3.4) |
12.9 (2.4) |
0.623 |
|
FSIQ |
105.3 (13.4) |
103.6 (12.9) (N=39) |
101.4 (13.5) (N=15) |
0.647 |
|
Married |
20 (71) |
23 (56) |
12 (75) |
0.269 |
|
Clinical Characteristics |
||||
|
WHO Grade 1 |
1 (4) |
3 (7) |
5 (31) |
0.151 |
|
WHO Grade 2 |
11 (39) |
24 (59) |
3 (19) |
|
|
WHO Grade 3 |
8 (29) |
8 (20) |
4 (25) |
|
|
WHO Grade 4 |
8 (29) |
6 (15) |
4 (25) |
|
|
Tumor Side – Left |
10 (36) |
15 (37) |
4 (25) |
0.840 |
|
Tumor Side – Right |
13 (46) |
18 (44) |
10 (63) |
|
|
Tumor Side - Both |
5 (18) |
8 (20) |
2 (13) |
|
|
Radiotherapy |
23 (82) |
29 (71) |
14 (88) |
0.309 |
|
Chemotherapy |
8 (29) |
13 (32) |
8 (50) |
0.319 |
|
Years since diagnosis** |
5 (2.0-10.5) |
6 (2.0-14.0) |
8 (5.5-16.5) |
0.410 |
|
Years since treatment** |
4.1 (1.5-6.0) |
4.5 (2.0-11.9) |
5.7 (2.0-11.0) |
0.653 |
|
Anti-epileptic drug use |
||||
|
Current use |
16 (57) |
28 (68) |
9 (56) |
0.551 |
|
Other medications |
||||
|
Steroid |
1 (4) |
5 (12) |
3 (9) |
0.265 |
|
Anti-depressant |
2 (7) |
3 (7) |
5 (31) |
0.052 |
|
Statin |
3 (11) |
5 (12) |
2 (13) |
0.999 |
|
Diabetes |
1 (4) |
5 (12) |
2 (13) |
0.540 |
|
Anti-anxiety |
2 (7) |
3 (7) |
1 (6) |
0.999 |
|
Blood pressure |
2 (7) |
4 (10) |
2 (13) |
0.890 |
Abbreviations: FSIQ Full Scale IQ estimated from the Test of Premorbid Functioning
*mean (SD), **median (interquartile range)
Table 2: Percentage of patients classified as impaired from physical tests
|
Test |
|
BFI Group N/Total N (%) |
p-value |
||
|
N |
None-Mild |
Moderate |
Severe |
||
|
EDSSa* |
84 |
1.8 (0.0-2.0) N=28 |
2.0 (2.0-3.0) N=40 |
3.5 (2.5-6.3) N=16 |
<0.0001 |
|
NHPTb |
79 |
4/28 (14%) |
14/38 (37%) |
7/13 (54%) |
0.026 |
|
TMWTb |
81 |
15/28 (54%) |
27/39 (69%) |
14 (100%) |
0.009 |
aHigher score = better performance bHigher score = poorer performance
Abbreviations: EDSSKurtzke Expanded Disability Status Scale; NHPT Nine Hole Peg Test; TMWT Ten Metre Walk Test.
*Continuous – median (interquartile range); Kruskal-Wallis Test
Table 3: Percentage of patients classified as impaired from cognitive tests
|
Test |
|
Fatigue Group N/Total N (%) |
p-value |
||
|
N |
None-Mild |
Moderate |
Severe |
||
|
HVLT-R |
81 |
|
|
|
|
|
Immediate Recalla |
|
11/26 (42%) |
25/39 (64%) |
9/16 (56%) |
0.223 |
|
Delayed Recalla |
|
9/26 (35%) |
18/39 (46%) |
7/16 (44%) |
0.645 |
|
Retention %a |
|
6/25 (24%) |
20/39 (51%) |
6/16 (38%) |
0.092 |
|
Delayed Recognitiona |
|
2/25 (8%) |
9/39 (23%) |
5/16 (31%) |
0.154 |
|
MAE COWATa |
80 |
7/26 (27%) |
13/39 (33%) |
9/16 (56%) |
0.142 |
|
TMT Ab |
81 |
3/28 (11%) |
11/38 (29%) |
10/15 (67%) |
0.0006 |
|
TMT Bb |
81 |
9/28 (32%) |
17/38 (45%) |
8/15 (53%) |
0.363 |
|
WDRTb |
79 |
13/27 (48%) |
13/37 (35%) |
4/15 (27%) |
0.345 |
aHigher score = better performance bHigher score = poorer performance
Abbreviations: HVLT-R Hopkins Verbal Learning Test-Revised; MAE COWAT Multilingual Aphasia Examination-Controlled Oral Word Association Test, TMT Trail making test, WDRT William’s Delayed Recall Test
|
Measure |
Domain |
|
Hopkins Verbal Learning Test-Revised (HVLT-R) [23] |
Verbal memory |
|
Williams Delayed Recall Test (WDRT) [22] |
Visual memory |
|
Controlled Oral Word Association Test (COWAT) [24] |
Verbal fluency |
|
Trail Making Test Part A(TMT-A) [25] |
Information processing speed |
|
Trail Making Test Part B(TMT-B) [25] |
Executive function |
|
Test of Premorbid Functioning (TOPF) [26] |
Estimated premorbid intelligence |
|
Cognitive Failures Questionnaire (CFQ) [27] |
Subjective cognitive impairment (completed by relatives) |
a10 patients did not complete a 1st appointment: two did not attend the appointment and did not want to reschedule; two cancelled the appointment due to health (n=2) or travel (n=1) reasons; five patients did not know their availability at the time of recruitment and requested a phone call to arrange an appointment time, but could not be reached via the contact details provided.
b10 patients missed their second appointment due to a work commitment (n=3), were unable to make travel arrangements (n=2), their symptoms were too bad (n=4) or were no longer interested (n=1).
References
- Aprile I, Chiesa S, Padua L, Di Blasi C, Arezzo MF et al. (2015) Occurrence and predictors of the fatigue in high-grade glioma patients. Neurol Sci 36: 1363-1369. [Crossref]
- Fox SW, Lyon D, Farace E (2007) Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh 39: 61-67. [Crossref]
- Lovely MP, Miaskowski C, Dodd M (1999) Relationship between fatigue and quality of life in patients with glioblastoma multiformae. Oncol Nurs Forum 26: 921-925. [Crossref]
- Pelletier G, Verhoef MJ, Khatri N (2002) Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol 57: 41-49. [Crossref]
- Van Coevorden-van Loon EMP, Coomans MB, Heijenbrok-Kal MH, Ribbers GM, van den Bent MJ (2017) Fatigue in patients with low grade glioma: systematic evaluation of assessment and prevalence. J Neurooncol 133: 237-246. [Crossref]
- Campos MP, Hassan BJ, Riechelmann R, Del Giglio A et al. (2011) Cancer-related fatigue: a practical review. Ann Oncol 22: 1273-1279. [Crossref]
- Powell C, Guerrero D, Sardell S, Cumins S, Wharram B et al. (2011) Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumors: a prospective study. Radiother Oncol 100: 131-136. [Crossref]
- Hinds PS, Hockenberry MJ, Gattuso JS, Srivastava DK, Tong X et al. (2007) Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer 110: 2321-2330. [Crossref]
- Struik K, Klein M, Heimans JJ, Gielissen MF, Bleijenberg G et al. (2009) Fatigue in low-grade glioma. J Neurooncol 92: 73-78. [Crossref]
- Taphoorn MJ, Heimans JJ, van der Veen EA, Karim AB et al. (1995) Endocrine functions in long-term survivors of low-grade supratentorial glioma treated with radiation therapy. J Neurooncol 25: 97-102. [Crossref]
- Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH et al. (2010) Risk factors for fatigue severity in primary brain tumor patients. Cancer 116: 2707-2715. [Crossref]
- Armstrong TS, Vera-Bolanos E, Acquaye AA, Gilbert MR, Ladha H et al. (2016) The symptom burden of primary brain tumors: evidence for a core set of tumor- and treatment-related symptoms. Neuro Oncol 18: 252-60. [Crossref]
- NCCN: Cancer-related fatigue (2014) NCCN Clinical Practice Guidelines in Oncology.
- Hanken K, Eling P, Hildebrandt H (2014) The representation of inflammatory signals in the brain–a model for subjective fatigue in multiple sclerosis. Front Neurol 5: 264. [Crossref]
- Bol Y, Duits AA, Lousberg R, Hupperts RM, Lacroix MH et al. (2010) Fatigue and physical disability in patients with multiple sclerosis: a structural equation modeling approach. J Behav Med 33: 355-363. [Crossref]
- Duncan F, Greig C, Lewis S, Dennis M, MacLullich A et al. (2014) Clinically significant fatigue after stroke: a longitudinal cohort study. J Psychosom Res 77: 368-373. [Crossref]
- Kroencke DC, Lynch SG, Denney DR (2000) Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler 6: 131-136. [Crossref]
- Schreurs KM, de Ridder DT, Bensing JM (2002) Fatigue in multiple sclerosis: reciprocal relationships with physical disabilities and depression. J Psychosom Res 53: 775-781. [Crossref]
- Grant R, Brown PD (2016) Fatigue in randomised controlled trials: How tired is "too tired" in patients undergoing glioma treatment? Neuro Oncol 18: 759-760. [Crossref]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA et al. (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85: 1186-1196. [Crossref]
- Kurtzke JF (1983) Rating Neurological Impairment in Multiple Sclerosis: an expanded disability status scale. Neurology 33: 1444-1452. [Crossref]
- Clyde Z, Chataway SJ, Signorini D, Gregor A, Grant R et al. (1998) Significant change in tests of neurological impairment in patients with brain tumors. J Neurooncol 39: 81-90. [Crossref]
- Benedict RHB, Schretlen D, Groninger L, Brandt J (1998) Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 12: 43-55. [Crossref]
- Ruff RM, Light RH, Parker SB, Levin HS (1996) Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol 11: 329-338. [Crossref]
- Arnett JA, Labovitz SS (1995) Effect of physical layout in performance of the Trails Making Test. Psychol Assessment 7: 220-221.
- Blair JR, Spreen O (1989) Predicting premorbid IQ: A revision of the national adult reading test. Clin Neuropsychol 3: 129-136.
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR (1982) The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol 21: 1-16. [Crossref]
- Zigmond AS, Snaith RP (1983) The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 67: 361-370. [Crossref]
- Rooney AG, Carson A, Grant R (2011) Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst 103: 61-76. [Crossref]
- Actiware: Actiwatch Spectrum. Bend, OR, Philips Respironics.
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC et al. (1992) Automatic sleep/wake identification from wrist activity. Sleep 15: 461-469. [Crossref]
- Jean-Louis G, Kripke DF, Cole RJ, Assmus JD, Langer RD et al. (2001) Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav 72: 21-28. [Crossref]
- Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14: 540-545. [Crossref]
- Holmes SJ, McKenna SP, Dorward LC (1995) Development of a questionnaire to assess the quality of life of adults with growth hormone deficiency. Qualit Life Res 4: 420-421.
- Day J, Yust-Katz S, Cachia D, Wefel J, Katz LH et al. (2016) Interventions for the management of fatigue in adults with a primary brain tumor. Cochrane Database Syst Rev 4: CD011376. [Crossref]
- Colosimo C, Millefiorini E, Grasso MG, Vinci F, Fiorelli M et al. (1995) Fatigue in MS is associated with specific clinical features. Acta Neurol Scand 92: 353-355. [Crossref]
- Tchen N, Juffs HG, Downie FP, Yi QL, Hu H et al. (2003) Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol 21: 4175-183. [Crossref]
- Andreasen AK, Spliid PE, Andersen H, Jakobsen J (2010) Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol 17: 212-218. [Crossref]
- Ben Ari Shevil E, Johansson S, Ytterberg C, Bergström J, von Koch L et al. (2014) How are cognitive impairment, fatigue and signs of depression related to participation in daily life among persons with multiple sclerosis? Disabil Rehabil 36: 2012-2018. [Crossref]
- (2017) NCCN Clinical Practice Guidelines in Oncology Cancer-related fatigue.
- Kessels E, Husson O, van der Feltz-Cornelis CM (2018) The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat 14: 479-494. [Crossref]
- Weber D, O’Brien K (2017) Cancer and Cancer-Related Fatigue and the Interrelationships with Depression, Stress, and Inflammation. J Evidence based Complement Alter Med 22: 502-512.
- Zarogoulidis P, Steiropoulos P, Perantoni E, Archontogeorgis K, Eleftheriadou Eet al. (2013) Subjective sleep quality in lung cancer patients before and after chemotherapy. Thorac Cancer 4: 138-142. [Crossref]
- Liu L, Rissling M, Natarajan L, Fiorentino L, Mills PJ et al. The Longitudinal Relationship between Fatigue and Sleep in Breast Cancer Patients Undergoing Chemotherapy. Sleep 35: 237-245. [Crossref]
- Siniscalchi A, Gallelli L, Russo E, De Sarro G (2013) A review on antiepileptic drugs-dependent fatigue: pathophysiological mechanisms and incidence. Eur J Pharmacol 718: 10-16. [Crossref]
- Day J, Ingle H, Tuck S (2015) Consider Levetiracetam as a potentially reversible cause of significant fatigue in patients with a stable primary brain tumor. Psychooncology 24: 8.
- Armstrong TS, Grant R, Gilbert MR, Lee JW, Norden AD (2016) Epilepsy in glioma patients: mechanisms, management, and impact of anticonvulsant therapy. Neuro Oncol 18: 779-789. [Crossref]
