Extraskeletal Osteosarcoma in the Thigh Recognized Following Acute Traumatic Injury: A Case Report and Review of the Literature
A B S T R A C T
Extraskeletal osteosarcoma is a rare mesenchymal malignancy arising in soft tissue that produces osteoid, neoplastic bone, or chondroid material without demonstrable attachments to bone or periosteum. We report an instructive case of extraskeletal osteosarcoma in a 42-year-old woman recognized following acute traumatic injury of her left thigh.
Keywords
Extraskeletal osteosarcoma, soft tissue, traumatic injury, thigh
Introduction
Extraskeletal osteosarcoma (ESOS) is a rare malignant mesenchymal neoplasm that produces osteoid, located in the soft tissues without skeletal or periosteal attachment [1-5]. Its most common location is the soft tissues of the thigh (46%), followed by the upper extremity (20%) and the retroperitoneum (17%); but can occur in any part of the body [3, 6-11]. Almost 700 cases of ESOS have been reported in English literature; and a history of prior trauma is present in 12.5% of patients [1, 8, 12, 13]. Here, we report a 42-year-old woman who presented with clinical, imaging and pathological features of osteoblastic extraskeletal osteosarcoma after a 3-month-episode of trauma of her left thigh.
Case Report
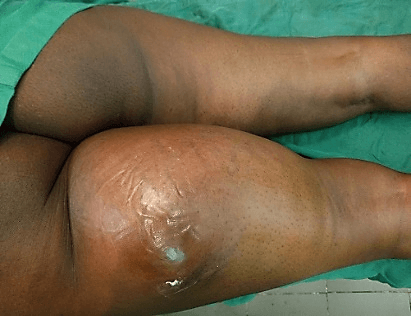
A 42-year-old woman, previously healthy, was initially treated in a private Clinic for a 1-month-trauma to her proximal left thigh (pedestrian stuck by a motorcycle while crossing the road). Her evaluation was suspicious for posttraumatic hematoma and was asked to return for follow up if symptoms were not improved. She returned 3 months later with a painless, rapidly enlarging mass at the posterior surface of the proximal left thigh. Due to this unusual presentation, she was thereafter transferred to our Clinic. On her arrival, she was healthy appearing with left limping and right-sided sitting position. There was a non-tender, well-circumscribed, fixed, elastic-firm soft tissue mass measuring 30 cm x 24 cm and adhering to the deep fascia with shiny skin arising from the posterior surface of the left thigh up to the gluteal fold. The diameter of the thigh at the top of the mass (5 cm from the distal gluteal fold) was 80 cm opposed to 62 cm on the contralateral thigh (Figure 1). The left knee was free with the range of motion (ROM) of 0/0/130°; but the flexion of the left hip was limited to 100°. The rest of the clinical examination was unremarkable.
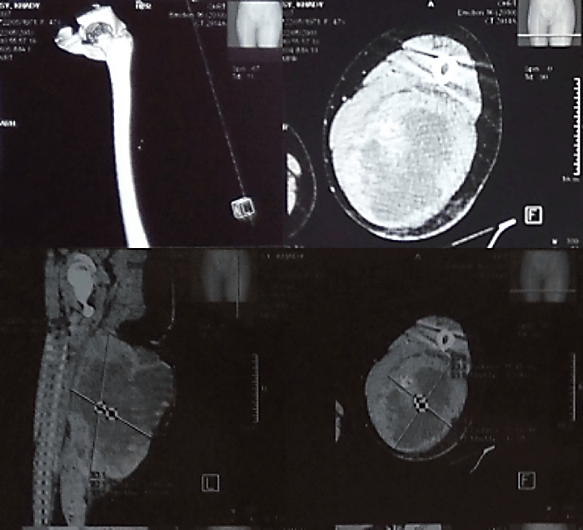
Plain radiographs of the left thigh showed a large deep-seated soft tissue mass with marked calcifications in a radial pattern and without apparent bone involvement. The complementary Computed Tomography (CT) scan revealed a significant mixed solid and cystic mass within the muscles of the posterior compartment of the left thigh with multiple nodules and septations. The mass measured 22 cm x 15 cm x 14 cm with necrotic centers (35 per 10 high-power fields), and was vascularized by a branch of the left deep femoral artery. No skeletal involvement was visualized (Figure 2).
Figure 1: Clinical aspect of the tumor after 3 months of onset. It was a well-circumscribed, fixed, elastic-firm soft tissue mass adhering to the deep fascia with shiny skin and arising from the posterior surface of the left thigh up to the gluteal fold. It measured 30 cm x 24 cm.
Figure 2: CT scan of the left thigh showing a significant deep-seated mixed solid and cystic mass within the muscle of the posterior compartment measuring 22 cm x 15 cm x 14 cm. There are tiny focal areas of ossification, which correspond with osteoid produced by the tumor and metallic foreign bodies are from prior trauma. No skeletal involvement was visualized.
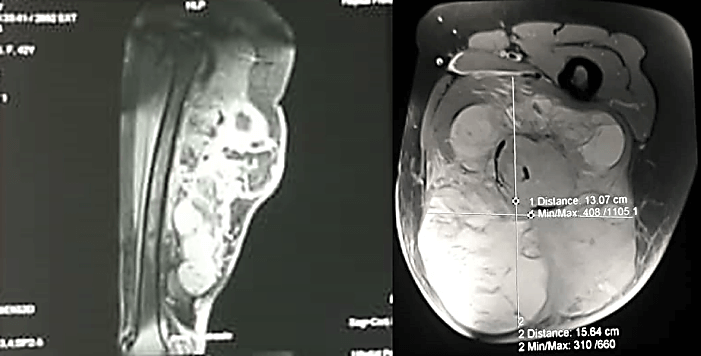
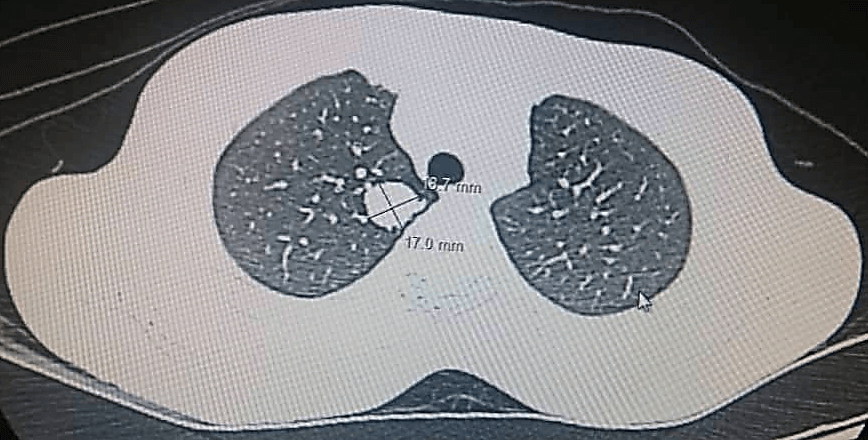
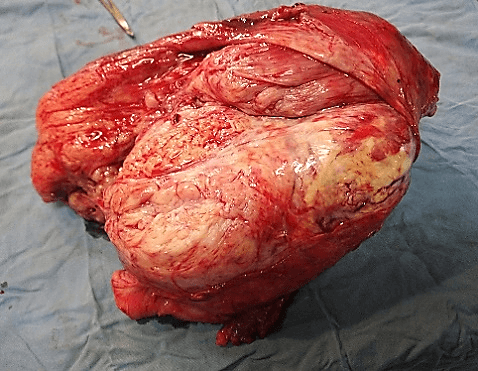
She underwent Magnetic Resonance Imaging (MRI) which showed a large marginated, multilobulated, heterogeneous mass of mixed intensity signal occupying the posterior compartment of the thigh measuring 15.64 cm x 13.07 cm axially and 26 cm x 11 cm coronally. It was a burgeoning mass with necrotic, fleshy and hemorrhagic components. There was no bone contact nor intraosseous invasion (Figure 3). Furthermore, her chest CT scan revealed the development of multiple large pulmonary masses, consistent with ‘cannonball’ metastasis. The largest was located in the right lung and measured 16.7 x 17.0 cm (Figure 4). Due to these findings, the consent was made with the patient to undergo wide margin surgical resection of the mass with subsequent pathology and immunohistochemistry analysis. Pathology results showed on gross pathology; tissue fragments of whitish appearance and fibrous locally myxoid (Figure 5).
Figure 3: Sagittal and axial T2-weighted MRI showing a large marginated, multilobulated, heterogeneous mass of mixed intensity signal occupying the posterior compartment of the thigh measuring axially 15.64 cm x 13.07 cm and coronally 26 cm x 11 cm.
Figure 4: Chest CT scan showing the development of multiple pulmonary masses with the largest measuring 16.7 x 17.0 in the right lung, consistent with ‘cannonball’ metastasis.
Figure 5: Gross surgical specimen cut section.
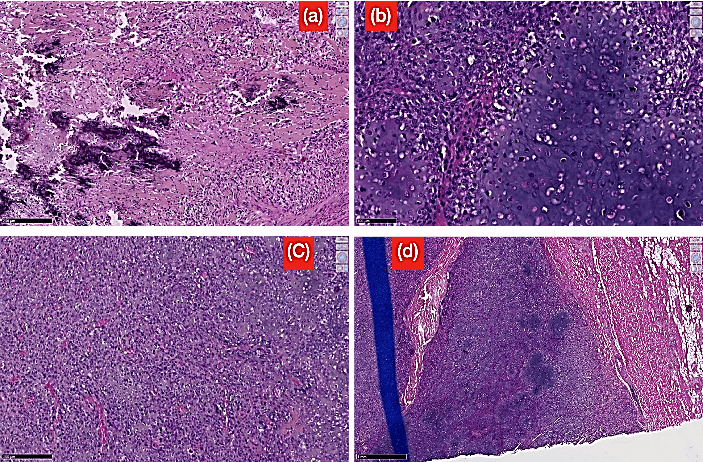
The micro-pathology found anaplastic spindle cell proliferation, cytological atypia, malignant chondroid areas, extensive areas of necrosis, mitotic activity and atypical mitotic figures with the presence of osteoid matrix formed by the neoplastic cells (Figure 6). Immunostaining for S100 protein was negative. The pathology results of the excision were significant for osteoblastic osteosarcoma. In the absence of bony involvement on imaging, we concluded to the diagnosis of osteoblastic extraskeletal osteosarcoma. Chemotherapy has been recommended; and the pre-therapeutic assessment has been carried out. The established chemotherapy protocol was a multiagent chemotherapy comprising Doxorubicin, Methotrexate and Cisplatin. The first treatment was done at third month (M3) postoperatively. The follow up was marked by a local recurrence with a budding wound at the surgical site after 45 days (D45) post excision. The patient died at the ninth month (M9) after the onset of the first symptoms which correspond to the fifth month (M5) post diagnosis of extraskeletal osteosarcoma.
Figure 6: Different high power histological sections showing: a) Pleomorphic malignant cells with extracellular matrix production (stain, hematoxylin and eosin; original magnification, 200x); b) Anaplastic spindle cell proliferation with the presence of osteoid matrix formed by the neoplastic cells; c) Polyhedral cells with cytological atypia, malignant chondroid areas, extensive areas of necrosis, mitotic activity and atypical mitotic figures; d) The tumor infiltrating the muscle.
Discussion
Extraskeletal osteosarcoma (ESOS) is a high-grade malignant mesenchymal soft tissue neoplasm composed of neoplastic cells (osteoblastic, chondroblastic and fibroblastic) that produce osteoid, neoplastic bone or chondroid matrix and has a clinically aggressive course [3, 5, 14, 15]. For a lesion to be defined as extraskeletal osteosarcoma, it must arise in the soft tissue and not attached to bone or periosteum, have a uniform sarcomatous pattern, and produce osteoid and/or cartilage matrix [5, 16-19]. Since it was originally described by Wilson in 1941 and further defined by Allan in 1971, less than 700 cases have been reported in the current modern English literature and this underlines its rarity [1, 3, 12, 13]. ESOS accounts for 1% of all soft tissue sarcomas and 4% of osteogenic osteosarcomas [3, 7, 18, 20, 21].
Known predisposing factors for development of ESOS include middle aged or elderly patients, involvement of lower extremities, a history of radiation and a controversial link to traumatic events [2-13]. ESOS is a tumor primarily of older age (mean age: 47.5 to 61 years) and male predominance (male to female ratio: 1.9:1) [2, 5, 7-22]. In a large series treated at a single institution, Wang et al. have found that 63% patients with ESOS were over 50 years [15]. However, pediatric patients have also been reported, for example, in the soft thigh tissue of a 6-year-old boy and a high-grade ESOS in the breast of a 16-year-old female [18, 23, 24]. Most extraskeletal osteosarcomas are deep seated; <10% are superficial, originating in the dermis or subcutis. The most common location is the lower extremity (75%; thigh and buttock), followed by the upper extremity (15-23%, shoulder girdle), and the retroperitoneum (17%); but can occur in any part of the body [3, 6-11]. Approximately 4-13% of reported cases occur secondary to radiation therapy, with the therapy taking place 2-40 years prior to presentation [6, 8-25]. Multiple cases of ESOS occurrences have been recognized after traumatic events. The time frame from trauma to diagnosis ranged from 10 months to 12 years and others diagnosed 25 and 36 years after the inciting traumatic event [1, 12, 21, 26, 27]. In our case, we have a 42-year-old woman (middle aged) with a deep seated painless tumor, episode of recent trauma (pedestrian stuck) and involvement of lower extremity (posterior surface of the left proximal thigh). No history of radiation exposure. In reported traumatic cases, the questionably development of ESOS has been primarily seen years later, which is in stark contrast to our patient’s rapid development in only 3 months.
Symptoms usually include a slowly growing painless mass in an extremity [5, 6-20]. In trauma patients, these symptoms often mask the ESOS diagnosis and are assumed to be hematoma or other traumatic diagnosis [21, 26, 27]. Radiographs may show a soft tissue mass with variable amounts of mineralization, and a lack of skeletal involvement. No calcification is seen in approximately 50% of lesions [12]. CT scans demonstrate a soft tissue mass often displacing fat planes, with variable degrees of calcification/ossification. MRI shows a soft tissue mass with the non-mineralized areas showing nonspecific intermediate to low signal intensity on T1 weighted images, and high signal intensity on T2 weighted images reflecting tumor edema. Foci of hemorrhage and necrosis are also common. The tumors often appear well circumscribed and pseudoencapsulated, or infiltrative into the surrounding soft tissues. Extraskeletal osteosarcomas are usually large at diagnosis, typically averaging 9 cm in diameter. Microscopically, by definition, they display at least some osteoid and often there are variable amounts of bone or cartilage, often blending into a high-grade sarcomatous stroma [1, 6-21]. All these features were found in our patient and the tumor was osteoblastic extraskeletal osteosarcoma in nature measuring 30 cm x 24 cm in diameters.
Wide margin surgical resection is the mainstay of treatment for extraskeletal osteosarcoma [4, 5-28]. Resection can be with amputation of limb salvage surgery if microscopically negative margins can be achieved [29, 30]. Treatment decisions regarding adjuvant chemotherapy or radiation therapy for ESOS should be individualized, based on the assessment of relative risk factors. However, the effectiveness of radiation and chemotherapy remains uncertain owing to conflicting results in previous reports [31]. More recent groups that have used multiagent chemotherapy and wide resection during surgical procedures have described overall survival rates of 66 to 77%, similar to skeletal osteosarcoma and there was a trend towards increased length of survival in patients who received chemotherapy compared to those who did not (16.4 months versus 9.3 months, p=0.16) [14-22]. Given the high grade malignant tumor and poor prognosis, aggressive treatment with preoperative radiation therapy and surgical resection for local tumor control, and (neo-) adjuvant multiagent chemotherapy to improve survival are current treatments of choice for the patients with extraskeletal osteosarcomas. Aggressive thoracotomy and resection of the pulmonary metastases may be necessary in patients with lung metastases. Our patient underwent wide margin surgical resection of the tumor and multiagent adjuvant chemotherapy (Doxorubicin, Methotrexate and Cisplatin).
The prognosis of the patients with extraskeletal osteosarcoma is poor; the 5-year survival ranges from 10-46%. More than 50% of the patients experience multiple local recurrences with wound-healing complications and distal metastases. Distal metastases are usually to the lungs (>80%), followed by the regional lymph nodes (4-29%), bone (8-19%), adrenal gland (<5%), brain, liver and skin [3, 5, 15-18]. Reported adverse prognostic factors include metastatic disease at presentation, large tumor size, and inability to achieve complete surgical resection [22]. Tumor size has also been reported as an important prognostic factor. In the study of Bane et al., comparing patients with a tumor size of <5 cm to size >5 cm, tumor size was an important predictive value of patient survival (p < 0.001). Only one tumor related death occurred in the <5 cm group out of 7 patients, whereas in comparison to 14 deaths in a >5 cm population of 16, at time of study production [6]. To add further evidence to the significance of tumor size in ESOS survival, Terence T. Sio et al. compared tumor size, with their variable of study being 10 cm. According to their published data, a 5-year-survival rate of 76% was appreciated in patients with a tumor size <10 cm, in comparison to 36% 5-year-survival rate in those patients with tumor size of >10 cm (p=0.002) [18]. Our patient had a significantly large tumor, measuring 30 cm x 24 cm at time of surgical excision. She had bilateral lung metastasis. The follow up was marked by a local recurrence with a budding wound at the surgical site after 45 days post-surgery, and unfortunately died 5 months after the diagnosis of extraskeletal osteosarcoma was made despite multiagent neo-adjuvant chemotherapy.
Conclusion
Through extensive review of the modern English literature, there is no question that extraskeletal osteosarcoma is a rare medical entity. Easy misinterpretation of what appears to be obvious traumatic injury, can lead to delays in accurate diagnosis and appropriate management of ESOS. Its diagnosis is made by a thoroughly clinical examination and confirmed by both imaging and pathology. Wide margin surgical resection is the mainstay of treatment. Adjuvant chemotherapy and/or preoperative radiation therapy may be useful. The case presented here displays particular attention to clinicians to regularly practice strict follow up visits after traumatic events to the lower extremities in middle aged and elderly patients.
Ethical Consideration
Written informed consent was obtained from the patient’s husband, given her death during production for publication of this paper. Ethical approval to report this case was obtained from the Ethical Board of Tamba Counda Hospital, Senegal on 26th November 2018.
Conflicts of Interest
None.
Author Contributions
Contributors to conception, design, acquisition and interpretation of data: ASS, SN. Manuscript writing and drafting: JMVH. Revising it critically for important intellectual content: JMVH, ASS. Final approval of the version to be published: JMVH, ASS, SN, JCS.
Funding
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Mon 09, Nov 2020Accepted: Wed 09, Dec 2020
Published: Thu 24, Dec 2020
Copyright
© 2023 Hope Jean Marie Vianney. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COCB.2020.02.03
Author Info
Aboubacar S. Sangaré Hope Jean Marie Vianney Serge Ntungwanayo J. Claude Sane
Corresponding Author
Hope Jean Marie VianneyDepartment of Orthopedics and Traumatology, Rwanda Military Hospital, Kigali, Rwanda
Figures & Tables
References
- Allan CJ, Soule EH (1971) Osteogenic sarcoma of the somatic soft tissues. Clinicopathologic study of 26 cases and review of literature. Cancer 27: 1121-1133. [Crossref]
- Curfman KR, Morrissey SL (2019) Extraskeletal Osteosarcoma Recognized following Acute Traumatic Injury. Case Rep Oncol 12: 282-288. [Crossref]
- Longhi A, Bielack SS, Grimer R, Whelan J, Windhager R et al. (2017) Extraskeletal osteosarcoma: A European Musculoskeletal Oncology Society study on 266 patients. Eur J Cancer 74: 9-16. [Crossref]
- Matsuo T, Shimose S, Kubo T, Mikami Y, Arihiro K et al. (2009) Extraskeletal Osteosarcoma with Partial Spontaneous Regression. Anticancer Res 29: 5197-5202. [Crossref]
- Mavrogenis AF, Papagelopoulos PJ (2014) Soft Tissue Tumours: Extraskeletal osteosarcoma. Atlas Genet Cytogenet Oncol Haematol 18: 443-446.
- Bane BL, Evans HL, Ro JY, Carrasco CH, Grignon DJ et al. (1990) Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer 65: 2762-2770. [Crossref]
- Choi LE, Healey JH, Kuk D, Brennan MF (2014) Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am 96: e2. [Crossref]
- Chung EB, Enzinger FM (1987) Extraskeletal osteosarcoma. Cancer 60: 1132-1142. [Crossref]
- Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ et al. (1995) A review of 40 patients with extraskeletal osteosarcoma. Cancer 76: 2253-2259. [Crossref]
- Nakamura T, Matsumine A, Nishimura K, Yokoyama H, Murata T et al. (2011) Extraskeletal subcutaneous osteosarcoma of the upper arm: A case report. Oncol Lett 2: 75-77. [Crossref]
- Narayanan ST, Gopalakrishnan MK, Ibrahim SS, Sanka R (2016) Extraskeletal Osteosarcoma- A Case Report. J Clin Diagn Res 10: ED03-ED04. [Crossref]
- Hoch M, Ali S, Agrawal S, Wang C, Khurana JS (2013) Extraskeletal osteosarcoma: a case report and review of the literature. J Radiol Case Rep 7: 15-23. [Crossref]
- Kajihara M, Sugawara Y, Hirata M, Kikuchi K, Miki H et al. (2005) Extraskeletal osteosarcoma in the thigh: a case report. Radiat Med 23: 142-146. [Crossref]
- Nystrom LM, Reimer NB, Reith JD, Scarborough MT, Gibbs Jr CP (2016) The treatment and outcome of extraskeletal osteosarcoma: Institutional experience and review of the literature. Iowa Orthop J 36: 98-103. [Crossref]
- Wang H, Miao R, Jacobson A, Harmon D, Choy E et al. (2018) Extraskeletal osteosarcoma: A large series treated at a single institution. Rare Tumors 10: 1-7. [Crossref]
- Fabbri N, Tiwari A, Umer M (2010) Extraskeletal Osteosarcoma: Clinicopathologic Features and Results of Multimodal Management. J Clin Oncol 28: 1-32.
- Mc Auley G, Jagannathan J, O’Regan K, Krajewski KM, Hornick JL et al. (2012) Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol 2012: W31-W37. [Crossref]
- Sio TT, Vu CC, Sohawon S, Houtte PV, Thariat J et al. (2016) Extraskeletal Osteosarcoma: An International Rare Cancer Network Study. Am J Clin Oncol 39: 32-36. [Crossref]
- Torigoe T, Yazawa Y, Takagi T, Terakado A, Kurosawa H (2007) Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci 12: 424 429. [Crossref]
- Lateef F, Kazi JI, Jamal S (2011) Extraskeletal Osteosarcoma; case report. J Coll Phys Surg Pak 21: 429-430.
- Sordillo P, Hajdu S, Magill G, Golbey RB (1983) Extraosseous osteogenic sarcoma: a review of 48 patients. Cancer 51: 727-734. [Crossref]
- Thampi S, Matthay KK, Boscardin WJ, Goldsby R, DuBois SG (2014) Clinical Features and Outcomes Differ between Skeletal and Extraskeletal Osteosarcoma. Sarcoma 1-9.
- Liu ZJ, Zhao Q, Zhang LJ (2010) Extraskeletal osteosarcoma near the hip: a pediatric case. J Pediatr Orthop B 19: 524-528. [Crossref]
- Zils K, Ebner F, Ott M, et al. (2012) Extraskeletal osteosarcoma of the breast in an adolescent girl. J Pediatr Hematol Oncol 34: e261-e263. [Crossref]
- Laskin WB, Silverman TA, Enzinger FM (1988) Postradiation soft tissue sarcomas: an analysis of 53 cases. Cancer 62: 2330-2340. [Crossref]
- Nagano A, Ohno T, Nishimoto Y, Yamada K, Shimizu K (2009) Extraskeletal osteosarcoma of the thigh: an autopsy case report. Sarcoma 2009: 186565. [Crossref]
- Sood N, Rewri S, Nigam JS (2014) Small cell extraskeletal osteosarcoma: a rare case report. Rare Tumours 6: 5029. [Crossref]
- Paludo J, Fritchie K, Haddox CL, Rose PS, Arndt CAS et al. (2017) Extraskeletal Osteosarcoma: Outcomes and the Role of Chemotherapy. Am J Clin Oncol. 41: 832-837. [Crossref]
- Goldstein Jackson SY, Gosheger G, Delling G, Berdel WE, Exner GU et al. (2005) Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol 131: 520-526. [Crossref]
- McCarter MD, Lewis JJ, Antonescus CR, Brennan MF (2000) Extraskeletal osteosarcoma: analysis of outcome of a rare neoplasm. Sarcoma 4: 119-123. [Crossref]
- Fan Z, Patel S, Lewis VO, Guadagnolo BA, Lin PL (2015) Should High-grade Extraosseous Osteosarcoma Be Treated with Multimodality Therapy Like Other Soft Tissue Sarcomas? Clin Orthop Relat Res 473: 3604-3611. [Crossref]
