Eviscerated Socket Challenge: Managing Orbital Myiasis in Emergency
A B S T R A C T
Maggots, larvae of diptera flies, thrive in environments with feces and decaying matter. They may infest vertebrates, causing myiasis. Human cases are sporadic and prevalent in rural areas. Orbital myiasis is the most severe form, encompassing extensive infestation of orbital tissue and progressing rapidly, potentially destroying orbital tissues within days. A 38-year-old male presented with severe pain and swelling in his right eye for six months, which increased over the past ten days. Ophthalmic examination revealed no light perception in the right eye, with redness, edema, and a 6 × 4 cm wound filled with larvae. CT and MRI scans confirmed orbital myiasis, leading to exenteration and successful removal of 301 larvae-the patient was diagnosed with necrotizing fasciitis. Parasite examination identified chrysomya species. Diverse management in ophthalmomyiasis, with successful single-extraction and oral ivermectin use, underscores tailored approaches. Reported cases aid understanding, emphasizing early identification and vigilance.
Keywords
Ophthalmomyiasis, maggots, orbital myiasis, chrysomya spp
Introduction
Maggots, the larvae of diptera flies, are primarily found in environments abundant with human and animal feces, decaying plants, and decomposing organic substances. In certain instances, these larvae can infest vertebrates, including humans, causing myiasis [1]. While myiasis predominantly affects animals like cattle, goats, and pigs, human occurrences are sporadic [2]. It is mainly observed in tropical and subtropical regions or developing nations with high population density and inadequate sanitation [1]. Risk factors for human myiasis include advanced age, compromised health, inadequate self-care, poor hygiene practices, and a rural background.
Ophthalmomyiasis, a manifestation of myiasis, can affect the orbit, eye, and periorbital tissues classified as external, internal, or orbital, depending on the location of larval infestation [3]. External ophthalmomyiasis involves limited superficial infestations of tissues, such as the palpebra and conjunctiva. Internal ophthalmomyiasis occurs when larvae invade deeply and migrate into the subretinal space. Orbital myiasis is the most severe form, encompassing extensive infestation of orbital tissue and progressing rapidly, destroying orbital tissues within days. Although, orbital myiasis is very rare, with only a few documented cases [4]. Managing orbital myiasis varies from the simple manual removal of maggots to more invasive surgical interventions targeting the globe and orbit [1]. This case study adds knowledge about orbital myiasis.
Case Description
In October 2023, a 38-year-old male sought emergency care with a complaint of severe pain and swelling in his right eye, persisting for six months, with a increase in intensity over the past ten days. Initially, there was pain in the right globe. The pain started after a minor trauma to his right lower eyelid, leading to a small unhealing ulcer and a 10-day history of symptoms aggravated along with the wriggling out of larvae. The patient was not able to recall the mode of injury, but he hasn't taken any treatment for the same. In the last two months, there has been a gradual loss of vision in the right eye. His painful symptoms worsened a few days before presenting to our hospital with complaints of bleeding, a crawling sensation, and larvae coming out from the right eye. He visited the primary health care setting and was immediately referred to a higher center for further management. He has had a habit of chewing tobacco since last 18 years. He denied any history of alcoholism, smoking, previous ocular surgery, or prolonged use of medications. He had no history of diabetes mellitus, tuberculosis, or systemic cancer. He was not a known HIV Positive individual, HBV HCV infection.
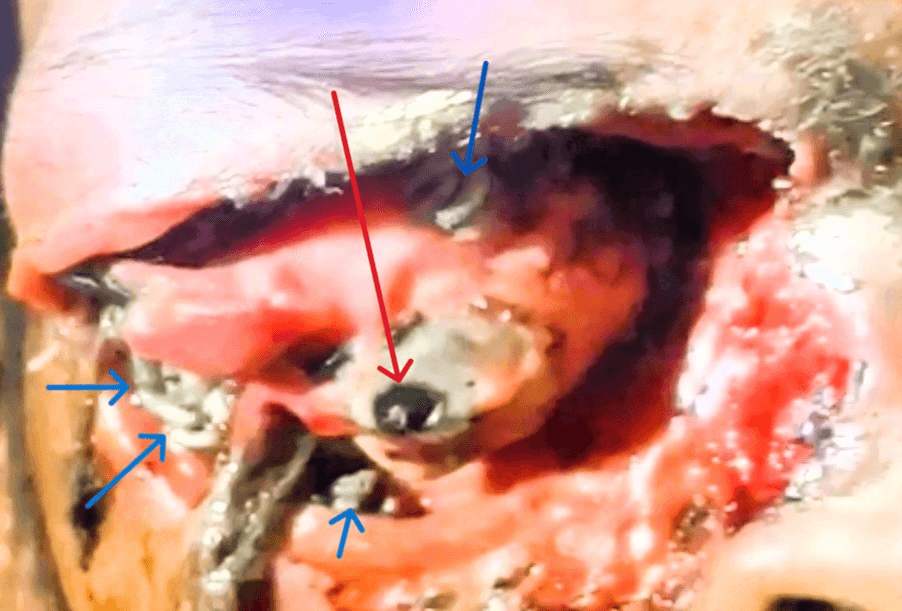
During ophthalmic examination, the visual acuity test indicated no light perception in the patient's right eye. The periorbital skin on the right side showed redness and edema, while the eyelid appeared thickened. A large wound, approximately 6 × 4 cm in size, and the deformed globe filled with many white larvae, some of which were crawling out in (Figure 1). The left eye was expected, with a visual acuity of 6/12. An ENT opinion for nasal cavity or sinus involvement, neurosurgery for frontal or cranial lobe involvement, ophthalmology, and dermatology opinion were sought. Routine investigations were sent.
Figure 1: Right eye of the patient blue arrows showing various larvae and red arrow showing the auto eviscerated globe.
A CT scan of the head + orbital + Right nasal cavity revealed mild communicating hydrocephalus of bilateral lateral ventricles (Evans Index - 0.41), 3rd and 4th ventricles noted. However, no obvious lesion was pointed out in the brain parenchyma; evidence of any intra/extra-axial bleed was reported. The contour of the right eyeball is lost with rarefaction of the anterior medial wall of the right orbit. Mucosal thickening is noted in the right frontal ethmoidal and sphenoidal sinuses.
A diagnosis of orbital myiasis was made. Based on imaging findings, there was destruction of the globe due to larval infiltration. He underwent exenteration of the right orbit. Approximately 301 larvae with necrotic tissues were extracted under local anaesthesia. Subsequently, the wound was monitored for infections and overlooked larvae. Oral tablet Ivermectin (12 mg stat), antibiotics, eye drops, and ointment for local application in the eviscerated right eye were advised. Considering potential infections, aerobic culture and sensitivity of right eye were done, revealing pus cells 10-20/oif and gram-positive cocci in pairs &chains seen; methicillin resistant staphylococcus aureus (MRSA) grown in culture. After obtaining a sensitivity report, appropriate organism-sensitive antibiotics were given. Larvae on parasite examination under a direct microscope revealed chrysomya species.
Three days later, MRI of brain + orbit revealed soft tissue thickening in the right pre-septal, premaxillary, and pre-frontal region with extension. However, no direct extension were seen in the brain parenchyma. The histopathological examination of the excised necrotic tissue involving orbit and adjacent tissue suggested necrotizing fasciitis and ruled out malignancy. The routine investigations had minimal abnormalities but clinically were normal. A comparison of normal blood workup on day 1 and day 3 of admission is shown in (Table 1). The neurosurgeon opined that there was no intervention as his GCS was 15/15. He obeyed commands and had no apparent abnormalities in brain parenchyma in NCCT and MRI brain. The patient recovered well and was referred to the ophthalmology unit on day 3 with daily wound dressing and for further management.
Table
1:
Comparison of blood parameters.
|
Complete blood count |
Day 1 |
Day 3 |
Normal Range |
Units |
|
Haemoglobin |
13.6 |
12.8 |
14-18 |
gm/dl |
|
Hematocrit |
41.2 |
36.3 |
37-47 |
percentage |
|
White Blood cells |
9.93 |
9.39 |
4-11 |
Thousand/Microliter |
|
Neutrophils |
83.5 |
88.8 |
40-70 |
percentage |
|
Lymphocytes |
7.3 |
7.6 |
20-40 |
percentage |
|
Monocytes |
8.3 |
3.2 |
2-8 |
percentage |
|
Basophils |
0.0 |
0.0 |
0-1 |
percentage |
|
Eosinophils |
0.9 |
0.4 |
1-6 |
percentage |
|
Platelet count |
228 |
242 |
150-450 |
Thousand/Microliter |
|
|
|
|
|
|
|
Liver Function test |
|
|
|
|
|
Total
Bilirubin |
0.67 |
0.72 |
0.3-1.2 |
mg/dl |
|
Direct Bilirubin |
0.18 |
0.17 |
<0.2 |
mg/dl |
|
AST |
63 |
59 |
<50 |
U/L |
|
ALT |
146 |
67 |
<50 |
U/L |
|
ALP |
85.4 |
88.3 |
30-120 |
U/L |
|
Total Protein |
5.57 |
5.61 |
6.6-8.3 |
gm/dl |
|
Serum
Albumin |
2.88 |
2.92 |
3.5-5.2 |
gm/dl |
|
|
|
|
|
|
|
Renal
Function test |
|
|
|
|
|
Serum Creatinine |
0.83 |
0.8 |
0.6-1.2 |
mg/dl |
|
Blood
Urea |
44.51 |
23.8 |
20-40 |
mg/dl |
|
|
|
|
|
|
|
Random
Blood Sugar |
132 |
136 |
70-140 |
mg/dl |
|
ESR |
71 |
- |
<15 mm/hr |
mm at first hour |
Discussion
Ophthalmomyiasis presents in three distinct forms: orbital myiasis, involving the eye socket; ophthalmomyiasis externa, which affects the cornea or conjunctiva; and ophthalmomyiasis interna, characterized by infestation within intraocular structures. The origin of this condition lies in the infestation of ocular structures by larvae, with the most common culprits being the sheep botfly (Oestrus ovis), screw-worm fly (Phaenicia lucilia, C. bezziana), human botfly (Dermatobia hominis), and cattle botfly (Hypoderma bovis). Ophthalmic involvement is observed in a relatively low percentage of cases, ranging from 5% to 14% [1, 5].
Humans are accidental hosts for dipterian flies, while sheep and goats are definitive hosts. The life cycle involves adult flies laying eggs, which then hatch into larvae over several days. The larvae or maggots undergo significant growth, constituting the primary feeding stage. Within 3-5 days, these larvae transform into a pupa, characterized by a hard shell protecting the developing fly. Subsequently, in another 4-6 days, adult flies emerge from the pupa. Alongside poor hygiene, risk factors for ectoparasitic infestations include local tissue necrosis, malignancies (such as basal cell carcinoma and squamous cell carcinoma), and ischaemia [2].
Orbital myiasis is a highly destructive disease, emphasizing the critical need for early identification. The larvae can penetrate the orbital tissues, the surrounding paranasal sinuses, and even the intracranial space. In cases where an intact eyeball is present, there is a notable risk of intraocular penetration, leading to the development of ophthalmomyiasis interna [5]. In our patient, the larvae had infiltrated the orbital tissues, but the surrounding paranasal sinuses and intracranial space were unaffected. Given the larvae's photosensitivity and mobility, immobilization strategies involving topical anaesthesia and various suffocating agents such as paraffin and turpentine oil have been recommended [4, 5].
The removal of larvae was conducted under local anaesthesia following exploration. This procedure was followed by frequent examinations throughout the day to remove any residual larvae. Daily wound examination, dressing, and cleaning were advised until the area was confirmed to be maggot-free. Standard practice involves surgical debridement of the wound and mechanical removal with plain forceps to extract the maggots. However, a drawback is that the larvae crawl deep into tissues when exposed to light, making removal challenging. To address this, suffocating formulations such as turpentine oil, liquid paraffin, and petroleum jelly temporarily immobilize the larvae, facilitating their mechanical extraction. [1, 4, 5].
Anaesthetic agents like xylocaine aid in immobilizing the crawling larvae and prevent their retreat into deeper tissues. Since larvae are only temporarily incapacitated, manual removal becomes necessary. Using larvicidal drugs like hydrogen peroxide and isopropyl alcohol effectively kills maggots and facilitates their mechanical removal [4]. In our case, the patient achieved maggot-free status with a single extraction. The potential for overlooking small-sized maggots existed. ivermectin, a larvicidal drug, proves highly effective in ensuring complete clearance of all larvae [2]. Additionally, there are documented cases of orbital myiasis secondary to cutaneous malignancies like SCC and BCC, but in our case the histopathological examination excluded malignancy of the excised tissues [5].
Puthran et al. reported a case of orbital myiasis in an anophthalmic socket in a patient from a rural background who had sustained a lid injury [6]. Khataminia et al. documented a case of orbital myiasis involving orbital destruction caused by C. bezziana in an 85-year-old female [7]. The treatment approach in this instance involved exenteration. Similarly, Sachdev et al. detailed a case of orbital myiasis in an immunocompetent patient with no apparent risk factors [8]. Similar to their approach, we used a combination of treatments, including removal, and oral ivermectin, but we didn’t used turpentine oil, we just used the povidone iodine and 2% lignocaine. The successful treatment in this case primarily involved mechanical removal aided by oral ivermectin.
This case report highlights the management of ophthalmomyiasis in immunocompetent patient. Successful outcomes, such as our patient's single-extraction resolution and use of ivermectin, underscore the importance of individualized approaches. Insights from reported cases contribute to understanding this rare condition, emphasizing early identification and vigilance.
Acknowledgements
None.
Funding
None.
Availability of Data and Materials
The data generated in the present study may be requested from the corresponding author.
Author Contributions
AK did patient evaluation, diagnosis, primary manuscript drafting and treatment planning. SS did through literature review and manuscript editing. RV did interpretation of clinical findings, contributing to discussions, and manuscript revisions. MY supervised the patient treatment, guided in case report preparation, manuscript review, corresponding author and guarantor of the article. AK and MY confirmed the authenticity of all the raw data. All authors read and approved the manuscript.
Ethics Approval and Consent to Participate
Not applicable (As it is a case report).
Patient Consent
Informed consent was taken from the patient (As per the principles of Declaration of Helsinki).
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 15, Jan 2024Accepted: Mon 05, Feb 2024
Published: Mon 26, Feb 2024
Copyright
© 2023 Md. Yunus. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJSCR.2024.01.02
Author Info
Amit Kumar Sourabh Singh Rashmi Verma Md. Yunus
Corresponding Author
Md. YunusDepartment of Trauma and Emergency Medicine, AIIMS, Bhopal, Madhya Pradesh, India
Figures & Tables
Table
1:
Comparison of blood parameters.
|
Complete blood count |
Day 1 |
Day 3 |
Normal Range |
Units |
|
Haemoglobin |
13.6 |
12.8 |
14-18 |
gm/dl |
|
Hematocrit |
41.2 |
36.3 |
37-47 |
percentage |
|
White Blood cells |
9.93 |
9.39 |
4-11 |
Thousand/Microliter |
|
Neutrophils |
83.5 |
88.8 |
40-70 |
percentage |
|
Lymphocytes |
7.3 |
7.6 |
20-40 |
percentage |
|
Monocytes |
8.3 |
3.2 |
2-8 |
percentage |
|
Basophils |
0.0 |
0.0 |
0-1 |
percentage |
|
Eosinophils |
0.9 |
0.4 |
1-6 |
percentage |
|
Platelet count |
228 |
242 |
150-450 |
Thousand/Microliter |
|
|
|
|
|
|
|
Liver Function test |
|
|
|
|
|
Total
Bilirubin |
0.67 |
0.72 |
0.3-1.2 |
mg/dl |
|
Direct Bilirubin |
0.18 |
0.17 |
<0.2 |
mg/dl |
|
AST |
63 |
59 |
<50 |
U/L |
|
ALT |
146 |
67 |
<50 |
U/L |
|
ALP |
85.4 |
88.3 |
30-120 |
U/L |
|
Total Protein |
5.57 |
5.61 |
6.6-8.3 |
gm/dl |
|
Serum
Albumin |
2.88 |
2.92 |
3.5-5.2 |
gm/dl |
|
|
|
|
|
|
|
Renal
Function test |
|
|
|
|
|
Serum Creatinine |
0.83 |
0.8 |
0.6-1.2 |
mg/dl |
|
Blood
Urea |
44.51 |
23.8 |
20-40 |
mg/dl |
|
|
|
|
|
|
|
Random
Blood Sugar |
132 |
136 |
70-140 |
mg/dl |
|
ESR |
71 |
- |
<15 mm/hr |
mm at first hour |
References
1.
Bhola
N, Jadhav A, Borle R, Adwani N, Khemka G et al. (2012) Primary oral myiasis: a
case report. Case Rep Dent 2012: 734234. [Crossref]
2.
Carvalho
RW, Santos TS, Antunes AA, Laureano Filho JR, Anjos ED et al. (2008) Oral and
maxillofacial myiasis associated with epidermoid carcinoma: a case report. J
Oral Sci 50: 103-105. [Crossref]
3.
Özyol
P, Özyol E, Sankur F (2016) External ophthalmomyiasis: a case series and review
of ophthalmomyiasis in Turkey. Int Ophthalmol 36: 887-891. [Crossref]
4.
Huang
YL, Liu L, Liang H, He J, Chen J et al. (2020) Orbital myiasis: A case report
and literature review. Medicine (Baltimore) 99: e18879. [Crossref]
5.
Kalamkar
C, Radke N, Mukherjee A (2016) Orbital myiasis in eviscerated socket and review
of literature. BMJ Case Rep 2016: bcr2016215361. [Crossref]
6.
Puthran
N, Hegde V, Anupama B, Andrew S (2012) Ivermectin treatment for massive orbital
myiasis in an empty socket with concomitant scalp pediculosis. Indian J
Ophthalmol 60: 225-227. [Crossref]
7. Khataminia G, Aghajanzadeh R, Vazirianzadeh B, Rahdar M (2011) Orbital myiasis. J Ophthalmic Vis Res 6: 199-203. [Crossref]
8. Sachdev MS, Kumar H, Roop, Jain AK, Arora R et al. (1990) Destructive ocular myiasis in a noncompromised host. Indian J Ophthalmol 38: 184-186. [Crossref]
