Evaluation of the Effect of an Advance Diabetes Support Product Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus
A B S T R A C T
Aim: Diabetes support product (ADSP) with phytocompounds was proposed to improve insulin secretion, avoid pancreatic beta cell apoptosis, and moderate beta cell differentiation and proliferation. In this research work, beta cell regenerative potential of ADSP was evaluated with STZ induced diabetes in rodents.
Method: Single dose of Streptozotocin (STZ) (70mpk; i.p.) was used to induce diabetes in the Wistar rats. The treatment of vehicle or test or GLB was continued for the next 28 days to assess sub-acute anti-diabetic potential. Fasting blood glucose levels (BGL) was monitored weekly once throughout the experiment. Bodyweight, Feed-water consumption was calculated on every day till end of the experiment. Interleukin-6, Interleukin-1β and Interferon-γ was also analysis from blood serum after 28 days of treatment in rats. Further, animal was humanely sacrifice and organs such as liver, kidney(s) and pancreas were isolated for histopathology analysis.
Result: Research showed that Insulin is Increased by 3 times in comparison with the diabetic group by ADSP after 28 days. After 28 days treatment of ADSP to rodents, Interleukin-6 decreased by 52%, Interleukin-1β decreased by 73% and Interferon-γ decreased by 28% in comparison with the diabetic control group. It is also observed in histopathology studies that there was a rise in the quantity of islets after 28 days treatment of ADSP in Streptozotocin carried diabetic rats.
Conclusion: Hence in conclusion, advance diabetes support product supposed to be appreciated as synergistic product of encouraging the β-cell regeneration in vivo.
Keywords
Beta cells, insulin, diabetes mellitus, regeneration
Introduction
Diabetes and metabolic disorders are becoming major global health issues due to their rising prevalence. Diabetes is predicted to impact 439 million people by 2030, up from an estimated 285 million cases in 2013 [1]. Type 2 diabetes affects more than 90% of diabetic patients, and the cost of care places a significant financial strain on many nations. Indeed, according to estimates, the cost of treating diabetes in the United States in 2012 was anticipated to be 245 billion US dollars, a 41 percent rise over the 2007 projection ($174 billion) [2]. Hyperglycemia, a characteristic of diabetes, can result in diabetic complications such as cardiovascular disease, nephropathy, retinopathy, and neuropathy [3].
The disruption of glucose homeostasis has a significant role in the emergence of hyperglycemia. The main hormone in charge of regulating the balance of glucose metabolism is insulin, which is secreted by pancreatic beta cells. Absolute or relative insulin insufficiency causes the development of hyperglycemia in both type 1 and type 2 diabetes. In type 1 diabetes, cytokines, macrophages, or T cells that have been activated by autoimmune reactions cause damage to pancreatic beta cells. Insulin resistance and a relative insulin shortage that cannot make up for the insulin resistance cause type 2 diabetes [4].
In type 2 diabetes, inflammatory mediators released from adipose tissue and the endoplasmic reticulum, oxidative stress, or persistently high glucose or lipid levels can induce damage to or malfunction of pancreatic beta cells. Therefore, preserving pancreatic beta-cell function may be a wise strategy for the management of diabetes. The development of new antidiabetics depends on the investigation of innovative and reasonably priced substances that can improve pancreatic beta-cell bulk or function [5]. A substitute approach to treat diabetic conditions is the use of different extracts of plant and Phytocompounds, because of their beneficial potential to manage hypoglycaemic properties. Comprehensive study of these molecules has exposed that some of them are origin of regeneration of β-cells, thus instigating a reverse of diabetes in human volunteers. Natural bio actives are a source of innovative medications due of their diversity, and it allows the production of drugs that diverge from additional chemical constituents in terms of their multifaceted structures along with biological effectiveness [6].
The purpose of the present research is to assess the antidiabetic efficacy of ADSP and its effect on pancreatic islet regeneration. In the current study, we use serum/blood parameters such as serum insulin level and interferon (IFN)- γ, interleukin (IL)-6, and IL-1β to examine the effects of ADSP on pancreatic b-cell function.
Material and Method
The rats were housed in animal house facility with adequate environmental conditions of temperature 20 + 3°C and relative humidity 30-70 %. The 12-hour light and 12-hour dark cycle was maintained manually throughout study. Each day floor of the experimental room was cleaned and mopped twice with disinfectant solution. Glibenclamide (GLB) is evaluated for sugar and insulin only as it has no role in other parameters. Rats were acclimatized for a period of seven days and observed for general health before the commencement of the experiment (Table 1).
Table 1: Animal allocation.
|
Groups |
Treatment |
Dose & Route |
No. of animals |
|
G1 |
Normal control (0.25% Na-CMC) |
10 ml/kg, p.o. |
10 |
|
G2 |
Disease control (0.25%
Na-CMC) |
10 ml/kg, p.o. |
10 |
|
G3 |
Glibenclamide (GLB) |
10 mpk, p.o. |
10 |
|
G4 |
ADSP (test compound) |
200 Mg/kg, p.o. |
10 |
I Induction of Diabetes
The diabetes was induced in the rats according to the modified protocol of Furman (2015). Single dose of Streptozotocin (STZ) (70mpk; i.p.) was used to induce diabetes in the Wistar rats. Animals was fast for 10-12 h before the Streptozotocin (STZ) injection. Freshly prepared, filtered (0.2-micron filter), ice chilled 100mM sodium citrate buffer (pH 4.5) was used as solvent. Different aliquots of STZ was prepared and kept in ice bath. The required quantity of ice chilled sodium citrate buffer was added to each STZ aliquot, vortexed for 0.5-1 min and injected intraperitoneally to each animal. The dose volume of 10mL/kg was kept constant for all the animals. Diabetes was recognized by symptoms like polydipsia and polyurea along with analysis of fasting blood glucose levels after 48 h of STZ administration. However, stable hyperglycemia (fasting blood glucose >200mg/dL) was observed after 4 days of STZ injection.
II Anti-Diabetic and Beta Cell Regeneration Effect
The treatment of vehicle or test or GLB was continued for the next 28 days to assess sub-acute anti-diabetic potential. Fasting blood glucose levels (BGL) was monitored weekly once throughout the experiment. Altogether the rats were kept at daily observation for the clinical signs and/or mortality if any. Bodyweight, Feed-water consumption was calculated on every day till end of the experiment. Interleukin-6, Interleukin-1β and Interferon-γ was also analysis from blood serum after 28 days of treatment in rats. At the end of 28 days, entirely the animals were anaesthetized, and blood was withdrawn. Serum was separated and processed for insulin analysis. Further, animal was humanely sacrifice and organs such as liver, kidney(s) and pancreas were isolated for histopathology analysis.
III Observations
Individual body weights of animal were recorded prior to dosing on every day and continue till end of experiment i.e., Day 28. Feed consumption of animal was calculated on everyday till end of experiment. water consumption of animal was calculated on everyday till end of experiment. Fasting Blood glucose level was monitored weekly once throughout the experiment. All the animals were observed daily for the clinical signs and/or mortality, if any.
IV Histopathological Observations
Liver, kidney and pancreas were divided out, excised and rinsed in cold saline solution. A portion of each tissue were fixed in 10% neutral buffered formalin. After fixation, tissues were processed to dehydrated in ascending grades of alcohol, clearing in xylene and fixed in paraffin; solid sections of 3-5µm thickness were analysis by using a microtome. The sections were stained with haematoxylin-eosin and histological observations were made using microscope. Blinded evaluations of coded slides were performed by veterinary pathologist to avoid bias in experiment. Images of the histological slides were took using Olympus Magnus Microscope camera and managed by Olympus MagVision Image analysis software. For Pancreas, Regeneration (cytoplasmic vacuolation of exocrine and endocrine pancreas-- Islet of Langerhans) Necrosis of exocrine, endocrine pancreas and surrounding adipose tissues, Atrophy, Hyperplasia of exocrine and endocrine pancreas, Any other lesion(s) was observed.
V Statistical Analysis
The facts and numbers were stated as Mean±SD for each group. The Statistical analysis was made by using GraphPad Prism version 7.0 software. The test called one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison t-test was considered to calculated statistical difference. Values of p<0.05 was measured statistically significant.
Results
These results indicate that individual treatment with ADSP (test compound) can positively influence b-cell function and beta-cell regeneration in rodents.
I Effect of ADSP on Blood Glucose and Insulin Level
ADSP (test compound) given as a daily dose for up to 28 days to animals, is able to reduce blood glucose by 44.93 % in 200 mg dose of ADSP and improve insulin levels. To get the insights for insulin secretory potential of pancreatic islets and its association with plasma glucose, we checked plasma insulin levels in diabetic animals and diabetic rats treated with the ADSP (test compound). The mean insulin level in plasma of diabetic rats was significantly reduced compared to the initial level. But in the ADSP (test compound) group rise in the mean plasma insulin levels amplified significantly after 28 days (Table 2, Figure 1).
Table 2: Fasting Blood glucose levels.
|
Groups |
Initial (Mean±SD) |
After 28 days (Mean±SD) |
|
Normal control |
115.5 (±7.60) |
139.625 (±12.96) |
|
Diabetics control |
354.125 (±50.98) |
245.3 (±105.61) |
|
Glibenclamide (GLB) |
402 (±32.89) |
133 (±20.09) |
|
ADSP (test compound) 200 mg |
377.50 (±73.38) |
169.63 (±24.41) |
Figure 1: Insulin level after 28 days.
The p-value is 0.010505.
NC: Normal Control; DC: Diabetic Control; STD: Glibenclamide; ADSP: 200mg/kg dose.
II Effect of ADSP on IFN-Y Level
Interferon-gamma (IFN-y) is playing a role in the destruction of beta cells which progress towards insulin-dependent diabetes mellitus. Upon induction of diabetes in the current research, the levels of IFN-γ were pointedly improved in diabetic rats compared to normal rats. These results suggest evidence of the marked influence of IFN-γ production on the β cell destruction pathway.
IFN-γ production was suggestively increased in streptozotocin diabetic rats when compared to normal and treated rats. Treatment of ADSP (test compound) caused a significant decrease in the levels of IFN-γ compared to diabetic rats. This indicates the protection of beta cells in ADSP (test compound) treated animals. these results state the evidence of the noticeable impact of IFN-γ secretion on the β cell destruction pathway i.e., low levels of IFN-γ suggest that B cell protection activity of ADSP (test compound) (Figure 2).
Figure 2: Effect of ADSP on IFN-Y level after 28 days.
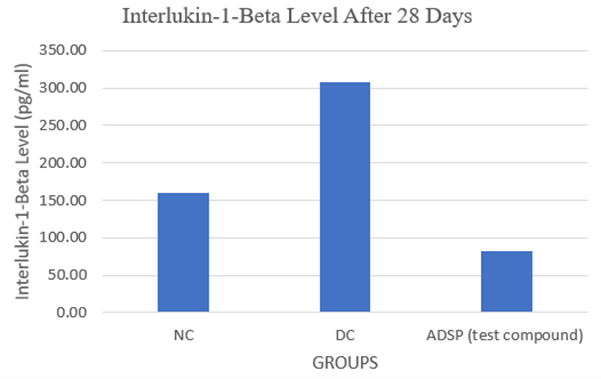
III Effect of ADSP on Interlukin-1-Beta Level
Interleukin 1Beta (IL1β) is the cytokine with the potential to destroy the islet of pancreases which has been observed in the diseases group but in the Treatment group, less amount of Interleukin 1Beta (IL1β) is detected and leads toward the regeneration of new beta cell or might be the protection of beta cells from destruction due to Interleukin 1Beta (IL1β).
Higher levels of the proinflammatory cytokine like Interleukin 1 beta (IL-1β) in diseased animals is concerned in decreased insulin production, decreased cell proliferation, and apoptosis of pancreatic beta cells whereas in the treatment group elevated IL-1β was reduced significantly ensuring improved insulin production, cell propagation, and decrease the apoptosis of pancreatic beta cells (Figure 3).
Figure 3: Interlukin-1-Beta Level After 28 Days.
The p-value is < .00001
NC: Normal Control; DC: Diabetic Control; ADSP: 200mg/kg dose.
IV Effect of ADSP on Interlukin-6 Level
Originally, it has proinflammatory and other properties on immune responses. In the existing research, the plasma levels of IL-6 in the diabetic rats noted significantly higher in levels which represents the necrosis and degeneration of Beta cells and inflammation in pancreases than those in normal rats. In the current experiment, the raise of IL-6 in the diabetic rats that were administered with ADSP (test compound) may be credited to the difference between immune-modulatory effectiveness in case of disease state and a healthy condition (Figure 4).
Figure 4: Interlukin-6 Level After 28 Days.
The p-value is .000101
NC: Normal Control; DC: Diabetic Control; ADSP: 200mg/kg dose.
V Histopathology
The histologic characteristics of islets present in the pancreas of diabetic rats is categorized by a considerable reduction in the quantity of islets, magnitude of inflammation, vacuolation of the islets, and degranulation of the beta cells (Figure 5). Apart from this, the systematic prearrangement of alpha and beta cells is disturbed and clumping of beta cells, pyknosis, and necrosis are perceived and observed in the islets. In Diabetic animals, Multifocal reduced number of Islet of Langerhans (3), Multifocal atrophy of Islet of Langerhans (3), Focal degeneration of cells of Islet of Langerhans (3). streptozotocin-induced diabetic rats for them test compounds was not given presented widespread destruction of islet cells as related with the sections of the pancreas of group of healthy rats. Further, there was a definite decrease in the number of islets in diabetic rats, compared to healthy animals.
Figure 5: Histopathology images of pancreas.
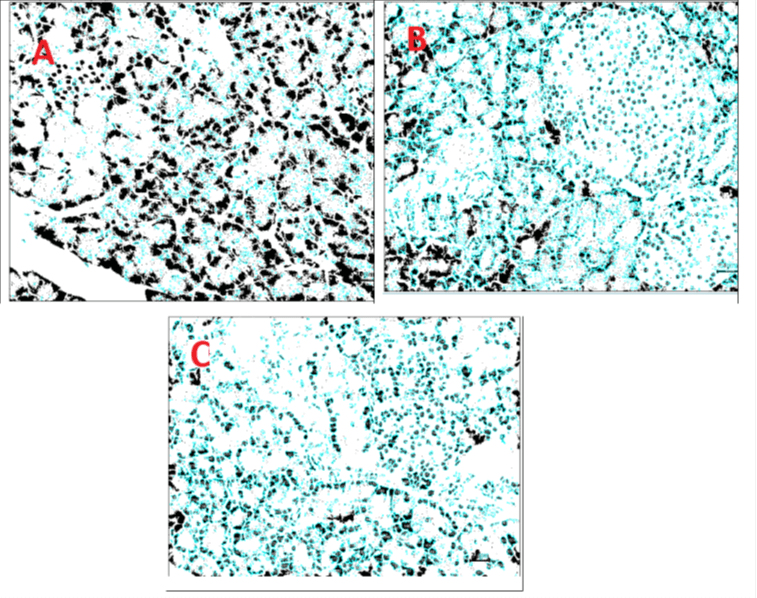
In animals dosed with 200 mg of ADSP (test compound), In Multifocal atrophy of Islet of Langerhans (1), Focal degeneration of cells of Islet of Langerhans (1), Focal atrophy of Islet of Langerhans (1). There was an increase in the quantity of number of islets in ADSP (test compound) treated group, 200 mg treatment, when compared to untreated group of diabetic rats as control group (Figure 6). Patho-morphological observation of pancreas, in present report suggests diabetes induction in the disease group produces inflammatory, degenerative, and atrophic changes in Islet of Langerhans. A reduced number of Islets of Langerhans of the pancreas is also seen in the disease control group. Rats treated with ADSP (test compound) revealed similar microscopic changes with less severity and distribution. It has been noted all the test compounds mitigate the inflammatory, degenerative, atrophic effect on Islet of Langerhans. These lesions along with loss of Islet of Langerhans are reduced in the treated group which could be due to mitigatory action of the test drug and efficacy of the test drug to regenerate cells of Islet of Langerhans.
Figure 6: Histopathology images of pancreas as per IMAGE-J software.
A- Diabetic control, N- Normal Control, and C- 200 mg ADSP (test compound).
Discussion
A variety of investigational and clinical studies have stated the advantageous effects of phytochemicals in the management of diabetic conditions [7]. Different mechanisms are involved in phytochemicals' anti-diabetic properties like reduced glucose absorption from cells, intestine, increasing insulin production from beta cells collective in pancreatic tissue regeneration [8, 9]. There is a large amount of controversy surrounding beta cell regeneration in diabetes studies and treatments. It is supposed that beta cells can regenerate from reproduction of pre-existing beta cells or through neogenesis of stem cells and progenitor cells on the outside or inside of the islets has been clearly demonstrated to regenerate via beta cell replication [10].
The greatest applicable effects of various phytocompounds originated from plants on pancreatic beta-cell function have been summarized by many researchers. The utmost appropriate potential of phytocompounds are improved insulin secretion, inhibition of beta-cell apoptosis, and modulation of beta-cell differentiation and proliferation [11]. ADSP is the synergistic blend of some key extract of Gymnema Sylvestre ext. (Gudmar), Trigonella Foenum-Graecum ext. (Methi), Cinnamomum verum ext. (Cinnamon), Curcuma longa ext. (Curcumin ext), Andrographis paniculata ext. (Kalmegh), Salacia Oblonga ext. (Saptarangi). Indeed, it is well reported that the foremost chemical compound found in G. sylvestre are triterpenoid saponins recognised as gymnemic acids, which exert the antidiabetic potential of the extracts [12, 13].
It was shown that curcumin stimulates insulin production by the pancreatic islets of Langerhans and resulted in an increase in beta cell function and protection from STZ-induced oxidative stress like free radical damage, protecting the pancreatic islets from oxidative stress, and significantly increasing cell viability and insulin secretion [14-16]. The curcumin also possesses antidiabetic potential and improved pancreatic islets regeneration [17].
An increase in pancreatic islet Beta-cell number and size was seen in histology production of pancreas of the animals treated with Trigonella, thus ameliorating symptoms of diabetes during advanced deterioration and improving glycemic control in diabetic rats treated with n-STZ [18]. As ADSP is the blend of extract many herbal compounds, It showed the comprehensive and synergistic effect in β cells function and regeneration.
Our results demonstrate the ability of ADSP (test compound) to promote b-cell function by acting islets to secret insulin levels. We believe that ADSP (test compound) acts by improving the production of insulin from the pancreas or increasing the glucose uptake and reducing gluconeogenesis, leading in the decreased plasma glucose levels. This study attentions on endogenous regeneration, which can shadow two pathways. Firstly, heightened reproduction of existing β cells and secondly formation of new β cells. Current studies discovered a astonishing regenerative dimensions of insulin-producing β cells in animals, signifying that regenerative treatment for human diabetes might in principle be accomplished.
The biochemical findings are constant with the histological conclusions found in this research. The pancreatic ß cells were devastated after introduction of streptozotocin to the rats. Streptozotocin has a damaging action on the beta cells of the pancreas. A histopathological study of diabetic untreated rats showed degeneration of pancreatic islet cells. ADSP (test compound) treatment qt 200mg/kg dose in the diabetic rat groups leads to augmented volume density of islets and an improved percentage of beta cells, which leads to the evidence of regeneration of ß cells. The pattern and signs of regeneration of ß cells, increase of insulin production from remaining ß cells of the islets of Langerhans, and a reduction in blood glucose have been described after treatment of ADSP (test compound). It has been surely predictable that oxidative burden estimates a vital role in beta-cell disfunction and cell death. As a consequence of deprived antioxidant potential, beta cells are exposed to the oxidative burden induced by both T1D insulitis and T2D glucotoxicity.
It remains unclear exactly how phytochemicals protect and regenerate pancreatic islets, but their antioxidant properties may explain in part why they inhibit apoptosis of pancreatic beta cells. Irrespective of the mechanisms at molecular level, it appears that subjects at the initial periods of diabetes may be able to benefit from these plants as a means of delaying or preventing the complete destruction of their pancreatic islets. Therefore, ADSP (test compound) that improves glycemia and/or oxidative stress ameliorates or prevents islet lesions. In this context, the protecting effect of ADSP (test compound) on the pancreas has been found to be facilitated with their antioxidant and anti-inflammatory potential. The particular mechanism accountable for the protective/regenerative properties ADSP (test compound) on pancreatic islets is yet to be explained.
Conclusion
Our most recent statistics indicate that the advance diabetes support product (ADSP) from Gplife Healthcare can dramatically improve experimental diabetes. In response to the 28-day therapy regimen given to diabetic rats in our investigation, pancreatic islets clearly regenerate. This may be linked to ADSP's anti-inflammatory and antioxidant properties, which promote pancreatic and systemic conditions that are favourable for islet neogenesis. Additionally, the function of ADSP in stem cell differentiation and proliferation may be relevant. Hence it has been concluded that advance diabetes support product (ADSP) can be the use and explore as treatment in diabetes.
Acknowledgment
The authors would like to acknowledge the research team and the back-office team involved in the research work. We would like to acknowledge the support provided by back office, Gplife Healthcare Pvt. Ltd.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 12, Jul 2022Accepted: Mon 25, Jul 2022
Published: Thu 18, Aug 2022
Copyright
© 2023 Dheeraj Nagore. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2022.01.03
Author Info
Shridhar Pandya Chetan Savaliya Kamlesh Thummar Dheeraj Nagore Vaishali Undale
Corresponding Author
Dheeraj NagoreMprex Healthcare P. Ltd, Wakad, Pune, India
Figures & Tables
Table 1: Animal allocation.
|
Groups |
Treatment |
Dose & Route |
No. of animals |
|
G1 |
Normal control (0.25% Na-CMC) |
10 ml/kg, p.o. |
10 |
|
G2 |
Disease control (0.25%
Na-CMC) |
10 ml/kg, p.o. |
10 |
|
G3 |
Glibenclamide (GLB) |
10 mpk, p.o. |
10 |
|
G4 |
ADSP (test compound) |
200 Mg/kg, p.o. |
10 |
Table 2: Fasting Blood glucose levels.
|
Groups |
Initial (Mean±SD) |
After 28 days (Mean±SD) |
|
Normal control |
115.5 (±7.60) |
139.625 (±12.96) |
|
Diabetics control |
354.125 (±50.98) |
245.3 (±105.61) |
|
Glibenclamide (GLB) |
402 (±32.89) |
133 (±20.09) |
|
ADSP (test compound) 200 mg |
377.50 (±73.38) |
169.63 (±24.41) |

The p-value is 0.010505.
NC: Normal Control; DC: Diabetic Control; STD: Glibenclamide; ADSP: 200mg/kg dose.

The p-value is < .00001
NC: Normal Control; DC: Diabetic Control; ADSP: 200mg/kg dose.

The p-value is .000101
NC: Normal Control; DC: Diabetic Control; ADSP: 200mg/kg dose.

A- Diabetic control, N- Normal Control, and C- 200 mg ADSP (test compound).
References
1. Saeedi P, Petersohn
I, Salpea P, Malanda B, Karuranga S et al. (2019) Global and regional diabetes
prevalence estimates for 2019 and projections for 2030 and 2045: Results from
the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin
Pract 157: 107843. [Crossref]
2. Zimmet P (2003) The
burden of type 2 diabetes: are we doing enough? Diabetes Metab 29:
6S9-6S18. [Crossref]
3. Mezil SA, Abed BA
(2021) Complication of Diabetes Mellitus. Annals Romanian Society Cell
Biology 25: 1546-1556.
4. Guo X, Li H, Xu H,
Woo S, Dong H et Al. (2012) Glycolysis in the control of blood glucose
homeostasis. Acta Pharmaceutica Sinica B 2: 358-367.
5. Burgos Morón E,
Abad Jiménez Z, Martínez de Marañón A, Iannantuoni F, Escribano López I et al.
(2019) Relationship between oxidative stress, ER stress, and inflammation in
type 2 diabetes: the battle continues. J Clin Med 8: 1385. [Crossref]
6. Salehi B, Ata A, V
Anil Kumar N, Sharopov F, Ramirez Alarcon K et al. (2019) Antidiabetic
potential of medicinal plants and their active components. Biomolecules
9: 551. [Crossref]
7. Ghorbani A (2013)
Best herbs for managing diabetes: A review of clinical studies. Braz J Pharm
Sci 49: 413-422.
8. Asgary S, Parkhideh
S, Solhpour S, Madani H, Mahzouni P et al. (2008) Effect of ethanolic extract
of Juglans regia L. on blood sugar in diabetes-induced rats. J Med Food
11: 533-538. [Crossref]
9. Kamyab H, Hejrati
S, Khanavi M, Malihi F, Mohammadirad A et al. (2010) Hepatic mechanisms of the
walnut antidiabetic effect in mice. Cent Eur J Biol 5: 304-309.
10. Bouwens L, Rooman I
(2005) Regulation of pancreatic betacell mass. Physiol Rev 85:
1255-1270. [Crossref]
11. Oh YS (2015)
Plant-Derived Compounds Targeting Pancreatic Beta Cells for the Treatment of
Diabetes, Evidence-Based Complement. Evid Based Complement Alternat Med 2015:
629863. [Crossref]
12. Kanetkar P, Singhal
R, Kamat M (2007) Gymnema sylvestre: a memoir. J Clin Biochem Nutr 41:
77-81. [Crossref]
13. Liu B, Asare Anane
H, Al Romaiyan A, Huang G, Amiel SA et al. (2009) Characterisation of the
insulinotropic activity of an aqueous extract of Gymnema sylvestre in mouse 𝛽-cells
and human islets of Langerhans. Cell Physiol Biochem 23: 125-132. [Crossref]
14. Best L, Elliott AC,
Brown PD (2007) Curcumin induces electrical activity in rat pancreatic 𝛽-cells
by activating the volume-regulated anion channel. Biochem Pharmacol 73:
1768-1775. [Crossref]
15. Meghana K, Sanjeev
G, Ramesh B (2007) Curcumin prevents streptozotocin-induced islet damage by
scavenging free radicals: a prophylactic and protective role. Eur J
Pharmacol 577: 183-191. [Crossref]
16. Hussain HEMA (2002)
Hypoglycemic, hypolipidemic and antioxidant properties of combination of
Curcumin from Curcuma longa, Linn, and partially purified product from Abroma
augusta, Linn. in streptozotocin induced diabetes. Indian J Clin Biochem
17: 33-43. [Crossref]
17. Abdel Aziz MT, El Asmar MF, Rezq AM, Mahfous SM, Wassef MA et al. (2013) The effect of a novel curcumin derivative on pancreatic islet regeneration in experimental type-1 diabetes in rats (long term study). Diabetol Metab Syndr 5: 75. [Crossref]
18. Kulkarni CP, Bodhankar SL, Ghule AE, Mohan V, Thakurdesai PA (2012) Antidiabetic activity of Trigonella foenumgraecum L. seeds extract (IND01) in neonatal streptozotocin-induced (n-STZ) rats. Diabetologia Croatica 1: 41.
