Evaluation of BRAF Gene Status in Gliomas
A B S T R A C T
Background: Development of different molecular markers has given a new insight in the glioma management. KIAA1549-BRAF gene fusion has a diagnostic and prognostic significance.
Aim: The aim of this study was to determine the KIAA1549-BRAF gene fusion in glioma and their correlation with various clinical parameters.
Material and Methods: Forty cases of glioma were studied for KIAA1549-BRAF gene fusion by reverse transcription-PCR (RT-PCR).
Results: Overall, KIAA1549-BRAF gene fusion was found in 22% (9/40) cases of glioma. Children had higher KIAA1549-BRAF fusion (72%; 8/11) as compared to adults (10%; 3/29) and this difference was statistically significant. Cerebellar location of tumor was significantly associated with KIAA1549-BRAF fusion. KIAA1549-BRAF fusion was highest in pilocytic astrocytoma (89%), and this difference was statistically significant. Statistically significant difference was noted between KIAA1549-BRAF fusion expression and WHO grade I glioma.
Conclusion: Overall, KIAA1549-BRAF gene fusion was found in 22% (9/40) cases of glioma. Childhood age, pilocytic astrocytoma histology, cerebellar location and WHO grade I tumor were significantly associated with KIAA1549-BRAF gene fusion.
Keywords
BRAF, glioma, pilocytic astrocytoma, brain tumor, MAPK Pathway
Introduction
Gliomas are the most frequent primary brain tumors and include a variety of different histological tumor types and malignancy grades. Histopathology is the gold standard for the typing and grading of gliomas. However, histological classification of glioma is associated with significant inter-observer variability. Recent progress in the molecular biology of gliomas, has acknowledged various markers of prognostic and predictive significance. Most crucial, however, are those markers that are used to predict response to certain therapies, thereby directing clinicians to a particular treatment while avoiding other potentially deleterious therapies. There has been an increasing use of molecular markers in the assessment and management of gliomas. Large-scale genome-wide surveys have been used to identify new biomarkers that have been rapidly developed as diagnostic and prognostic tools [1, 2].
Recent data from a phase I clinical trial with the specific inhibitor PLX4032 in patients with metastasized BRAF V600E mutant malignant melanoma nourishes the hope that BRAF V600E mutation may become a prime target of cancer therapy in the near future. The aim of this study was to determine the KIAA1549-BRAF gene fusion in glioma and their correlation with various clinical parameters. Our results have been discussed in the light of relevant literature [3].
Materials and Methods
In this prospective study, forty-one cases of glioma were included from March 2015 to December 2018. One patient was excluded from the study due to inadequate tumor tissue. Thus total 40 cases were considered for the study. Their clinical details in form of age, sex, duration of symptom, clinical features, radiological findings and operative details were noted. The slides were reviewed, and the histological diagnosis was established. Representative sections with tumor area were selected for RNA extraction from tissues using the RNeasy mini kit (QIAGEN), reverse transcribed, and amplified by PCR using BRAF and KIAA1549 primers. RT-PCR was performed with 40 cycles of denaturation for 50s at 94°C, annealing for 45s at 58°C and extension for 50 s at 72°C. PCR products were visualized by 8% acrylamide-gel electrophoresis, staining with gel red. The results of fusion products were correlated with clinico-pathological parameters. Statistical analysis of data was done using SPSS software version 13.0.
Table 1: Summary of our glioma cases (n=40).
|
Parameters |
Number of cases |
|
Age (years) |
|
|
Mean |
14 years |
|
Range |
8-75 years |
|
<16
year (Children) |
11 |
|
>16
year (Adult) |
29 |
|
Sex |
|
|
Male |
24 |
|
Female |
16 |
|
Location of tumor |
|
|
Frontal |
12 |
|
Temporal |
7 |
|
Temporo-parietal |
3 |
|
Parieto-occipital |
3 |
|
Cerebellum |
7 |
|
Optic
nerve |
2 |
|
Hypothalamus |
2 |
|
Brain stem |
3 |
|
Lateral
ventricle |
1 |
|
Histopathology |
|
|
Fibrillary
astrocytoma |
11 |
|
Pilocytic
astrocytoma |
10 |
|
Glioblastoma |
8 |
|
Anaplastic
astrocytoma |
5 |
|
Ependymoma |
3 |
|
Oligodendroglioma |
3 |
|
RT-PCR (KIAA1549-BRAF fusion) |
|
|
Positive |
9 |
|
Negative |
31 |
Results
Out of forty study cases, there were 24 males and 16 females. Age range was 8-75 years with mean age of 30.4 years. There were 11 pediatric cases (age below 16 years) and 29 adult cases (age above 16 years). The most common clinical feature was seizure (n=12) and cerebellar signs (n=7). Most common location of glioma was frontal lobe (n=12) followed by cerebellum (n=7) and temporal lobe (n=7). Glioma was having contrast enhancement (n=38), and cystic degeneration (n=18). Surgical intervention in form of near total tumor excision (n=12), gross total tumor excision (n=25), tumor decompression (n=2) and tumor biopsy (n=1) was performed. The most common histopathology was astrocytic tumors (n=34) followed by oligodendroglial tumors (n=3) and ependymal tumor (n=3). The most common astrocytic tumor was fibrillary astrocytoma (n=11), pilocytic astrocytoma (n=10), glioblastoma (n=8) followed by anaplastic astrocytoma (n=5). Overall, the most common WHO grade of tumor was grade II (n=17), followed by grade I (n=10) and grade IV (n=8). Table 1 summarizes the details of our cases.
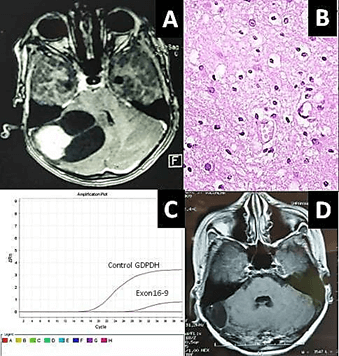
KIAA1549-BRAF fusion was positive in 22% (9/40) cases of glioma. On correlating KIAA1549-BRAF fusion with various clinical parameters, it was observed that KIAA1549-BRAF fusion was higher in children (72%; 8/11) as compared to adults (0.3%; 1/29) and this difference was statistically significant (Table 2; P=<0.001). However, KIAA1549-BRAF fusion was higher in males (25%; 6/24) as compared to and females (19%; 3/16) and this difference was statistically not significant (Table 2; P=0.106). For different sites of tumor location, KIAA1549-BRAF fusion ranged from 0% (Lateral ventricle, optic nerve, temporal, temporo-parietal and parieto-occipital) to 86% (cerebellum). On applying Fisher’s exact test, cerebellar location of tumor was significantly associated with KIAA1549-BRAF fusion (Table 3; P=0.002). KIAA1549-BRAF fusion was highest in pilocytic astrocytoma (80%) followed by and oligodendroglioma (33%) and was not encountered in any case of fibrillary or anaplastic astrocytoma, glioblastoma and ependymoma, and this difference was statistically significant (Table 4; P=0.001; Figure 1). In one of our cases, the radiological and intraoperative impression was cerebellar pilocytic astrocytoma. The histopathology revealed tumor cells disposed in sheet displaying uniform fibrillary astrocytic cells suggestive of diffuse fibrillary astrocytoma. As radiological and intraoperative findings were suggestive of pilocytic astrocytoma, hence, BRAF fusion by RT-PCR (Taqman assay) was applied which was positive for KIAA1549-BRAF (exon 16-9) fusion. Thus, the final diagnosis of pilocytic astrocytoma was given (Figure 2). KIAA1549-BRAF fusion was highest in WHO grade I glioma (80%; 8/10) followed by grade II glioma (0.6%; 1/17). KIAA1549-BRAF fusion was not seen in any case of WHO grade III and IV glioma. Statistically significant difference was noted between KIAA1549-BRAF fusion expression and WHO grade I glioma (Table 5; P=<0.001).
Table 2: Association of KIAA1549-BRAF gene fusion with demographic
variables.
|
No. |
Variable |
BRAF fusion present |
BRAF fusion absent |
Total |
P value |
||
|
No. |
% |
No. |
% |
||||
|
1. |
Age |
||||||
|
<16 years |
8 |
73 |
3 |
27 |
11 |
<0.001 |
|
|
>16 years |
1 |
0.3 |
28 |
99.7 |
29 |
||
|
Total |
9 |
22 |
31 |
78 |
40 |
|
|
|
2. |
Gender |
||||||
|
Male |
6 |
25 |
18 |
75 |
24 |
0.106 |
|
|
Female |
3 |
19 |
13 |
81 |
16 |
||
|
Total |
9 |
22 |
31 |
78 |
40 |
|
|
Chi-square test used/Fisher exact test used.
Table 3: Association of KIAA1549-BRAF gene fusion with tumor location.
|
No. |
Tumor location |
BRAF fusion present |
BRAF fusion absent |
Total |
P value
|
||
|
No. |
% |
No. |
% |
||||
|
1. |
Frontal |
1 |
8 |
11 |
92 |
12 |
0.002 |
|
2. |
Cerebellar |
6 |
86 |
1 |
14 |
7 |
|
|
3. |
Temporal |
0 |
0 |
7 |
100 |
7 |
|
|
4. |
Temporo-parietal |
0 |
0 |
3 |
100 |
3 |
|
|
5. |
Parieto-occipital |
0 |
0 |
3 |
100 |
3 |
|
|
6. |
Hypothalamus |
1 |
50 |
1 |
50 |
2 |
|
|
7. |
Optic nerve |
0 |
0 |
2 |
100 |
2 |
|
|
8. |
Brain stem |
1 |
33 |
2 |
67 |
3 |
|
|
9. |
Lateral ventricle |
0 |
0 |
1 |
100 |
1 |
|
|
|
Total |
9 |
22 |
31 |
78 |
40 |
|
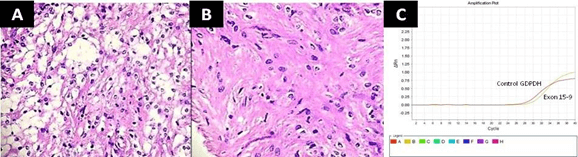
Figure 1: Photomicrograph (Haematoxylin and eosin stain) of pliocytic astrocytoma showing Typical biphasic pattern of compact, A) fiber-rich areas and hypocellular areas with microcysts, B) compacted piloid cells with long, bipolar tumor cells, KIAA1549- BRAF fusion (exon 15-9) is positive by RT-PCR, C) compared with the positive control GAPDH.
Table 4: Association of BRAF gene fusion with glioma histological subtype.
|
No. |
Histopathology |
BRAF fusion present |
BRAF fusion absent |
Total |
P value |
||
|
No. |
% |
No. |
% |
||||
|
1. |
Fibrillary astrocytoma |
0 |
00 |
11 |
100 |
11 |
0.001 |
|
2. |
Pilocytic astrocytoma |
8 |
80 |
2 |
20 |
10 |
|
|
3. |
Glioblastoma |
0 |
00 |
8 |
100 |
8 |
|
|
4. |
Anaplastic astrocytoma |
0 |
00 |
5 |
100 |
5 |
|
|
5. |
Ependymoma |
0 |
00 |
3 |
100 |
3 |
|
|
6. |
Oligodendroglioma |
1 |
33 |
2 |
67 |
3 |
|
|
Total |
9 |
22 |
31 |
78 |
40 |
||
Fisher exact test used.
Table 5: Association of BRAF gene fusion with WHO grade of glioma.
|
No. |
WHO grade |
BRAF fusion present |
BRAF fusion absent |
Total |
P value
|
||
|
No. |
% |
No. |
% |
||||
|
1. |
I |
8 |
80 |
2 |
20 |
10 |
<0.001 |
|
2. |
II |
1 |
6 |
16 |
94 |
17 |
|
|
3. |
III |
0 |
00 |
5 |
100 |
5 |
|
|
4. |
IV |
0 |
00 |
8 |
100 |
8 |
|
|
Total |
9 |
22 |
31 |
78 |
40 |
||
Fisher exact test used.
Figure 2: A) MRI axial section of a 7-year-old child presenting with cerebellar mass with enhancing mural nodule suggestive of pilocytic astrocytoma, B) Photomicrograph (Haematoxylin and eosin stain) showing tumor cells disposed in sheet displaying uniform fibrillary astrocytic cells suggestive of diffuse fibrillary astrocytoma, KIAA1549- BRAF fusion (exon 16-9) is positive by RT-PCR, C) compared with the positive control GAPDH, D) Follow up MRI axial section after 3 years, showing no recurrence of tumor.
Discussion
BRAF, a proto-oncogene, located in the chromosome 7 (7q34), is a member of RAF (ras activating factor) family of proteins which includes ARAF, BRAF and CRAF. These proteins have serine-threonine kinase domain and once they are activated by ras proteins, phosphorylate the proteins of MAPK/ERK pathway. This pathway in turn regulates cellular differentiation, proliferation, and migration, and is thought to contribute to the tumorigenesis of a variety of human malignancies [1-4]. Approximately, 7% of all cancers show mutations in BRAF whereas those occurring in ARAF and CRAF are rare. BRAF has been identified as a highly ‘druggable’ target protein (i.e., capable of being bound by drug-like compounds) in melanomas and analogous clinical trials in gliomas has already begun [2]. There are two ways of turning on BRAF gene in gliomas. The most frequent BRAF alteration in gliomas is tandem or focal duplication/fusion. This tandem duplication on 7q34 occurs as result of fusion between a gene BRAF and another gene of unknown function which lies centromeric to BRAF. This fusion oncogene has loss of the ras-binding domain on BRAF [5, 6]. The end result is to delete the N-terminal ras-binding regulatory domain, producing constitutive BRAF (or CRAF) activity [5, 7-14]. The most common tumor related fusion occurs in BRAF followed by CRAF and not at all in ARAF.
BRAF fusions are present in approximately 70% of all pilocytic astrocytomas (PAs) compared with about 15% of all other low-grade gliomas (diffuse grade II astrocytoma, ganglioglioma, and so forth) [3, 5, 6, 8, 15-17]. They are hardly ever seen in high-grade pediatric gliomas [6]. In our study, 80% of PAs showed KIAA1549-BRAF fusion positive by RT-PCR. None of our high-grade glioma demonstrated KIAA1549-BRAF fusion. Frequency of BRAF fusion also changes with the site. Nearly 80% of cerebellar PAs have these fusions, compared to only 50-55% of non-cerebellar PAs [3, 5, 6, 9, 15, 16, 18, 19]. In our study, 86% (6/7) of our cerebellar PAs cases had KIAA1549-BRAF fusion.
In addition to histological features and site, age also affects the likelihood of a BRAF fusion being present. The occurrence of BRAF fusions appears to decline with increase in patient’s age, from about 80% in the first decade to 50% in the second decade, with less than 10% of PAs in patient over 40 years [20]. We also noted that KIAA1549-BRAF fusion was higher in children (72%; 8/11) as compared to adults (10%; 3/29) and this difference was statistically significant. Another way to turn on BRAF is valine to glutamate (V600E) point mutation in exon 15, less commonly in exon 11 at 600 residues, which activates MEK without first needing upstream ras phosphorylation leading to constitutive BRAF activity. BRAF V600E exists in diverse tumors, including melanocytic nevi, melanoma, colon cancer, and papillary thyroid cancer. BRAF mutation has been reported in 10-15% of grade II-IV diffusely infiltrative pediatric astrocytomas, but in less than 2% of comparable adult gliomas [12, 21-25]. In both children and adults, less than 10% of all PAs have BRAF mutation, and it is present in only 2% of cerebellar PA [5, 6, 8, 15-18, 21, 22]. Rarely, concomitant mutation and BRAF fusion has also been reported in the same tumor [8, 26]. V600E mutation is more associated with tumors that come in the differential diagnosis of PAs such as 20-25% of pediatric and adult gangliogliomas and 60-80% of pleomorphic xanthoastrocytomas in both age groups [8, 17, 21, 25, 26]. We did not study BRAF mutation in our study.
Thus, over 75% of sporadic PAs have some sort of BRAF alteration, compared to about 40-50% of all other gliomas that come in the differential diagnosis of PA [13, 18, 26-28]. We also noted KIAA1549-BRAF fusion in one of our frontal oligodendroglioma case. Low grade gliomas under microscopy from a diagnostics standpoint if show a BRAF fusion is suggestive of a PA, but whether it can be used to definitively prove PA is still unclear.
In contrast, detecting a V600E mutation in low grade gliomas is more associated with other tumors in the PA differential like ganglioglioma, pleomorphic xanthoastrocytoma or diffuse fibrillary astrocytoma, but again cannot be used to definitively prove one type of tumor versus another. In one patient of our series, the radiological and intraoperative impression was of cerebellar pilocytic astrocytoma. The histopathology was suggestive of diffuse fibrillary astrocytoma. As radiological and intraoperative findings were suggestive of pilocytic astrocytoma, hence, BRAF fusion by RT-PCR was applied which was positive. Thus, the final diagnosis of pilocytic astrocytoma was given. This shows the importance of BRAF fusion test in differentiating PA from low grade glioma.
Some of the studies have shown that regardless of the histology, presence of a BRAF fusion is associative with favourable prognosis [8, 26, 29, 30]. However, few contradict this finding by reporting no association with progression-free survival independent of location [6, 31]. From an outcome-based perspective, recent work shows that supratentorial low-grade pediatric gliomas with BRAF fusion usually behave as typical grade I PAs irrespective of histology and status of surgery i.e., whether they are completely resected or not; whereas PAs without BRAF fusion are more likely to behave like grade II diffuse astrocytomas [9]. Similar trend was seen in PAs in the cerebellum [16]. In another study, 118 pediatric WHO grade II diffuse astrocytomas and oligodendrogliomas, 6 had tumor in posterior fossa had BRAF fusion and all 6 were extremely indolent despite their grade II microscopic appearance [6]. However, two studies that included pediatric low-grade gliomas from both the supratentorium and posterior fossa did not show BRAF fusion to be an independent prognostic variable on multivariate analysis [26, 31]. There is not yet a consensus on the prognostic impact of BRAF fusion in gliomas. It appears to be at least a neutral biomarker, and perhaps even a favourable marker in certain contexts.
In contrast, data on BRAF V600E mutations and outcome are very sparse. Recent work comparing both types of BRAF alterations in the same cohort of pediatric low-grade gliomas suggested that there is a trend toward divergence in prognosis- i.e., BRAF fusions tend to be associated with longer progression free survival, while BRAF V600E mutations suggest shorter progression free survival [26]. Interestingly, in that same cohort neither alteration was more powerful than the presence of p16 deletion, which was a significant independent adverse prognostic marker. This makes sense because loss of p16 inhibits BRAF-induced tumor senescence [32, 33].
The potential for rapid implementation of targeted therapeutics against continuative BRAF activity fusion/mutation is higher than typical because there are already many pharmacologic inhibitors that theoretically should work on these tumors. The MEK inhibitor AZD6244 (Selumetinib) is undergoing various phase I and II trials for BRAF mutant solid tumors, including pediatric gliomas. Certainly, the experience with melanomas and PLX4032 (Vemurafenib) is reason for some optimisms [34].
Limitations
The main limitation of our study is small number of cases. Further studies with large cohorts are required to validate the role of BRAF gene status in glioma patients.
Conclusion
In our study, overall, KIAA1549-BRAF fusion was found in 22% (9/40) cases of glioma. Childhood age, pilocytic astrocytoma histology, cerebellar location and WHO grade I tumor were significantly associated with KIAA1549-BRAF gene fusion as demonstrated in other studies.
Acknowledgement
The authors thankfully acknowledge the Research Cell, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India for providing financial support for this study.
Conflicts of Interest
None.
Funding
The study was funded by intramural research grant from Research cell, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 17, Aug 2020Accepted: Thu 29, Jul 2021
Published: Mon 16, Aug 2021
Copyright
© 2023 Awadhesh Kumar Jaiswal. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2021.03.02
Author Info
Awadhesh Kumar Jaiswal Sarita Agrawal Sushila Jaiswal Kuntal Kanti Das Sanjay Behari Swasti Tewari Madam Mohan Godbole Prabhakar Misra
Corresponding Author
Awadhesh Kumar JaiswalDepartment of Neurosurgery, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow, Uttar Pradesh, India
Figures & Tables
Table 1: Summary of our glioma cases (n=40).
|
Parameters |
Number of cases |
|
Age (years) |
|
|
Mean |
14 years |
|
Range |
8-75 years |
|
<16
year (Children) |
11 |
|
>16
year (Adult) |
29 |
|
Sex |
|
|
Male |
24 |
|
Female |
16 |
|
Location of tumor |
|
|
Frontal |
12 |
|
Temporal |
7 |
|
Temporo-parietal |
3 |
|
Parieto-occipital |
3 |
|
Cerebellum |
7 |
|
Optic
nerve |
2 |
|
Hypothalamus |
2 |
|
Brain stem |
3 |
|
Lateral
ventricle |
1 |
|
Histopathology |
|
|
Fibrillary
astrocytoma |
11 |
|
Pilocytic
astrocytoma |
10 |
|
Glioblastoma |
8 |
|
Anaplastic
astrocytoma |
5 |
|
Ependymoma |
3 |
|
Oligodendroglioma |
3 |
|
RT-PCR (KIAA1549-BRAF fusion) |
|
|
Positive |
9 |
|
Negative |
31 |
Table 2: Association of KIAA1549-BRAF gene fusion with demographic
variables.
|
No. |
Variable |
BRAF fusion present |
BRAF fusion absent |
Total |
P value |
||
|
No. |
% |
No. |
% |
||||
|
1. |
Age |
||||||
|
<16 years |
8 |
73 |
3 |
27 |
11 |
<0.001 |
|
|
>16 years |
1 |
0.3 |
28 |
99.7 |
29 |
||
|
Total |
9 |
22 |
31 |
78 |
40 |
|
|
|
2. |
Gender |
||||||
|
Male |
6 |
25 |
18 |
75 |
24 |
0.106 |
|
|
Female |
3 |
19 |
13 |
81 |
16 |
||
|
Total |
9 |
22 |
31 |
78 |
40 |
|
|
Chi-square test used/Fisher exact test used.
Table 3: Association of KIAA1549-BRAF gene fusion with tumor location.
|
No. |
Tumor location |
BRAF fusion present |
BRAF fusion absent |
Total |
P value
|
||
|
No. |
% |
No. |
% |
||||
|
1. |
Frontal |
1 |
8 |
11 |
92 |
12 |
0.002 |
|
2. |
Cerebellar |
6 |
86 |
1 |
14 |
7 |
|
|
3. |
Temporal |
0 |
0 |
7 |
100 |
7 |
|
|
4. |
Temporo-parietal |
0 |
0 |
3 |
100 |
3 |
|
|
5. |
Parieto-occipital |
0 |
0 |
3 |
100 |
3 |
|
|
6. |
Hypothalamus |
1 |
50 |
1 |
50 |
2 |
|
|
7. |
Optic nerve |
0 |
0 |
2 |
100 |
2 |
|
|
8. |
Brain stem |
1 |
33 |
2 |
67 |
3 |
|
|
9. |
Lateral ventricle |
0 |
0 |
1 |
100 |
1 |
|
|
|
Total |
9 |
22 |
31 |
78 |
40 |
|
Table 4: Association of BRAF gene fusion with glioma histological subtype.
|
No. |
Histopathology |
BRAF fusion present |
BRAF fusion absent |
Total |
P value |
||
|
No. |
% |
No. |
% |
||||
|
1. |
Fibrillary astrocytoma |
0 |
00 |
11 |
100 |
11 |
0.001 |
|
2. |
Pilocytic astrocytoma |
8 |
80 |
2 |
20 |
10 |
|
|
3. |
Glioblastoma |
0 |
00 |
8 |
100 |
8 |
|
|
4. |
Anaplastic astrocytoma |
0 |
00 |
5 |
100 |
5 |
|
|
5. |
Ependymoma |
0 |
00 |
3 |
100 |
3 |
|
|
6. |
Oligodendroglioma |
1 |
33 |
2 |
67 |
3 |
|
|
Total |
9 |
22 |
31 |
78 |
40 |
||
Fisher exact test used.
Table 5: Association of BRAF gene fusion with WHO grade of glioma.
|
No. |
WHO grade |
BRAF fusion present |
BRAF fusion absent |
Total |
P value
|
||
|
No. |
% |
No. |
% |
||||
|
1. |
I |
8 |
80 |
2 |
20 |
10 |
<0.001 |
|
2. |
II |
1 |
6 |
16 |
94 |
17 |
|
|
3. |
III |
0 |
00 |
5 |
100 |
5 |
|
|
4. |
IV |
0 |
00 |
8 |
100 |
8 |
|
|
Total |
9 |
22 |
31 |
78 |
40 |
||
Fisher exact test used.
References
1. Dhomen N, Marais R
(2007) New insight into BRAF mutations in cancer. Curr Opin Genet Dev
17: 31-39. [Crossref]
2. Keller TH, Pichota
A, Yin Z (2006) A practical view of 'druggability'. Curr Opin Chem Biol
10: 357-361. [Crossref]
3. Bar EE, Lin A,
Tihan T, Burger PC, Eberhart CG (2008) Frequent gains at chromosome 7q34
involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol 67:
878-887. [Crossref]
4. Roberts PJ, Der CJ
(2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for
the treatment of cancer. Oncogene 26: 3291-3310. [Crossref]
5. Forshew T,
Tatevossian RG, Lawson AR, Ma J, Neale G et al. (2009) Activation of the
ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic
astrocytomas. J Pathol. 218: 172-181. [Crossref]
6. Jones DTW,
Kocialkowski S, Liu L, Pearson DM, Bäcklund LM et al. (2008) Tandem duplication
producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic
astrocytomas. Cancer Res 68: 8673-8677. [Crossref]
7. Hasselblatt M,
Riesmeier B, Lechtape B, Brentrup A, Stummer W et al. (2011) BRAF-KIAA1549
fusion transcripts are less frequent in pilocytic astrocytomas diagnosed in
adults. Neuropathol Appl Neurobiol 37: 803-806. [Crossref]
8. Hawkins C, Walker E, Mohamed N, Zhang C, Jacob
K et al. (2011) BRAF-KIAA1549 fusion predicts better clinical outcome in
pediatric low-grade astrocytoma. Clin Cancer Res 17: 4790-4798. [Crossref]
9. Jacob K, Albrecht
S, Sollier C, Faury D, Sader E et al. (2009) Duplication of 7q34 is specific to
juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway
tumours. Br J Cancer 101: 722-733. [Crossref]
10. Korshunov A, Meyer J, Capper D, Christians A,
Remke M et al. (2009) Combined molecular analysis of BRAF and IDH1
distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta
Neuropathol 118: 401-405. [Crossref]
11. Lawson ARJ,
Tatevossian RG, Phipps KP, Picker SR, Michalski A et al. (2010) RAF gene
fusions are specific to pilocytic astrocytoma in a broad paediatric brain
tumour cohort. Acta Neuropathol 120: 271-273. [Crossref]
12. Schiffman JD,
Hodgson JG, VandenBerg SR, Flaherty P, Polley MYC et al. (2010) Oncogenic BRAF
mutation with CDKN2A inactivation is characteristic of a subset of pediatric
malignant astrocytomas. Cancer Res 70: 512-519. [Crossref]
13. Yu J, Deshmukh H,
Gutmann RJ, Emnett RJ, Rodriguez FJ et al. (2009) Alterations of BRAF and HIPK2
loci predominate in sporadic pilocytic astrocytoma. Neurology 73:
1526-1531. [Crossref]
14. Tatevossian RG,
Lawson AR, Forshew T, Hindley GFL, Ellison DW et al. (2010) MAPK pathway
activation and the origins of pediatric low-grade astrocytomas. J Cell
Physiol 222: 509-514. [Crossref]
15. Pfister S, Janzarik
WG, Remke M, Ernst A, Werft W et al. (2008) BRAF gene duplication constitutes a
mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin
Invest 118: 1739-1749. [Crossref]
16. Horbinski C,
Hamilton RL, Nikiforov Y, Pollack IF (2010) Association of molecular
alterations, including BRAF, with biology and outcome in pilocytic
astrocytomas. Acta Neuropathol 119: 641-649. [Crossref]
17. Sievert AJ, Jackson
EM, Gai X, Hakonarson H, Judkins AR et al. (2009) Duplication of 7q34 in
pediatric low-grade astrocytomas detected by high-density single-nucleotide
polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain
Pathol 19: 449-458. [Crossref]
18. Cin H, Meyer C,
Herr R, Janzarik WG, Lambert S et al. (2011) Oncogenic FAM131B-BRAF fusion
resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway
activation in pilocytic astrocytoma. Acta Neuropathol 121: 763-774. [Crossref]
19. Huang H, Okamoto Y,
Yokoo H, Heppner FL, Vital A et al. (2004) Gene expression profiling and
subgroup identification of oligodendrogliomas. Oncogene 23: 6012-6022. [Crossref]
20. Hasselblatt M,
Riesmeier B, Lechtape B, Brentrup A, Stummer W et al. (2011) BRAF-KIAA1549
fusion transcripts are less frequent in pilocytic astrocytomas diagnosed in
adults. Neuropathol Appl Neurobiol 37: 803-806. [Crossref]
21. Schindler G, Capper
D, Meyer J, Janzarik W, Omran H et al. (2011) Analysis of BRAF V600E mutation
in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic
xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta
Neuropathol 121: 397-405. [Crossref]
22. Nicolaides TP, Li H,
Solomon DA, Hariono S, Hashizume R et al. (2011) Targeted therapy for
BRAFV600E malignant astrocytoma. Clin Cancer Res 17: 7595-7604. [Crossref]
23. Basto D, Trovisco
V, Lopes JM, Martins A, Pardal F et al. (2005) Mutation analysis of B-RAF gene
in human gliomas. Acta Neuropathol 109: 207-210. [Crossref]
24. Knobbe CB,
Reifenberger J, Reifenberger G (2004) Mutation analysis of the Ras pathway
genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol 108:
467-470. [Crossref]
25. Dias Santagata D, Lam Q,
Vernovsky K, Vena N, Lennerz JK et al. (2011) BRAF V600E mutations
are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic
implications. PloS One 6: e17948. [Crossref]
26. Horbinski C,
Nikiforova MN, Hagenkord JM, Hamilton RL, Pollack IF (2012) Interplay among
BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro Oncol 14:
777-789. [Crossref]
27. Eisenhardt AE,
Olbrich H, Roring M, Janzarik W, Van Anh TN et al. (2010) Functional
characterization of a BRAF insertion mutant associated with pilocytic
astrocytoma. Int J Cancer 129: 2297-2303. [Crossref]
28. Jentoft M, Giannini
C, Cen L, Scheithauer BW, Hoesley B et al. (2010) Phenotypic variations in
NF1-associated low grade astrocytomas: possible role for increased mTOR
activation in a subset. Int J Clin Exp Pathol 4: 43-57. [Crossref]
29. Lin A, Rodriguez
FJ, Karajannis MA, Williams SC, Legault G et al. (2012) BRAF alterations in
primary glial and glioneuronal neoplasms of the central nervous system with
identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp
Neurol 71: 66-72. [Crossref]
30. Ida CM, Lambert SR,
Rodriguez FJ, Voss JS, Mc Cann BE et al. (2012) BRAF alterations are frequent
in cerebellar low-grade astrocytomas with diffuse growth pattern. J
Neuropathol Exp Neurol 71: 631-639. [Crossref]
31. Tihan T, Ersen A,
Qaddoumi I, Sughayer MA, Tolunay S et al. (2011) Pathologic characteristics of
pediatric intracranial pilocytic astrocytomas and their impact on outcome in 3
countries: a multi-institutional study. Am J Surg Pathol 36: 43-55. [Crossref]
32. Jacob K, Quang
Khuong DA, Jones DT, Witt H, Lambert S et al. (2011) Genetic aberrations
leading to MAPK pathway activation mediate oncogene-induced senescence in
sporadic pilocytic astrocytomas. Clin Cancer Res 17: 4650-4560. [Crossref]
33. Raabe EH, Lim KS, Kim JM, Meeker A, Mao XG et al. (2011) BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res 17: 3590-3599. [Crossref]
34. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507-2516. [Crossref]
