Journals
Ethyl acetate fraction from Passiflora foetida promotes rat femoral fracture healing by the BMP-2 signaling pathway
A B S T R A C T
Aim: To evaluate the effect of ethyl acetate fraction (EAF) from Passiflora foetida on bone regeneration following bone and marrow injury.
Materials and methods: EAF was administered for two weeks at 50, 100 and 200mg/kg doses orally to adult female Sprague-Dawley rats having a drill-hole injury in femur. Analysis of calcein labelling, BMD, micro-architectural parameters, tissue morphology at the drill-hole site, and serum level of bone turnover markers, mineralized nodule formation, expression of osteogenic genes and localization of BMP-2 protein was performed.
Results: EAF dose-dependently accelerated bone regeneration mainly by increasing BMD at the fracture site, serum levels of PINP, OCN, and also mineralized nodule formation in BMCs. In addition, EAF also enhanced microarchitecture of the regenerating bone evident from increased bone volume fraction, trabecular thickness, trabecular number, connective density and decreased trabecular separation and degree of anisotropy. The mechanism studies, EAF accelerated fracture healing in rats by the recruitment of osteoblasts through up-regulation of the BMP-2 signaling pathway at the fracture site.
Conclusion: EAF accelerated fracture healing in rats by the recruitment of osteoblasts through up-regulation of the BMP-2 signaling pathway at the fracture site, therefore could be taken as an alternative therapy for fracture healing.
K E Y W O R D S
Bone regeneration, trabecular microarchitecture, micro-computed tomography
I N T R O D U C T I O N
Bone fractures, one of the most common orthopaedic diseases, eventuate due to accidents or pathological conditions such as osteoporosis [1]. Being more and more common, the expenses of musculoskeletal injuries involved cause significant burdens on public health planning [2]. Repairing of bone at the fracture site is a complex physiological process, commencing after the local bleeding and inflammation, which is accompanied by the complicated activities of mesenchymal precursor cells leading to the formation of soft extracellular matrix tissue, cartilage and the bone [3]. It involves a well-orchestrated pattern of events which is responsible for the migration, proliferation and differentiation of osteoprogenitor cells to endothelial cells and osteoblast cells, which form vascular tissues and bone tissues, respectively, at the fracture site [3].
Metabolic bone disorders involving primary and secondary osteoporosis fundamentally occur due to the reduction in osteoblast function, which leads to high risk of fragility and fracture [4, 5]. A few number of pharmacological interventions which minimize the risk of fractures in osteoporosis contain bisphosphonates, selective estrogen receptor modulators (SERMs) and calcitonin [6-8]. Parathyroid hormone (PTH 1-34) treatment is the only anabolic therapy available in the market for postmenopausal osteoporosis but it has a black-box warning issued by the US FDA because it’s long term use increases the risk of osteosarcoma in rats [9]. Although these pharmacological interventions are available for clinical use, yet the research is ongoing due to the lack of sufficient benefit-to-risk ratio.
Since there is non-availability of pharmacological interventions as oral administration for rapid fracture repair but traditional mode of plant-based medicine globally has huge mention of herbal extracts which shows positive effect on fracture repair [10]. However, the action of making these effects acceptable via controlled studies is inadequate in the literature. Medicinal plants are the only source of health care management for a huge part of the world’s population and continue to play an exuberant role in the health delivery systems. Most of the people in several developing countries still utilize medicinal plants to treat bone related disorders due to the side effects of synthetic drugs and their higher cost. The efficacy of the few medicinal plants extracts to enhance the bone fracture repair process has been reported including Dalbergia sissoo, Spinacia oleracea and Peperomia pellucida [10-12].
Passiflora foetida, commonly known as stinking passion flower, an Indian medicinal plant from the family of Passifloraceae is a herbaceous climber, native of tropical America and found in many parts of India [13]. Traditionally it is used for diarrhoea, intestinal tract, throat, ear infections, fever, skin diseases, vomiting, eczema, chronic ulcer, asthma, biliousness and nervous disorders [14]. It is also reported for Passiflora foetida to have sedative, hypnotic, antispasmodic, hepatoprotective and anodyne properties [15]. Recently, we have reported the anti-osteoporotic activity of butanolic fraction from Passiflora foetida in ovariectomy-induced bone loss in mice [15]. To the best of our knowledge, fracture healing effect of Passiflora foetida has not been scientifically evaluated. Therefore, we demonstrated the effects of ethyl acetate fraction (EAF) from Passiflora foetida on fracture healing in rats using a drill-hole injury model of bone and bone marrow. In this study, fracture healing potential of EAF was investigated in rats using a drill-hole injury model of bone.
Materials and Methods
Reagents and chemicals
All chemicals, cell culture media, supplements and PTH were purchased from Invitrogen (Carlsbad, CA), Sigma-Aldrich (St Louis, MO) and Calbiochem (San Diego, CA).
Plant material
Ariel parts of Passiflora foetida (L.) were collected from Telangana, India in January 2012, identified and authenticated by a botanist, Dr. N. Venkateshwrlu. A voucher specimen has been preserved in the investigator’s laboratory with specimen number CDRI–25012.
Animal study
All the animal studies were designed and approved by the Institutional Animal Ethics Committee (IAEC), Council of Scientific and Industrial Research-Central Drug Research Institute (CSIR-CDRI) and performed according to the regulations of the Council for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India. Fifty female SD rats (200±20 g) were taken from the National Laboratory Animal Centre of CSIR-CDRI, Lucknow India. Animals were divided into five groups of equal number as follows: control + vehicle (gum acacia in distilled water), EAF (50, 100 and 200 mg/kg/day) and PTH. Treatment to the PTH group was based on our previous published study on SD rats [12]. Animals were kept in a 12h light–dark cycle, with controlled temperature (22–24°C) and humidity (50–60%) and free access to standard rodent food and water.
Drill-hole injury in femur
A drill-hole injury was done in vehicle as well as treatment groups as described earlier [11, 12]. The front skin of the mid portion of the femur in rats was cut straight and longitudinally at 1 cm in length under anesthesia. Femoral bone surface was exposed by stripping the periosteum after splitting the muscle. 2 cm above the knee joint, a drill-hole injury was created with a drill bit of 0.8 mm diameter in the diaphysis of femur. The treatment was started after one day of drill-hole injury and continued for two weeks. One day before autopsy, all animals received intraperitoneal injection of a fluorochrome calcein (20 mg/kg). After the treatment period of the various groups described earlier, all the animals were euthanized and autopsied to collect the femur bones for micro-CT analysis and dynamic histomorphometric study.
Bone mineral density (BMD)
Analysis of the volumetric bone mineral density (vBMD) at the fracture site was conducted by using micro-CT scan following the previously published protocols [16, 17]. Machine was calibrated by using 2 mm diameter hydroxyapatite (HA) phantom rods with known BMD (0.25 g/cm3 and 0.75 g/cm3) [16]. Analysis was done based on a linear correlation between BMD and micro-CT attenuation coefficient [16].
Micro-computed tomography (μCT)
2-D and 3-D analysis of bone internal microstructure of the mineralized tissue at the drill-hole site was performed by using μCT Sky Scan 1076 CT scanner (Aartselaar, Belgium). Bones were scanned with X-ray source of 70KV, 100mA with a pixel size of 18μm. Reconstruction of images was done by using Sky Scan Nrecon software. Network-distributed reconstruction was done with the help of Nrecon software in four personal computers running simultaneously. Bone callus formation area at the fracture site was selected with CT analyzer software by outlining ellipsoid contour. 3-D micro-architecture parameters of bone such as BV/TV (bone volume fraction, %), Tb.Th (trabecular thickness, mm), Tb.Sp (trabecular separation, mm), Tb.N (trabecular number, 1/mm), DA (degree of anisotropy) and Conn.Dn (connection density, 1/mm3) were analysed as described earlier [11, 12].
Bone strength testing
To assess the bone biomechanical properties, we performed the three-point bending test using a bone strength tester (model TK-252C; Muromachi Kikai, Co. Ltd., Tokyo Japan). Femur bones were kept horizontally on the fixture (14 mm span). Vertical rounded point was employed to load on fracture site (drill-hole site). The force displacement curve was noted during loading at the drill-hole site. The maximum force required to break the bone, energy and the stiffness was documented.
Ex-vivo culture of BMCs (Bone marrow cells)
For mineralization study, we followed our previous published study [18]. Bone marrow cells were collected from femur bone and cultured in osteogenic differentiation medium containing 10-7M dexamethasone. Cells were plated 12-well plates and cultured for 21 days. The medium was refreshed once every 48h. Alizarin Red S dye was used to stain the mineralized nodule formation and quantified by taking optical density at 405nm.
Real-time polymerase chain reaction (qPCR)
Gene expression analysis of BMP-2, Col-I and OCN was performed at the fracture site after EAF treatment for 2 weeks. The drill-hole region of bones including marrow tissue were carefully cut with 2 mm margins with the help of a surgical scalpel, and then crushed in liquid nitrogen [11]. Total RNA was isolated by using TRIzol (Invitrogen) according to the procedure describe by the manufacturer. The concentration of RNA samples was determined by using a spectrophotometer (NANO-Drop). Synthesis of cDNA was done with RevertAid first strand cDNA synthesis kit (Fermentas, Austin, USA) from 2μg of total RNA. SYBR green chemistry was done for quantitative determination of gene expression and the housekeeping gene GAPDH [19, 20]. Primer sequences of the genes used in this study are shown in (Table 1).
Bone turnover markers
Serum bone formation markers such as PINP (procollagen type I N-terminal propeptide) and OCN (osteocalcin) levels were measured after EAF treatment in the fracture healing progress. During autopsy, blood samples were taken from all the animals followed by serum collection by centrifuging blood samples at 2000g for 20 min at 4°C and stored at -80°C for further analysis [21]. The serum PINP and OCN levels were determined using enzyme-linked immunosorbent assay kit (Qayee Bio-Technology Co. Ltd., Shanghai, China) by following the manufacturer's protocols.
Table
Table 1: Primer sequence of various genes used for qPCR.
|
Gene Symbol |
Gene Name |
Primer Sequence |
Accession Number |
|
GAPDH |
glyceraldehyde-3-phosphate dehydrogenase |
F-CAGCAAGGATACTGAGAGCAAGAG R-GGATGGAATTGTGAGGGAGATG |
NM-017008 |
|
BMP-2 |
Bone morphogenetic protein-2 |
F- CCCCTATATGCTCGACCTGT R-AAAGTTCCTCGATGGCTTCTT |
NM_017178.1 |
|
COL1 |
Type I Collagen |
F- CATGTTCAGCTTTGTGGACCT R- GCAGCTGACTTCAGGGATGT |
NM_053304 |
|
OCN |
Osteocalcin |
F-ATAGACTCCGGCGCTACCTC R-CCAGGGGATCTGGGTAGG |
NM-013414 |
qPCR; quantitative polymerase chain reaction, F; forward, R; reverse
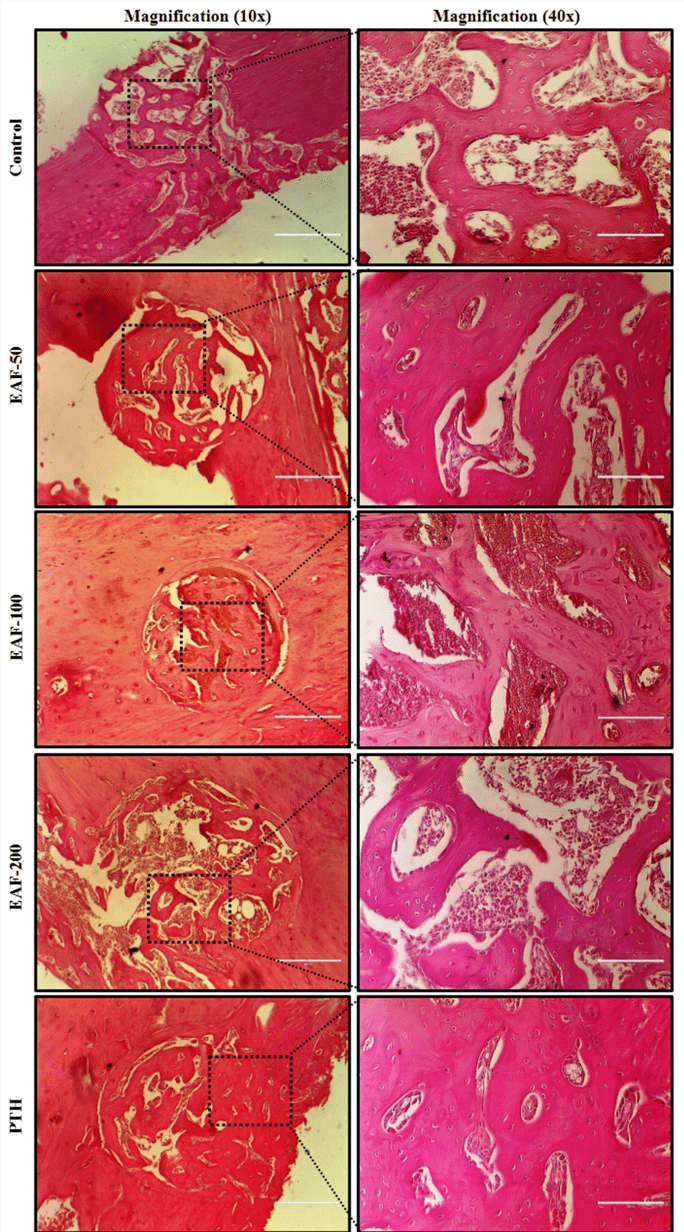
Histological staining
Morphology of the bone tissue at the fracture site was observed after EAF treatment in the fracture healing progress. During autopsy, femur bones with drill-hole injury were dissected out and cleaned. Bones were decalcified in 1% EDTA and subsequently embedded in paraffin. For Histological analysis of the bone at the fracture site, sections of 5μm size were cut through the long axis of femur. Paraffin sections were de-waxed, re-hydrated and stained with haematoxylin and eosin (H&E) [21]. Representative images of the H&E stained sections were selected for observation.
Immunofluorescence
BMP-2 protein expression at the fracture site after EAF treatment was measured by using immunofluorescence method [19, 22, 23]. Femur bones were separated from the knee joint followed by cleaning of soft tissues. Femur bones with drill-hole sites were fixed in 4% paraformaldehyde, followed by decalcification in 1% EDTA. Then, bones were embedded in paraffin wax to cut 5μm sections. Immunofluorescence analysis of the localization of BMP-2 protein in femoral bone sections at the injury site was performed by using specific antibodies of BMP-2. Deparaffinization and rehydration of the sections were carried out using xylene and ethanol gradient, respectively and permeabilized with 0.1% Triton X-100, then followed by blocking with 1% BSA. Sections were incubated with BMP-2 antibody diluted in 0.5% BSA (1:500) at 4°C for overnight. Then, sections were washed in PBS (pH 7.4), and incubated with Cy-3 goat anti-rabbit antibody (1:300) for 2h at room temperature. Counter staining was done with DAPI for 15 minutes, followed by washing in PBS and mounting with antifade mounting media ((life technologies, Carlsbad, CA, USA). Visualization of the sections was done using Cell Imaging Station (life technologies, Carlsbad, CA, USA).
Antioxidant activity
DPPH free radical scavenging activity assay
DPPH (2, 2-diphenyl-1-picryl-hydrazyl-hydrate), a free radical dye is used to evaluate the antioxidant properties of the plant materials. In this assay, DPPH produces free radicals that gives a violet colour solution and this violet solution is reduced in the presence of an antioxidant molecule, changes measured in spectrophotometric measurement. DPPH was dissolved in methanol, and EAF from Passiflora foetida was serially diluted from 1000 μg/ml to 1.95 μg/ml. DPPH radicals and different concentrations of EAF were used in 1:1 ratio. The DPPH solution with or without EAF concentrations was allowed to keep at room temperature for 30 minutes, then measured at 517nm. The DPPH scavenging effect (anti-oxidant activity) of EAF was measured as follows: DPPH scavenging effect (%) = (Abc – Abs) / Abc × 100. (Abc is value of DPPH without the sample (EAF); Abs is value of DPPH with EAF concentrations from 1.95 μg/ml to 1000 μg/ml [24].
NO (Nitric Oxide) free radical scavenging activity assay
Sodium Nitroprusside is a nitric oxide donor. To check the nitric oxide free radical scavenging activity, EAF was serially diluted from 1000 μg/ml to 1.95 μg/ml. 1ml volume was taken from each concentration and 2.0 mL of sodium nitroprusside (10mM) was added in each tube. All samples with or without EAF were incubated for 150 minutes. After the incubation of all samples, 5.0 mL of griess reagent was added to each sample and the absorbance of chromophore was measured at 546nm. The percentage scavenging activity was calculated: % Scavenging = [𝐴bc – 𝐴bs]/𝐴bc× 100, where 𝐴bc is absorbance of control and 𝐴bs for EAF sample [25].
Statistical analysis
Data obtained from this study are represented as the mean ± SEM. Analysis of all the data obtained in the experiments with multiple treatments was done by using one-way ANOVA followed by the Newman–Keuls test of significance with the help of GraphPad Prism version 5.0 software. Qualitative observations were represented following assessments made by three individuals blinded to the experimental designs. Probability values of p < 0.05 were considered to be statistically significant.
Results
Effect of EAF on bone regeneration
Quantitative analysis of bone regeneration by using calcein label (mineral deposition) at the drill-hole site was done to check the effect of EAF from Passiflora foetida. Oral administration of EAF for 2 weeks enhanced significant mineral deposition than control group. Figure 1A-1C shows representative 2D, 3D drill-hole and 3D callus images of femur bones including drill-hole site by μCT. Figure 1D shows confocal microscopy images of calcein deposition at the same site in different experimental groups. The increase in calcein intensity, compared to the control, was ~48.23% (at 50 mg/kg/day), ~79.25% (at 100 mg/kg/day) and ~69.03% (at 200 mg/kg/day) and was comparable to PTH (Fig. 1E).
Effect of EAF on microarchitecture of regenerated bone at the drill-hole site
After oral administration of EAF for 2 weeks, μCT analysis of the various experimental groups at the drill-hole site was executed. Quantification of micro-architectural parameters of bone revealed that, EAF treatment at a dose of 200 mg/kg/day significantly enhanced the bone micro-architecture by increasing BV/TV (~28.02%), Tb.Th (~27.5%), Tb.N (~22.9%), Conn.Dn (~81.7%) and by decreasing Tb.Sp (~32.22%) and DA (~17.73%), compared to the control group and these results were comparable with PTH (Fig. 2A-2F). Furthermore, two other doses of EAF including 50 and 100mg/kg/day significantly enhanced the bone micro-architectural parameters in the same manner except Tb.Th (Fig. 2B).
Effect of EAF on BMD and bone strength
Volumetric bone mineral density (vBMD) measurement results at the drill-hole site of the femur bones from the different experimental groups were calculated (Fig. 3A). μCT analysis was performed on excised bones of different experimental groups to compare the volumetric BMD. When vBMD of the control group was compared with the results from the EAF (50, 100 and 200 mg/kg/day) treatment groups, a significant decrease by ~30.83%, ~34.16% and ~49.34% could be noticed in the control group, respectively (Fig. 3A). Furthermore, PTH group showed significantly higher vBMD values by ~61.38%, compared to the control group (Fig. 3A). Bone biomechanical strength was also assessed at the drill-hole site, and parameters like power, energy and stiffness were evaluated (Fig. 3B-D). It was noticed that EAF treatment revealed increased power (EAF-50, 100 and 200 mg/kg/day by ~19.45%, ~28.64% and ~31.12%), energy (EAF-50, 100 and 200 mg/kg/day by ~23.32%, ~35.29% and ~40.97%) and stiffness (EAF-50, 100 and 200 mg/kg/day by ~18.19%, ~23.46% and ~37.11%) at the drill site in a dose dependent manner, compared to control. The increase noticed in energy at 200 mg/kg/day dose was comparable to PTH (Fig. 3C).
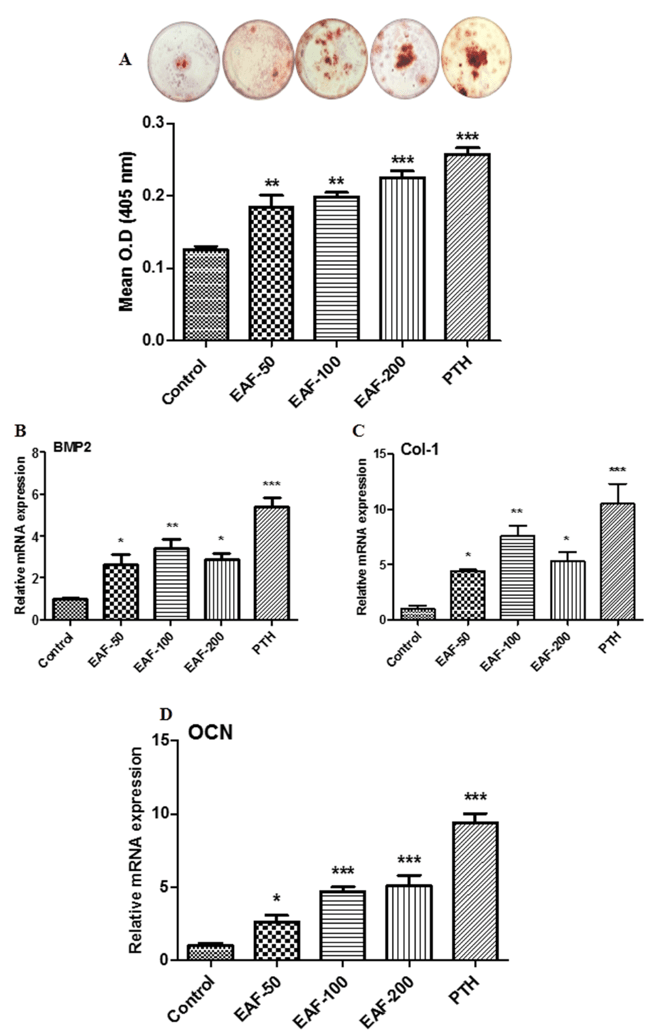
Effect of EAF on mineralized nodule formation in BMCs
Bone marrow cells (BMCs) from the femurs of all experimental groups were harvested and cultured to induce mineralized nodules formation [26, 27]. Upper panel of figure 4A shows the representative photomicrograph of alizarin red-S stained cells to show the formation of mineralized nodules. Lower panel of figure 4A represents the quantification of alizarin red-S stained cells. Data from this study showed that EAF and PTH treatment exhibited increase in mineralized nodules formation over control (Fig. 4A). The maximum increase in mineralization was ~79.91% for 200 mg/kg/day dose followed by ~47.26% and ~58.42% for 50 and 100 mg/kg/day doses compared to the control group. PTH the positive control showed the best response by increasing the nodules formation by ~105.73%.
Figures
Figure 1: Morphology of dynamic observation of ENSCs culture. A, Cell colony formed after 3-5 days culture. B, The NLBs appeared after 6-7 days culture.C. Free floating NLBs appeared at 9-10 days culture. D, ENSCs migrated from NLBs.
Figure 2: Immunocytochemistry characterization of ENSCs. A, NLBs nestin expression detected by FITC-conjugated antibody. B, NLBs TUJ-1 expression detected by CY-3-conjugated antibody. C, ENSCs expressed GFAP positive after differentiation. D. ENSCs expressed SMA positive after differentiation. Nuclei were counterstained with Hoechst33258.
Figure 3: PKH-26 labeled ENSCs pylorus transplantation. A, Injection spot of pylorus. B, PKH-26 labeled ENSCs under white light. C, PKH-26 labeled ENSCs under fluorescent light.
Figure 4: Survival and migration of ENSCs after transplantation. A, Frozen section of pylorus tissue under white light. B, PKH-26 labeled ENSCs were observed under fluorescent light. C, Transplantated ENSCs were observed by figure A and B overlap. D, The precise location of transplanted ENSCs were observed after H&E counterstaining.
Figure 5: Survival of ENSCs 2 weeks after transplantation. A, Frozen section of pylorus tissue showed the PKH-26 labeled ENSCs under fluorescent light. B, The same section could be observed with the Hoechst33258 nuclei staining. C, ENSCs kept the normal cellular morphology after transplantation. D, PKH-26 labeled ENSCs . E, Host’s enteric neural cells and grafted ENSCs expressed TUJ-1 by FITC. F, Figure D merged with figure E showing some grafted cells expressed Tuj-1 by overlap.
Figure 6: Survival of ENSCs 2 weeks after transplantation. A, Frozen section of pylorus tissue showed the PKH-26 labeled ENSCs under fluorescent light. B, The same section could be observed with the Hoechst33258 nuclei staining. C, ENSCs kept the normal cellular morphology after transplantation. D, PKH-26 labeled ENSCs . E, Host’s enteric neural cells and grafted ENSCs expressed TUJ-1 by FITC. F, Figure D merged with figure E showing some grafted cells expressed Tuj-1 by overlap.
Figure 7: Survival of ENSCs 2 weeks after transplantation. A, Frozen section of pylorus tissue showed the PKH-26 labeled ENSCs under fluorescent light. B, The same section could be observed with the Hoechst33258 nuclei staining. C, ENSCs kept the normal cellular morphology after transplantation. D, PKH-26 labeled ENSCs . E, Host’s enteric neural cells and grafted ENSCs expressed TUJ-1 by FITC. F, Figure D merged with figure E showing some grafted cells expressed Tuj-1 by overlap.
Discussion
Passiflora foetida, an Indian medicinal plant of the family passifloraceae is a herbaceous climber, traditionally used in asthma, eczema, chronic ulcer [13, 28]. Recently, we have reported the anti-osteoporotic activity of butanolic fraction from Passiflora foetida in ovariectomy-induced bone loss in mice [15]. However, there is no systematic report available to verify the ability of EAF from Passiflora foetida to speed up the process of fracture healing. Currently, there is no availability of orally given agent having potential to treat bone fractures. In this study, dynamic histomorphometric analysis of bone was done to check whether EAF from Passiflora foetida could induce the regeneration of bone at the fracture site, followed by the static histomorphometric analysis of bone by using μ-CT to check the quality of the newly formed bone (callus) at the fracture site [10, 11, 29, 30]. In addition, its osteogenic effect was also assessed in ex-vivo cultured BMCs. The results from this study showed that EAF from Passiflora foetida given orally to rats enhanced the mineral deposition at the fracture site and exhibited the improvement in quality of callus formation, which was plausible due to its stimulatory effect on osteoblast cells.
Resembling the in vivo bone formation at the cellular level, the EAF treatment enhanced new bone formation by increasing differentiation of the bone marrow osteoprogenitor cells cultured ex-vivo as well as by increasing expression of osteogenic genes in bones. BMP-2 is used in several animal models and clinical studies to enhance the process of fracture healing. Clinically, human recombinant BMP-2 is employed for the open tibial fractures to accelerate healing and reduce the need for secondary intervention by applying it directly to the fracture site as this cytokine has no oral bioavailability [31, 32]. EAF from Passiflora foetida significantly enhanced the BMP-2 mRNA levels as well as other osteogenic genes (OCN and Col-1) in femur bone. It seemed that the mechanism required in speeding up the process of fracture healing by the EAF involved the endogenous BMP-2 production. Furthermore, EAF treatment also enhanced the expression of BMP-2 protein at the fracture site analysed by the immunofluorescence analysis. In addition, histological analysis by H&E staining had shown a larger area of regenerating bone in EAF treated animals, and it was same as that of PTH treated group. Data taken together, suggest the potential of the EAF to promote the recruitment of osteoblast at the fracture site and preserve the quality and the integrity of the bone.
Micro-CT analysis exhibited increase in BV/TV, Tb.Th, Tb.N and Conn.Dn and decrease in Tb.Sp and DA of the callus by EAF treatment resulting in better representation of the newly formed bone over the control group. In addition, EAF from Passiflora foetida significantly improved vBMD at the fracture site after the oral administration for two weeks, indicating that EAF is capable of increasing the bone mass in fracture healing. Bone strength testing was also performed at the fracture site having newly regenerated bone. It was observed that EAF treatment led to increased bone strength parameters (power, energy and stiffness).
Procollagen type-I N-terminal propeptide (PINP) and the non-collagenous protein (OCN) play the important roles in bone formation. PINP provides the bone with its basic fabric and tensile biomechanical properties and serum concentration of PINP is directly proportional to the amount of new collagen produced by osteoblasts [33, 34]. OCN maintains the normal bone mineralization, suppression of abnormal formation of hydroxyapatite crystals and the effects of cartilage mineralization [34, 35]. In this study, we found that serum PINP and OCN levels were enhanced by the treatment of EAF. All the results suggested that EAF promoted the secretion of serum bone formation markers to promote bone formation at the fracture site.
Oxidative stress is associated with the pathogenesis of bone loss leading to fractures or osteoporosis. An antioxidant has capacity to protect bone against fractures or osteoporosis via its antioxidant properties [36]. Free radicals are involved in the process of fracture healing and their higher levels may be harmful for fracture healing [37]. Osteoporosis itself may intensify oxidative stress as noticed in postmenopausal osteoporotic women, who were observed to be under oxidative stress [38, 39]. We found that EAF has anti-oxidant potential, because it was able to scavenge free radicals, which was evaluated by DPPH and NO free radical scavenging activity assays. Data show that EAF has anti-oxidant activity in a concentration dependant manner. It is surmised that EAF was able to control oxidative stress at the fracture site to generate an ideal environment for fracture healing to take place.
In folk medicines, Passiflora foetida is used in the form of decoction for asthma and biliousness [28]. Earlier, we have shown that butanolic fraction from Passiflora foetida given orally has anti-osteoporotic activity in ovariectomy-induced bone loss in mice [15]. Phytochemistry of Passiflora species shows the presence of alkaloids, phenols, glycosyl flavanoids and cyanogenic compounds [13]. It is believable that flavonoids and phytoestrogens found in the plant contribute to its osteoblast stimulating effect and resultant faster fracture healing process in-vivo. Overall, our results suggest that EAF from Passiflora foetida stimulates fracture healing and improves callus quality by enhancing the production of BMCs, followed by their differentiation to osteogenic lineage cells as a result increasing the recruitment of osteoblast cells to the fracture site as well as improving the ability of the cells to increase the production of osteogenic cytokine, BMP-2 and deposition of matrix protein, col1, eventually speed up the process of fracture healing.
Conclusion
In a preclinical set up, this study certainly showed that daily oral administration of EAF from Passiflora foetida stimulates the process of fracture healing. The effect of accelerating the process of fracture healing at the injury site appears to be due to the osteogenic effect of the EAF as a result stimulating the recruitment and differentiation of osteoblast cells at the injury site. This study rationalizes the traditional use of the plant in folk medicines, therefore could be taken as an alternative therapy for fracture healing. Further studies will be needed to determine the bio-active markers and their mechanism of action of the EAF from Passiflora foetida.
Conflict of Interest
The authors declare no conflict of interests.
Supporting grants
Council of Scientific and Industrial Research, Government of India.
Acknowledgements
This work was supported in part by Council of Scientific and Industrial Research as BSC0201, BSC0111 and university grant commission, Government of India.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 11, Apr 2018Accepted: Wed 02, May 2018
Published: Tue 15, May 2018
Copyright
© 2023 Ritu Trivedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2018.10.004
Author Info
Priyanka Kothari Anirudha Karvande Ashish Kumar Tripathi Naseer Ahmad Priyanka Kushwaha Raju Chillara Rakesh Maurya Ritu Trivedi Sulekha Adhikary
Corresponding Author
Ritu TrivediDivision of Endocrinology, Central Drug Research Institute (Council of Scientific and Industrial Research), Sector 10, Jankipuram Extension, Sitapur Road, Lucknow 226031, Uttar Pradesh, India
Tables
Table 1: Primer sequence of various genes used for qPCR.
|
Gene Symbol |
Gene Name |
Primer Sequence |
Accession Number |
|
GAPDH |
glyceraldehyde-3-phosphate dehydrogenase |
F-CAGCAAGGATACTGAGAGCAAGAG R-GGATGGAATTGTGAGGGAGATG |
NM-017008 |
|
BMP-2 |
Bone morphogenetic protein-2 |
F- CCCCTATATGCTCGACCTGT R-AAAGTTCCTCGATGGCTTCTT |
NM_017178.1 |
|
COL1 |
Type I Collagen |
F- CATGTTCAGCTTTGTGGACCT R- GCAGCTGACTTCAGGGATGT |
NM_053304 |
|
OCN |
Osteocalcin |
F-ATAGACTCCGGCGCTACCTC R-CCAGGGGATCTGGGTAGG |
NM-013414 |
qPCR; quantitative polymerase chain reaction, F; forward, R; reverse
Figures





References
1. Doblare M, JM Garcia (2003) On the modelling bone tissue fracture and healing of the bone tissue. Acta Cient Venez 54: 58-75. [Crossref]
2. Peng LH, Ko CH, Siu SW, Koon CM, Yue GL, et al. (2010) In vitro & in vivo assessment of a herbal formula used topically for bone fracture treatment. J Ethnopharmacol 131: 282-289. [Crossref]
3. Matsuyama J, Ohnishi I, Kageyama T, Oshida H, Suwabe T, et al. (2005) Osteogenesis and angiogenesis in regenerating bone during transverse distraction: quantitative evaluation using a canine model. Clin Orthop Relat Res (433): 243-250. [Crossref]
4. Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, et al. (2008) Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone 42: 535-546. [Crossref]
5. Liu MJ, Li Y, Pan JH, Liu H, Wang SJ, et al. (2011) Effects of zuogui pill (see text) on Wnt singal transduction in rats with glucocorticoid-induced osteoporosis. J Tradit Chin Med 31: 98-102. [Crossref]
6. Gerstenfeld LC, TA Einhorn (2003) Developmental aspects of fracture healing and the use of pharmacological agents to alter healing. J Musculoskelet Neuronal Interact 3: 297-303; discussion 320-321. [Crossref]
7. Delaney MF (2006) Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am J Obstet Gynecol 194: 12-23. [Crossref]
8. Gass M, B Dawson-Hughes (2006) Preventing osteoporosis-related fractures: an overview. Am J Med 119: 3-11. [Crossref]
9. Vahle JL, Sato M, Long GG, Young JK, Francis PC, et al. (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 30: 312-321. [Crossref]
10. Ngueguim FT, Khan MP, Donfack JH, Siddiqui JA, Tewari D, et al. (2012) Evaluation of Cameroonian plants towards experimental bone regeneration. J Ethnopharmacol 141: 331-337. [Crossref]
11. Khedgikar V, Kushwaha P1, Ahmad N1, Gautam J1, Kumar P, et al. (2017) Ethanolic extract of Dalbergia sissoo promotes rapid regeneration of cortical bone in drill-hole defect model of rat. Biomed Pharmacother 86: 16-22. [Crossref]
12. Adhikary S, Choudhary D, Ahmad N, Kumar S, Dev K, et al. (2017) Dried and free flowing granules of Spinacia oleracea accelerate bone regeneration and alleviate postmenopausal osteoporosis. Menopause 24:686-698. [Crossref]
13. Dhawan K, S Dhawan, A Sharma (2004) Passiflora: a review update. J Ethnopharmacol 94: 1-23. [Crossref]
14. Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, et al. (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126: 2049-2063. [Crossref]
15. Ahmad N, Chillara R, Kushwaha P, Khedgikar V, Karvande A, et al. (2017) Evaluation of anti-osteoporotic activity of butanolic fraction from Passiflora foetida in ovariectomy-induced bone loss in mice. Biomed Pharmacother 88: 804-813. [Crossref]
16. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, et al. (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25: 1468-1486. [Crossref]
17. Ahmad N, Thomas GN, Gill P, Torella F (2016) The prevalence of major lower limb amputation in the diabetic and non-diabetic population of England 2003-2013. Diab Vasc Dis Res 13: 348-53. [Crossref]
18. Trivedi R, Kumar S, Kumar A, Siddiqui JA, Swarnkar G, et al. (2008) Kaempferol has osteogenic effect in ovariectomized adult Sprague-Dawley rats. Mol Cell Endocrinol 289: 85-93. [Crossref]
19. Dharmendra Choudhary, Ashutosh Pandey, Sulekha Adhikary, Naseer Ahmad, Chitra Bhatia, et al. (2016) Genetically engineered flavonol enriched tomato fruit modulates chondrogenesis to increase bone length in growing animals. Sci Rep 6: 21668. [Crossref]
20. Kushwaha P, Khedgikar V, Sharma D, Yuen T, Gautam J, et al. (2016) MicroRNA 874-3p Exerts Skeletal Anabolic Effects Epigenetically during Weaning by Suppressing Hdac1 Expression. J Biol Chem 291: 3959-3966. [Crossref]
21. Yang B, Lin X, Tan J, She X, Liu Y, et al. (2016) Root bark of Sambucus Williamsii Hance promotes rat femoral fracture healing by the BMP-2/Runx2 signaling pathway. J Ethnopharmacol 191: 107-114. [Crossref]
22. Bostrom MP, Lane JM, Berberian WS, Missri AA, Tomin E, et al. (1995) Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res 13: 357-367. [Crossref]
23 Dixit M, Raghuvanshi A, Gupta CP, Kureel J, Mansoori MN, et al. (2015) Medicarpin, a Natural Pterocarpan, Heals Cortical Bone Defect by Activation of Notch and Wnt Canonical Signaling Pathways. PLoS One 10: 0144541. [Crossref]
24. Lu Y, Xue Y, Chen S, Zhu H, Zhang J, et al. (2016) Antioxidant Lignans and Neolignans from Acorus tatarinowii. Sci Rep 6: 22909. [Crossref]
25. Yadav NK, Arya RK, Dev K, Sharma C, Hossain Z, et al. (2017) Alcoholic Extract of Eclipta alba Shows In Vitro Antioxidant and Anticancer Activity without Exhibiting Toxicological Effects. Oxid Med Cell Longev 2017: 9094641. [Crossref]
26. Kushwaha P, Khedgikar V, Ahmad N, Karvande A, Gautam J, et al. (2016) A neoflavonoid dalsissooal isolated from heartwood of Dalbergia sissoo Roxb. has bone forming effects in mice model for osteoporosis. Eur J Pharmacol 788: 65-74. [Crossref]
27. Khedgikar V, Gautam J, Kushwaha P, Kumar A, Nagar GK, et al. (2012) A standardized phytopreparation from an Indian medicinal plant (Dalbergia sissoo) has antiresorptive and bone-forming effects on a postmenopausal osteoporosis model of rat. Menopause 19: 1336-46. [Crossref]
28. Krishnaveni A, SRThaakur (2008) Pharmacognostical and preliminary phytochemical studies of Passiflora foetida. Anc Sci Life 27: 19-23. [Crossref]
29. Tanaka K, Tanaka S, Sakai A, Ninomiya T, Arai, et al. (2010) Deficiency of vitamin A delays bone healing process in association with reduced BMP2 expression after drill-hole injury in mice. Bone 47: 1006-1012. [Crossref]
30. Kumar P, Kushwaha P, Ahmad N, Maurya SW, Dev K, et al. (2017) Design and synthesis of dalbergin analogues and evaluation of anti-osteoporotic activity. Bioorg Med Chem Lett 27: 1765-1775. [Crossref]
31. Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, et al. (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84: 2123-34. [Crossref]
32. Ngueguim FT, Khan MP, Donfack JH, Tewari D, Dimo T, et al. (2013) Ethanol extract of Peperomia pellucida (Piperaceae) promotes fracture healing by an anabolic effect on osteoblasts. J Ethnopharmacol 148: 62-68. [Crossref]
33. Linkhart SG, Linkhart TA, Taylor AK, Wergedal JE, Bettica P, et al. (1993) Synthetic peptide-based immunoassay for amino-terminal propeptide of type I procollagen: application for evaluation of bone formation. Clin Chem 39: 2254-2258. [Crossref]
34. Hammett-Stabler CA (2004) The use of biochemical markers in osteoporosis. Clin Lab Med 24: 175-197. [Crossref]
35. Yilmaz N, Bayram M, Erbåğci AB, Kilinçer MS (1999) Diagnostic value of biochemical markers of bone turnover and postmenopausal osteoporosis. Clin Chem Lab Med 37: 137-143. [Crossref]
36. Muhammad N, Luke DA, Shuid AN, Mohamed N, Soelaiman IN (2013) Tocotrienol supplementation in postmenopausal osteoporosis: evidence from a laboratory study. Clinics (Sao Paulo) 68: 1338-43. [Crossref]
37. Göktürk E, Turgut A, Bayçu C, Günal I, Seber S, et al. (1995) Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand 66: 473-475. [Crossref]
38. Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, et al. (2003) Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab 88: 1523-1527. [Crossref]
39. Sontakke AN, RS Tare (2002) A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta 318: 145-148. [Crossref]
