Endolymphatic Sac Tumor: A Rare Case
A B S T R A C T
Endolymphatic sac tumor is rare, locally aggressive and non-metastasizing neoplasm arising from the endolymphatic sac of the petrous portion of the temporal bone. Most occur in adult and present with ipsilateral hearing loss. They can be sporadic or associated with Von-Hippel-Lindau disease. Patient with endolymphatic sac tumor should be screened for VHL disease. We report a case of 52-year-old female with dizziness and headache. Histopathology was consistent with typical features of endolymphatic sac tumor. This was confirmed by cytokeratin and EMA positivity and TTF-1 negativity. This case is presented for its rarity with only few cases reported.
Keywords
Endolymphatic sac tumor, temporal bone
Introduction
Endolymphatic sac tumor (ELST) is a rare neoplasm with benign histopathological appearance and clinically destructive behaviour which occurs in the skull base and frequently invades the posterior petrous bone, the mastoid, semicircular canal, cerebellopontine angle structures and cranial nerve. These tumors can be encountered sporadically or in Von Hippel-Lindau (VHL) disease [1].
Case Report
I Clinical History
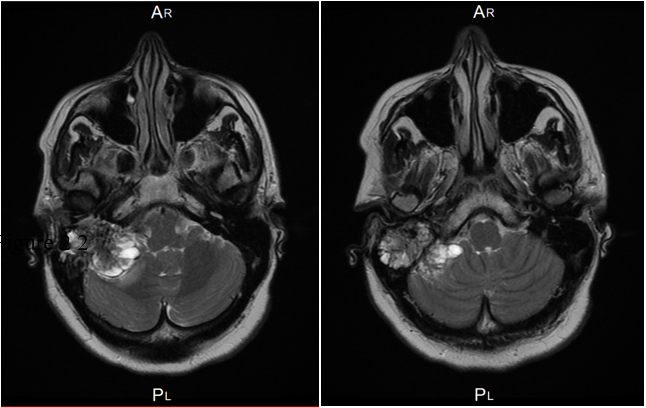
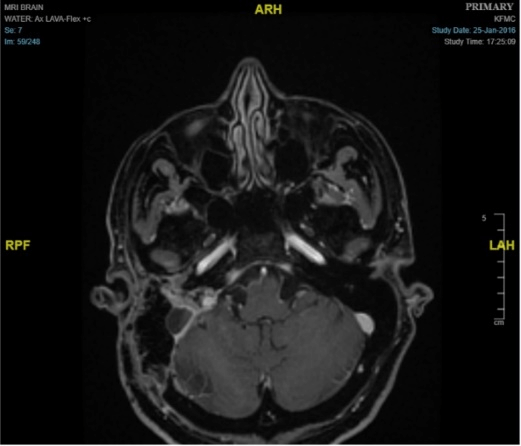
52-year-old female presented with history of dizziness and headache. Contrast enhanced MRI of brain demonstrated a large heterogeneous enhancing mass in the right cerebellopontine angle measures 6.0 x 4.0 x 3.5 cm with extensive moth-eaten destruction of the right petrous bone and mastoid air cells in addition to extension into middle ear and external auditory canal. There was a large exophytic component in the posterior fossa causes mass effect on the middle cerebral peduncle and the cerebellar hemisphere. It also exerted a mass effect on the fourth ventricle. No invasion of the brain was seen (Figure 1). The differential diagnosis was paraganglioma/glomus tumor and endolymphatic sac tumor. Right retro-sigmoid trans-labyrinthine approach under neuro-navigation guidance and intraoperative neurophysiology monitoring of the 5, 7, 8th and lower cranial nerves together with motor evoked potentials and somatosensory evoked potentials. Gross total resection of the CP angle component was achieved with resection of big part of the intraosseous component. Postoperatively there was no new neurological deficit except mild right facial nerve weakness which improved with time and physiotherapy (Figure 2).
Figure 1: Axial view of MRI pre-operative demonstrates large enhancing mass with destruction of right petrous bone.
Figure 2: Axial view of MRI demonstrates shows gross total resection of the tumor.
II Materials and Methods
A formalin-fixed, paraffin-embedded tissue was available. These were routinely processed and stained with hematoxylin and eosin (H&E). In addition to H&E, selected sections were stained with Periodic Acid-Schiff (PAS). An immunohistochemical study was performed. The antibodies used were cytokeratin (CK7 and CK19), epithelial membrane antigen (EMA), cytokeratin 5/6, and vimentin, glial fibrillary acidic protein (GFAP), S-100, synaptophysin, TTF-1, CA125, WT1, ER, PR and Ki-67. Appropriate positive and negative control stained slides were performed.
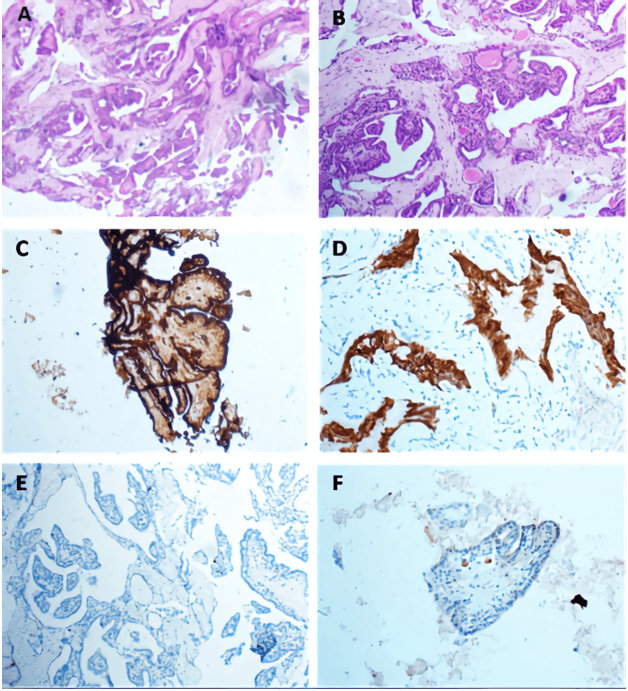
Figure 3: A & B) Histological sections show papillary architecture lined by single layer of cuboidal cells with colloid like material. C) The tumor cells are immunoreactive for CK7, D) EMA, E) very low Ki-67 index, F) negative for TTF-1.
III Pathological Findings
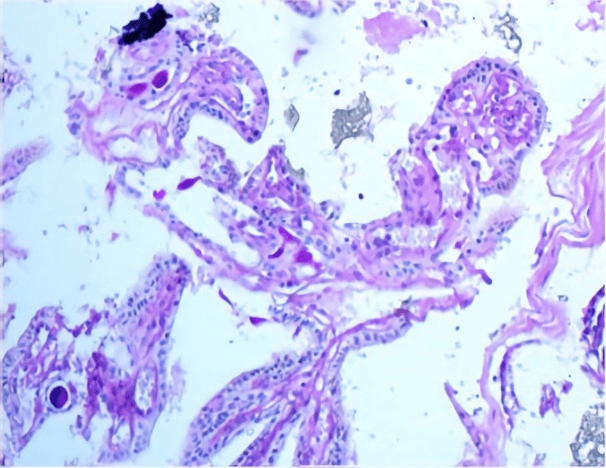
Grossly, received fresh was a fragment of grey soft tissue measuring 0.8 cm in greatest dimension. An intraoperative consultation was reported as papillary lesion with no high-grade features, possibility of endolymphatic sac tumor to be considered. Further immune-histochemical assessment was required. Morphology, the tumor showed bland looking papillary growth with thyroid like follicles filled with inspissated eosinophilic colloid like material. On higher magnification, the papillary and glandular structures were lined by a single layer of flattened cuboidal to columnar cells. Cellular pleomorphism, mitotic activity and necrosis were not observed (Figures 3A & 3B).
IV Immunohistochemistry and Special Stains Findings
The tumor cells showed positive reactivity for CK7, CK19, vimentin and EMA, while Ki-67 immunostain was low (Figure 3). CK20, WT1, CA125, TTF1, GFAP, S100, synaptophysin, ER and PR were negative. The colloid-like material showed positive reactivity with PAS (Figure 4).
Figure 4: PAS stain highlights colloid like material.
Discussion
Endolymphatic sac tumor (ELST) is a low-grade papillary epithelial neoplasm arising within the endolymphatic sac/ duct, showing a high association (up to one-third) with Von Hippel-Lindau (VHL) syndrome and usually presenting between 30 and 40 years of age, with an equal sex distribution. The most common symptoms include ipsilateral hearing loss (sensorineural > conductive, although it can be mixed), tinnitus, facial nerve palsy and vestibular dysfunction (vertigo, ataxia) [2]. The best radiographic studies are a combination of MR and CT. Retrospective study of 31 adult patients with imaging (computed tomography [CT], magnetic resonance imaging [MRI], and angiography), They established reliable radiologic criteria that exist in the majority of endolymphatic sac tumors to differentiate them from other lesions of the posterior temporal bone included retro-labyrinthine location, intratumoral calcification on CT scan, hyper-intense focal signals on T1-weighted MRI scan and heterogeneous signal on T2-weighted MRI scan [3].
Table 1: Differential diagnosis of CNS papillary tumors.
|
Tumor Type |
Location |
Positive Antibodies |
|
Meningioma, papillary type GIII |
Dura based extra axial |
EMA cytoplasmic, PR nuclear |
|
Ependymoma, papillary type GII, GIII |
Wall of the ventricles and spine |
GFAP, EMA dot |
|
Myxopapillary Ependymoma GI |
Spine, filum terminal |
GFAP, EMA dot |
|
Papillary glioneuronal tumor GI |
Temporal |
GFAP, OLIG2 in perivascular astrocytes Synaptophysin, Neu-N in neurocytes |
|
Choroid plexus neoplasm GI, GII, GIII |
Lateral ventricles |
CK, VIMENTIN, CK7 |
|
Papillary tumor of Pineal Region |
Pineal region |
Cam5.2, CK18 S100, CD56 |
|
Pituitary adenoma |
Sellar, Suprasellar
|
Synaptophysin Pit. Hormones Transcription Factor |
|
Craniopharyngioma, papillary type GI |
Suprasellar or 3rd ventricle |
EMA, P63, CK7, Cludin B-Catenin membranous. BRAF- V600E mutation |
|
Endolymphatic sac tumor |
Posterior medial petrous ridge of temporal bone (site of endolymphatic sac) |
CK, CK7 PAS positive colloid like material. |
|
Metastatic Papillary Carcinoma |
Anywhere |
CK7, CA125, WT-1 Ovary CK7, TTF1, Napsin-A lung CK7, TTF-1, Thyroglobulin Thyroid CK7, CD117, AMACAR Papillary Renal |
In 1990, Manski and Heffner considered hearing loss and ELST are part of the VHL syndrome and advocated awareness of the association between VHL, hearing loss and ELSTs by patients and physicians and use of the diagnostic evaluations to allow early detection of ELSTs and hearing loss [4]. The prevalence of VHL gremlin mutations in apparently sporadic ELSTs was 39%. The prevalence of ELSTs in patients with VHL was 3.6%. ELST was the initial manifestation in 32% of patients with VHL-ELST [5]. The histopathological differential diagnosis of ELST include paraganglioma based on the location, and all the papillae forming tumors as choroid plexus tumor, papillary ependymoma, middle ear adenoma, and metastatic papillary carcinoma of the thyroid, kidney, lung and ovary. Paragangliomas of the middle ear demonstrate papillary structures, at least focally characteristic nest arrangement and positive for chromogranin and synaptophysin antibodies in addition to S100 positive sustentacular cells. Choroid plexus tumor originates in the ventricle and does not invade the bone. Transthyretin, a marker for choroid plexus epithelium, was found to show differential expression between choroid plexus papillomas and tumors of the endolymphatic sac or duct.
Papillary ependymoma shows perivascular pseudo rosettes with EMA dot like positivity and GFAP-positive tumor cell processes. Metastatic carcinoma can be eliminated by radiologic screening and immunohistochemical study, for example, reactivity with thyroglobulin and TTF1 confirms a metastatic papillary thyroid carcinoma. TTF-1 and Napsin A are positive in lung adenocarcinoma. Metastatic papillary renal cell carcinoma is immunoreactive for AMACR (diffuse) EMA/MUC1 (membranous), RCC, CD10 (often apical staining), CK8/CK18, CK19, vimentin CD117/C- KIT (cytoplasmic), PAX8, PAX2. Being a female, metastatic papillary serous adenocarcinoma of the ovary or endometrium should be also considered. Radiologic correlation in addition to positive reaction to CA 125 antibody will be helpful to reach to the definitive diagnosis. ELST treatment of choice is complete surgical resection of the tumor. The adjuvant therapy has no role in the management of these tumors [6]. A study of 14 cases of ELST occurred sporadically and associated with VHL syndrome concluded that sporadic tumors are more aggressive and the surgical removal was more extensive than those associated with VHL syndrome [7].
Conclusion
We report a rare case of endolymphatic sac tumor (ELST) which is a locally aggressive tumor and could be a part of the VHL syndrome. Late recurrences are common, so long follow up is necessary. ELST should be considered in the differential diagnosis of papillary neoplasm of the base of the skull.
Conflicts of Interest
None.
Funding
None.
Informed Consent
Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration
Not applicable, because this article does not contain any clinical trial.
Article Info
Article Type
Case ReportPublication history
Received: Tue 07, Jul 2020Accepted: Thu 16, Jul 2020
Published: Mon 31, Aug 2020
Copyright
© 2023 Amal Algarni. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2020.04.04
Author Info
Amal Algarni Yaser Orz Sofia Muzzafar Wafa Alshakweer
Corresponding Author
Amal AlgarniPathology Resident, Pathology and Clinical Pathology Administration, King Fahad Medical City, Riyadh, Saudi Arabia
Figures & Tables
Table 1: Differential diagnosis of CNS papillary tumors.
|
Tumor Type |
Location |
Positive Antibodies |
|
Meningioma, papillary type GIII |
Dura based extra axial |
EMA cytoplasmic, PR nuclear |
|
Ependymoma, papillary type GII, GIII |
Wall of the ventricles and spine |
GFAP, EMA dot |
|
Myxopapillary Ependymoma GI |
Spine, filum terminal |
GFAP, EMA dot |
|
Papillary glioneuronal tumor GI |
Temporal |
GFAP, OLIG2 in perivascular astrocytes Synaptophysin, Neu-N in neurocytes |
|
Choroid plexus neoplasm GI, GII, GIII |
Lateral ventricles |
CK, VIMENTIN, CK7 |
|
Papillary tumor of Pineal Region |
Pineal region |
Cam5.2, CK18 S100, CD56 |
|
Pituitary adenoma |
Sellar, Suprasellar
|
Synaptophysin Pit. Hormones Transcription Factor |
|
Craniopharyngioma, papillary type GI |
Suprasellar or 3rd ventricle |
EMA, P63, CK7, Cludin B-Catenin membranous. BRAF- V600E mutation |
|
Endolymphatic sac tumor |
Posterior medial petrous ridge of temporal bone (site of endolymphatic sac) |
CK, CK7 PAS positive colloid like material. |
|
Metastatic Papillary Carcinoma |
Anywhere |
CK7, CA125, WT-1 Ovary CK7, TTF1, Napsin-A lung CK7, TTF-1, Thyroglobulin Thyroid CK7, CD117, AMACAR Papillary Renal |
References
- Sun YH, Wen W, Wu J, Song J, Guan H et al. (2012) Endolymphatic sac tumor: case report and review of the literature. Diagn Pathol 7: 36. [Crossref]
- Benign Neoplasms of the Ear and Temporal Bone. Head Neck Pathol 2019: 453.e2-477.e2.
- Patel NP, Wiggins RH 3rd, Shelton C (2006) The radiologic diagnosis of endolymphatic sac tumors. Laryngoscope 116: 40-46. [Crossref]
- Manski TJ, Heffner DK, Glenn GM, Patronas NJ, Pikus AT et al. (1997) Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA 277: 1461-1466. [Crossref]
- Bausch B, Wellner U, Peyre M, Boedeker CC, Hes FJ et al. (2016) Characterization of endolymphatic sac tumors and von Hippel-Lindau disease in the International Endolymphatic Sac Tumor Registry. Head Neck 1: E673- E679. [Crossref]
- Poletti AM, Dubey SP, Colombo G, Cugini G, Mazzoni A (2016) Treatment of endolymphatic sac tumour (Papillary adenocarcinoma) of the temporal bone. Rep Pract Oncol Radiother 21: 391-394. [Crossref]
- Nevoux J, Nowak C, Vellin J, Lepajolec C, Sterkers O et al. (2014) Management of endolymphatic sac tumors: Sporadic cases and von Hippel-Lindau disease. Otol Neurotol 35: 899-904. [Crossref]
