Efficacy of Single Pre-Operative Dose of Glucocorticoid (125mg of Solumedrol Intravenous) in Terms of Seroma Formation in Patients Undergoing Mastectomy with Axillary Clearance For Breast Cancer: A Randomized Control Trial
A B S T R A C T
Objective: The study aimed to determine the efficacy of single dose of glucocorticoid (125 mg of Solumedrol intravenous) in terms of seroma formation after mastectomy in patient with carcinoma of breast.
Study Design: Randomized control trial.
Place and Duration of Study: Study was conducted in the Department of General Surgery, Liaquat National Hospital Karachi, Pakistan from july 1 to dec 31, 2010.
Patients and Methods: Patients were randomly divided in two groups (study and control) each group consisting of 30 patients. Randomization was done by opening of a sealed envelope which had a slip bearing the name of study medication (solumederol or saline as placebo) to be administered. The study group received a single dose of inj 125mg solumedrol IV half an hour prior to surgery by resident scrub in surgery. A similar procedure was applied to the control group and patients in controlled group were administered an equal volume of saline intravenously. After drain removal patients in both groups were observed for a duration of 2 weeks for sermoa formation. Detection of seroma formation was based on clinical grounds by absence of any fluid collection at mastectomy bed as detected by manual palpation. SPSS 10 was used for analysis.
Results: Seroma formation was observed in 66.7% (40/60) women 2 weeks post drain removal. Rate of seroma formation was significantly low in study groups than control groups (33.3% vs. 100%; p=0.0001).
Conclusion: Single dose of steroid is efficacious in reducing the post mastectomy seroma formation.
Keywords
Post mastectomy seroma formation, steroid and seroma, factors for seroma formation, how to reduce seroma formation post, mastectomy, mastectomy, seroma
Introduction
Seroma is a post-operative fluid collection requiring one or more aspirations or subsequent drain placement following surgery. It is a common occurrence following surgery. The incidence rate of seroma formation following breast surgery is up to 30% to 92 % [1]. It is often an ongoing problem after removal of the suction drain, and repeated skin puncture is necessary to remove the seroma. Seroma formation is most likely the result of the inflammatory response due to wound healing.
Comparison of drain fluid to plasma ratios with known lymph to plasma values for biochemical parameters showed that this fluid is compositionally different from lymph but is similar to inflammatory exudates [2]. In the seroma fluid several factors have been detected that support this assumption. These factors are: high levels of IgG, leucocytes, granulocytes, proteinases, proteinases inhibitors, different kinds of cytokines (tPA, uPA, uPAR, PAI-1, PAI-2, IL-6, IL-1).
The modulation of acute phase response may have important implications for patients with cancer undergoing surgery [3]. On the basis of this understanding, an inhibition of the inflammatory response might result in a decrease in seroma formation, and perhaps improve quality of life after mastectomy. Steroids inhibit the inflammatory response for example by inhibition of the cytokine function. It has been shown that a high single dose of steroid infusion (30mg/kg solu-medrol) inhibits the normal IL 6 response after colon resection. While there are no studies on the use of solumederol in breast cancer, the use of triamcinalone in breast reconstruction surgery demonstrated than 55% did not have seroma formation whereas 95% developed seroma in those administered saline [4]. It is the possibility of decreased seroma formation with the use of steroids that we propose to undertake this study. It is proposed to use a single dose of 125 mg solumedrol intravenously in this study.
Operational Definitions
Seroma: Presences of clear fluid filled pockets in the body post operatively. Efficacy: Efficacy will be deemed posture as when no seroma formation at the end of 4 week. Carcinoma Breast: Biopsy proven breast cancer lobular or infiltrative ductal.
Materials and Methods
I Study Design
Randomized control trial.
II Setting
Study was conducted in the Department of General Surgery, Liaquat National Hospital Karachi, Pakistan from july 1 to dec 31, 2010.
III Duration of Study
6 months, patients in each group were observed for seroma formation for 2 weeks after drain removal.
IV Sample Technique
Non-Probability purposive.
V Sample Selection
i Inclusion criteria
i. Women with primary breast cancer up to stage III, planned for a mastectomy with axillary dissection.
ii. Age over 25 years.
iii. Signed informed consent.
ii Exclusion Criteria
i. Men
ii. Treatment with glucocorticoids within the last month before surgery, including inhalation products
iii. Pregnant.
iv. Severe heart disease
v. Treatment with carbamazepine, phenytoin, Phenobarbital, rifampicin, salicylates and cyclosporine
vi. Uremia
vii. Diabetes
viii. Post neo-adjuvant and adjuvant chemotherapy.
VI Data Collection Procedure
Patients found eligible as per inclusion criteria were offered to participate in the study. Those giving informed consent were included. A proforma was filled for each patient to record. Mastectomy with axillary clearance was performed by consultant (with 15 years of experience) in breast surgery. Each patient enrolled in the study was eligible to be enrolled into either arm of the study following the opening of a sealed envelope which had a slip bearing the name of study medication (solumederol or saline as placebo) to be administered.
The study group received a single dose of inj 125mg solumedrol IV half an hour prior to surgery by resident scrub in surgery. A similar procedure was applied to the control group and patients in controlled group were administered an equal volume of saline intravenously. Patients in both groups were followed postoperatively until 2 weeks after drain removal. Absence of clear fluid filled pocket in the mastectomy bed was documented in the proforma by a resident attending the OPD.
VII Data Analysis Procedure
Data was entered and analyzed by using statistical package for social sciences (SPSS) version 11.0 software. Frequencies and percentages were computed for categorical variables like age groups, stages of breast cancer, seroma formation and efficacy. Mean, standard deviations, 95% confidence interval, median with IQR were calculated for quantitative variables like age of patient. Chi-square test and fisher’s exact test was applied to compare proportion difference between groups for efficacy. Independent sample t test was applied to compare mean difference between groups for age. P≤ 0.05 was considered significant. Confounding variables like age and stages of carcinoma were controlled by stratification to observed effect on outcome.
Results
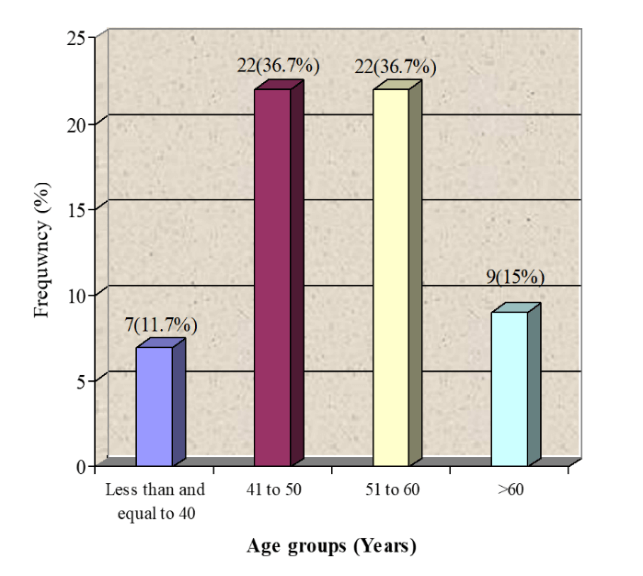
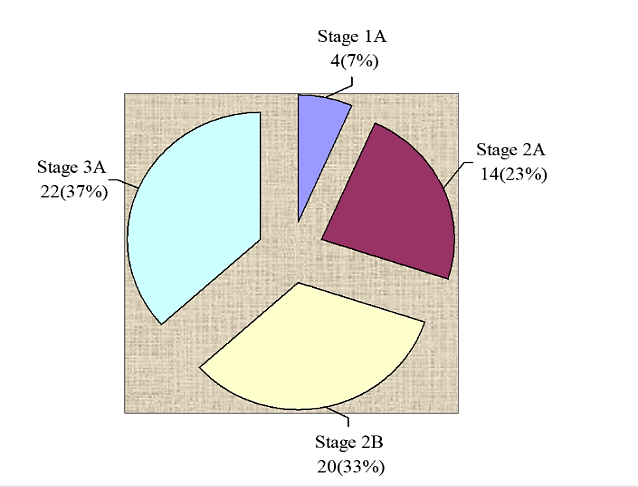
A total of 60 women with primary breast cancer planned for a mastectomy with axillary dissection were included in this study. Patients were equally divided into two groups. The study group received a single dose of inj 125 mg solumedrol IV half an hour prior to surgery and similar procedure was applied to the control group who were administered an equal volume of saline. Overall age distribution of the patients is presented in (Figure 1). The average age of the patients was 52.32 ± 9.86 years (95%CI: 49.77 to 54.86) as shown in (Table 1). Significant difference was not observed between group in average age (52.62±8.42 vs. 52±11.25; p>0.05) as presented in (Figure 2). Regarding stages of carcinoma, 4(7%) women were in stage 1A, 14(23%) were in stage 2A 22(37%) were in stage 3A and 20(33%) women were observed with stage 2B carcinoma as presented in (Figure 3).
Seroma formation was observed in 66.7% (40/60) women 2 weeks post drain removal. Rate of seroma formation was significantly low in study groups than control groups (33.3% vs. 100%; p=0.0001) as shown in (Table 2). Comparisons of efficacy in term of seroma formation in women with primary breast cancer after mastectomy with axillary dissection are presented in (Table 3). Efficacy of the treatments was significantly high in study group (Solumedrol) than control (66.7% vs. 0%; p=0.0001). Similarly, effectiveness of solumendrol was significantly high with respect to age as presented in (Tables 4 & 5) and it was also significantly effective in stage 2A, stage 3A and stage 2B as presented in (Table 7-9).
Figure 1: Age distribution of the patients n=60.
Table 1: Descriptive statistics of age of the patients N=60.
|
Statistics |
Age (Years) |
|
Mean ± SD |
52.32 ± 9.86 |
|
95% Confidence Interval |
49.77 to 54.86 |
|
Median (IQR) |
52(14.75) |
|
Maximum Age |
75 |
|
Minimum Age |
32 |
Figure 2: Comparison of age between groups.
Figure 3: Stages of breast cancer N=60.
Table 2: Comparison of seroma formation between groups.
|
Seroma Formation |
Solumedrol n=30 |
Normal Saline n=30 |
Total n=60 |
P-value |
|
Yes |
10(33.3%) |
30(100%) |
40(66.7%) |
0.0001 |
|
No |
20(66.7%) |
0(0%) |
20(33.3%) |
Chi-Square = 30, df= 1.
Table 3: Comparison of efficacy between groups.
|
Efficacy |
Solumedrol n=30 |
Normal Saline n=30 |
Total n=60 |
P-value |
|
Yes |
20(66.7%) |
0(0%) |
20(33.3%) |
0.0001 |
|
No |
10(33.3%) |
30(100%) |
40(66.7%) |
Fisher’s exact test applied.
Table 4: Comparison of efficacy between groups for below and equal to 50 years of age.
|
Efficacy |
Solumedrol n=14 |
Normal Saline n=15 |
Total n=29 |
P-value |
|
Yes |
9(64.3%) |
0(0%) |
9(31%) |
0.0001 |
|
No |
5(35.7%) |
15(100%) |
20(69%) |
Fisher exact test applied.
Table 5: Comparison of efficacy between groups for above 50 years of age.
|
Efficacy |
Solumedrol n=16 |
Normal Saline n=15 |
Total n=31 |
P-value |
|
Yes |
11(68.8%) |
0(0%) |
9(31%) |
0.0001 |
|
No |
5(31.3%) |
15(100%) |
20(69%) |
Fisher exact test applied.
Table 6: Comparison of efficacy between groups for stage 1a of breast cancer.
|
Efficacy |
Solumedrol n=2 |
Normal Saline n=2 |
Total n=4 |
P-value |
|
Yes |
1(50%) |
0(0%) |
1(31%) |
0.99 |
|
No |
1(50%) |
2(100%) |
3(75%) |
Fisher exact test applied.
Table 7: Comparison of efficacy between groups for stage 2a of breast cancer.
|
Efficacy |
Solumedrol n=7 |
Normal Saline n=7 |
Total n=14 |
P-value |
|
Yes |
6(85.7%) |
0(0%) |
6(42.9%) |
0.005 |
|
No |
1(14.3%) |
7(100%) |
8(75.1%) |
Fisher exact test applied.
Table 8: Comparison of efficacy between groups for stage 2b of breast cancer.
|
Efficacy |
Solumedrol n=10 |
Normal Saline n=10 |
Total n=20 |
P-value |
|
Yes |
4(40%) |
0(0%) |
4(20%) |
0.025 |
|
No |
6(60%) |
10(100%) |
16(80%) |
Fisher exact test applied.
Table 9: Comparison of efficacy between groups for stage 3a of breast cancer.
|
Efficacy |
Solumedrol n=10 |
Normal Saline n=10 |
Total n=20 |
P-value |
|
Yes |
9(81.8%) |
0(0%) |
9(40.9%) |
0.0005 |
|
No |
2(18.2%) |
11(100%) |
13(59.1%) |
Fisher exact test applied.
Discussion
Breast cancer is the most common malignancy in women. Surgery is the mainstay of treatment. Modified radical mastectomy with or without reconstruction or breast preservation in addition to axillary lymph node dissection are common surgical procedures in breast cancer. Surgery of the axilla is associated with numerous complications, including infection, lymphedema of the ipsilateral upper extremity and collection of fluid in surgical site (seroma) [5]. Most common complication after breast cancer surgery is wound seroma [6]. The exact etiology of seroma formation remains controversial. Several interventions have been reported with the aim of reducing seroma formation including the use of ultrasound scissors in performing quilting, buttress suture, lymphadenectomy fibrin glue, fibrin sealant, bovine thrombin application, and altering surgical technique to close dead space [7-13].
The seroma formation after breast cancer surgery is independent of duration of drainage, compression dressing and other known prognostic factors in breast cancer patients except the type of surgery, i.e. there is a 2.5 times higher risk of seroma formation in patients undergoing MRM compared to BP [14]. The documented effect of triamcinolone as a potent anti-inflammatory agent could perhaps be suggestive of its mechanism in seroma management. It is a synthetic glucocorticoid frequently used to treat autoimmune and allergic conditions. It suppresses the inflammatory process by formation of phospholipase inhibitor lipocortin, which diminishes the supply of arachidonic acid available for synthesis of prostaglandin and leukotrienes. As a result, capillary permeability, oedema, migration of leucocytes, and later signs of capillary proliferation, and fibroblast and collagen deposition are inhibited. This study has demonstrated the effect of solumedrol in reducing seroma re-accumulation, which would perhaps suggest the inflammatory mechanism to be significant in seroma formation. The use of solumedrol is therefore an inexpensive and simple means of seroma management. Moreover, we found no added significant complications with this technique, in particular infective complications given the potential immunocompromising effect of steroid.
In this study a total of 60 women with primary breast cancer planned for a mastectomy with axillary dissection were included. Patients were equally divided into two groups. The study group received a single dose of inj 125mg solumedrol IV half an hour prior to surgery and similar procedure was applied to the control group who were administered an equal volume of saline. The average age of the patients was 52.32 ± 9.86 years (95%CI: 49.77 to 54.86). Significant difference was not observed between group in average age (52.62±8.42 vs. 52±11.25; p>0.05).
Regarding stages of carcinoma, 4 (7%) women were in stage 1A, 14(23%) were in stage 2A 22(37%) were in stage 3A and 20(33%) women were observed with stage 2B carcinoma. Seroma formation was observed in 66.7% (40/60) women. Rate of seroma formation was significantly low in study groups than control groups (33.3% vs. 100%; p=0.0001). Efficacy of the treatments was significantly high in study group (Solumedrol) than control (66.7% vs. 0%; p=0.0001). Similarly, effectiveness of solumendrol was significantly high with respect to age and it was also significantly effective in stage 2A, stage 3A and stage 2B.
While there are no studies on the use of solumedrol (but easily available in our setup) in breast cancer, the use of triamcinalone (which has same mode of action and duration like solumedrol) in breast reconstruction surgery demonstrated than 55% did not have seroma formation whereas 95% developed seroma in those administered saline [12]. It is the possibility of decreased seroma formation with the use of steroids that we propose to undertake this study. The small sample size of present study is a limitation and hence the power of the study is low. A number of questions remain unanswered and more research is needed to answer these.
Conclusion
Single dose of steroid is efficacious in reducing the post mastectomy seroma formation which helps in timely administration of chemotherapy and reduce the risk of infection, necrosis and sepsis and improve quality of life. Steroids may have applications in managing other seromas of similar etiology, such as those after abdominoplasty and hernia mesh repair.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 17, Feb 2020Accepted: Wed 18, Mar 2020
Published: Mon 30, Mar 2020
Copyright
© 2023 Aliya Ishaq. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2020.01.05
Author Info
Abida Parveen Aliya Ishaq Mariya Ishaq Muhammad Jamshaid Hussain Khan Nazia lodhi Rufina Soomro Shadab Ishaq
Corresponding Author
Aliya IshaqLiaquat National Hospital and medical college Karachi, Pakistan
Figures & Tables
Table 1: Descriptive statistics of age of the patients N=60.
|
Statistics |
Age (Years) |
|
Mean ± SD |
52.32 ± 9.86 |
|
95% Confidence Interval |
49.77 to 54.86 |
|
Median (IQR) |
52(14.75) |
|
Maximum Age |
75 |
|
Minimum Age |
32 |
Table 2: Comparison of seroma formation between groups.
|
Seroma Formation |
Solumedrol n=30 |
Normal Saline n=30 |
Total n=60 |
P-value |
|
Yes |
10(33.3%) |
30(100%) |
40(66.7%) |
0.0001 |
|
No |
20(66.7%) |
0(0%) |
20(33.3%) |
Chi-Square = 30, df= 1.
Table 3: Comparison of efficacy between groups.
|
Efficacy |
Solumedrol n=30 |
Normal Saline n=30 |
Total n=60 |
P-value |
|
Yes |
20(66.7%) |
0(0%) |
20(33.3%) |
0.0001 |
|
No |
10(33.3%) |
30(100%) |
40(66.7%) |
Fisher’s exact test applied.
Table 4: Comparison of efficacy between groups for below and equal to 50 years of age.
|
Efficacy |
Solumedrol n=14 |
Normal Saline n=15 |
Total n=29 |
P-value |
|
Yes |
9(64.3%) |
0(0%) |
9(31%) |
0.0001 |
|
No |
5(35.7%) |
15(100%) |
20(69%) |
Fisher exact test applied.
Table 5: Comparison of efficacy between groups for above 50 years of age.
|
Efficacy |
Solumedrol n=16 |
Normal Saline n=15 |
Total n=31 |
P-value |
|
Yes |
11(68.8%) |
0(0%) |
9(31%) |
0.0001 |
|
No |
5(31.3%) |
15(100%) |
20(69%) |
Fisher exact test applied.
Table 6: Comparison of efficacy between groups for stage 1a of breast cancer.
|
Efficacy |
Solumedrol n=2 |
Normal Saline n=2 |
Total n=4 |
P-value |
|
Yes |
1(50%) |
0(0%) |
1(31%) |
0.99 |
|
No |
1(50%) |
2(100%) |
3(75%) |
Fisher exact test applied.
Table 7: Comparison of efficacy between groups for stage 2a of breast cancer.
|
Efficacy |
Solumedrol n=7 |
Normal Saline n=7 |
Total n=14 |
P-value |
|
Yes |
6(85.7%) |
0(0%) |
6(42.9%) |
0.005 |
|
No |
1(14.3%) |
7(100%) |
8(75.1%) |
Fisher exact test applied.
Table 8: Comparison of efficacy between groups for stage 2b of breast cancer.
|
Efficacy |
Solumedrol n=10 |
Normal Saline n=10 |
Total n=20 |
P-value |
|
Yes |
4(40%) |
0(0%) |
4(20%) |
0.025 |
|
No |
6(60%) |
10(100%) |
16(80%) |
Fisher exact test applied.
Table 9: Comparison of efficacy between groups for stage 3a of breast cancer.
|
Efficacy |
Solumedrol n=10 |
Normal Saline n=10 |
Total n=20 |
P-value |
|
Yes |
9(81.8%) |
0(0%) |
9(40.9%) |
0.0005 |
|
No |
2(18.2%) |
11(100%) |
13(59.1%) |
Fisher exact test applied.

References
- S Vijayalakshmi (2018) A comprehensive study on the effect of injection methylprednisolone in post mastectomy seroma. IAIM 5: 43-47.
- Qvamme G, Axelsson CK, Lanng C, Mortensen M, Wegeberg B et al. (2015) Randomized clinical trial of prevention of seroma formation after mastectomy by local methylprednisolone injection. Br J Surg 102: 1195-1203. [Crossref]
- Does a Single Steroid Injection Reduce the Formation of Postmastectomy Seroma (2015) ClinicalTrials.gov Identifier: NCT00307606.
- Taghizadeh R, Taimur Shoaib, Andrew M, Hart,Eva M, Weiler- Mithoff (2008) Triamcinolone reduces seroma re-accumulation in the extended latissimus dorsi donor site. J Plastic Reconstr Aesthet Sur 61: 636-642. [Crossref]
- Hadi N, Soltanipour S, Talei A (2012) Impact of modified radical mastectomy on health-related quality of life in women with early stage breast cancer. Arch Iran Med 15: 504-507. [Crossref]
- Axelsson CK, Qvamme GM, Okholm M, Lanng C, Arpi M et al. (2019) Bacterial colonization of seromas after breast cancer surgery with and without local steroid prophylaxis. World J Surg Oncol 17: 120. [Crossref]
- ten Wolde B, van den Wildenberg FJ, Keemers-Gels ME, Polat F, Strobbe LJ (2014) Quilting Prevents seroma formation following axillary lymph node dissection and mastectomy. Ann Surg Oncol 21: 802-807. [Crossref]
- Lumachi F, Burelli P, Basso SM, Iacobone M, Ermani M (2004) Usefulness of ultrasound scissors in reducing serous drainage after axillary dissection for breast cancer: a prospective randomized clinical study. Am Surg 70: 80-84. [Crossref]
- Schuijtvlot M, Sahu AK, Cawthorn SJ (2002) A prospective audit of the use of a buttress suture to reduce seroma formation following axillary node dissection without drains. Breast 11: 94-96. [Crossref]
- Gilly FN, Francois Y, Sayag-Beaujard AC, Glehen O, Brachet A et al. (1998) Prevention of lymphorrhea by means of fibrin glue after axillary lymphadenectomy in breast cancer: prospective randomized trial. Eur Surg Res 30: 439-443. [Crossref]
- Jain PK, Sowdi R, Anderson AD, MacFie J (2004) Randomized clinical trial investigating the use of drains and fibrin sealent following surgery for breast cancer. Br J Surg 91: 54-60. [Crossref]
- Burak WE, Goodman PS, Young DC, Farrar WB (1997) Seroma formation following axillary dissection for breast cancer: risk factors and lack of influence of bovine thrombin. J Surg Oncol 64: 27-31. [Crossref]
- McCaul JA, Aslaam A, Spooner RJ, Louden I, Cavanagh T et al. (2000) Aetiology of seroma formation in patients undergoing surgery for breast cancer. Breast 9: 144-148. [Crossref]
- Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H et al. (2004) Seroma formation after surgery for breast cancer. World J Surg Oncol 2: 44. [Crossref]
