Efficacy of Lower Fixed Dose Pembrolizumab in the Treatment of Non-Small Cell Lung Cancer Patients in a Lower-Middle Income Country: Jordan Experience
A B S T R A C T
Background: Pembrolizumab was approved as first line treatment of advanced non-small cell lung cancer (NSCLC) at standard dose of 200mg every 3 weeks. We aim to assess the efficacy of a lower fixed dose of 100mg and review the Jordanian experience in first line setting.
Methods: We conducted a retrospective study of 88 NSCLC patients, including 27 who received low fixed dose pembrolizumab (Pem100) and 61 received standard fixed dose (Pem200) from September 2016 to February 2022.
Results: Objective response rate was (48.8%), including (5.6%) with complete response, and (43.2%) with partial response. The median progression free survival (PFS) and median overall survival (OS) for this Jordanian population were consistent with published clinical trials; 8.00 months (95% CI, 7.19 to 8.80) and 17.48 months (95 CI, 15.53 to 19.44), respectively. The PFS and OS were not statistically different between the Pem100 and Pem200 groups (8.00 vs. 8.00 months, p=0.73, and 17.02 vs 17.60 months, p=0.66, respectively). PFS and OS were significantly affected by the programmed cell death ligand (PD-L1) tumor proportion score (TPS) (12.40 vs. 8.00 vs. 5.80 months, p<0.001, and 22.70 vs. 16.47 vs. 12.71 months, p<0.001, in TPS ≥ 50% vs. TPS 1-49% vs. TPS < 1%, respectively). OS was not statistically different according to age, gender, histology, or smoking status.
Conclusion: Lower fixed dose pembrolizumab (100mg 3-weekly) appears to be effective in first line NSCLC. A randomized trial should be done to investigate this low dose with significant cost reduction potential.
Keywords
Lung cancer, pembrolizumab, lower fixed dose
Introduction
Immune checkpoint inhibitors (ICIs) have become a standard treatment in advanced non-small cell lung cancer (NSCLC) patients. In phase 3 trials, NSCLC patients treated with ICI monotherapies had higher median overall survival (OS) than those treated with docetaxel in second-line treatment [1-4]. Moreover, the KEYNOTE-024 and KEYNOTE-042 trials revealed that NSCLC patients with programmed cell death ligand (PD-L1) tumor proportion score (TPS) > 50% who were treated with first line pembrolizumab monotherapy had a longer median OS than those treated with first-line platinum-based chemotherapy [5, 6].
Other phase 3 trials showed that NSCLC patients treated with ICI plus platinum-based chemotherapy as first-line treatment had a higher objective response rate (ORR), longer median progression-free survival (PFS), and longer median OS than those treated with chemotherapy alone, regardless of the PD-L1 TPS status [7-11]. The incorporation of ICIs, especially pembrolizumab in NSCLC treatment guidelines is of high importance in countries like Jordan which is reporting a progressive increase in NSCLC incidence and mortality. It is the third most common type of cancer after breast and colorectal cancers (7.5%), and most common cause of death (15.1%) among cancers in Jordan [12]. Jordan is a lower-middle income country with limited resources; this restricts access to new high-cost therapies like pembrolizumab.
Therefore, we retrospectively reviewed the clinical data of NSCLC patients who were treated in three different Jordanian hospitals with pembrolizumab-based therapy in first-line setting. This was done to assess pembrolizumab’s efficacy and to compare results between patients who received the standard fixed dose of 200mg every 3 weeks versus a lower fixed dose of 100mg at same frequency because of financial restrains. Previous retrospective studies showed similar efficacy between the two doses in an Asian lung cancer population with significant reduction in cost [13, 14].
Methods
I Patients
Patients who were diagnosed with advanced NSCLC and treated with pembrolizumab monotherapy (in case of PD-L1 TPS ≥ 50%) or pembrolizumab plus platinum-based chemotherapy (in case of PD-L1 TPS < 50%) as first line treatment were included in this retrospective study. 88 patients were treated at three hospitals (Al-Khalidi medical center, Jordan Hospital, and Farah medical center) between September 2016 to February 2022. The diagnosis of lung cancer was based on pathology findings. The clinical stage was established according to the 8th edition of the TNM classification. All patients were negative for epidermal growth factor receptor (EGFR) mutation, and anaplastic lymphoma kinase (ALK) rearrangement. The PD-L1 TPS was assessed by means of the PD-L1 immunohistochemistry 22C3 pharmDx assay. Expression was categorized according to the tumor proportion score [15].
According to their financial ability and after detailed discussion about the standard dose in clinical trials and practice guidelines, patients received pembrolizumab as standard fixed dosed of 200mg every 3 weeks or lower fixed dose of 100mg every 3 weeks. All patients were required to sign an informed consent before receiving their treatment. Treatments were administered until disease progression, death, intolerable toxicity, or patient refusal. Tumor imaging was done on weeks 6 and 12, then every 9 weeks through week 48 and every 12 weeks thereafter. Patients were contacted every 12 weeks to assess survival during follow-up.
II Study Design
We retrospectively investigated patient characteristics, ORR, PFS, and OS. Clinical data were collected from medical records. Tumor evaluation was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [16]. ORR was defined as the percentage of patients achieving complete response (CR) or partial response (PR) as their best response. OS was measured from first day of treatment to death from any cause, and PFS was measured from first day of treatment to disease progression or death, whichever occurred first. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Al-Khalidi medical center, which waived the necessity to obtain informed consent.
III Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation, and categorical variables were presented as counts and percentages. The progression-free survival (PFS) or overall survival (OS) were generated by the Kaplan–Meier method and compared by log-rank test. Multivariate Cox regression analyses were performed to determine independent prognostic factors associated with OS and PFS outcomes. All statistical analyses were performed using IBM SPSS V.28.0 (IBM) and p-value level of ≤0.05 was considered statistically significant (two-sided).
Results
I Patient Characteristics
88 patients with advanced NSCLC who underwent first line pembrolizumab therapy were included. The median age was 64 years. 72 (81.8%) patients were males versus 16 (18.2%) females. 69 (79.3%) patients were smokers. 63 (71.6%) patients had non-squamous histology versus 25 (28.4%) patients had squamous histology. PD-L1 was positive in 62 (70.5%) patients, with TPS score of (1-49%) in 30 (48.4%) patients, and score of (50-100%) in 32 (51.6%) patients. 26 (29.5%) of the whole patient population were negative for PD-L1. Because of financial restrains, 27 (30.6%) patients received a lower fixed dose of pembrolizumab (100mg) versus 61 (69.4%) patients received the standard fixed dose of (200mg) every 3 weeks.
II Efficacy
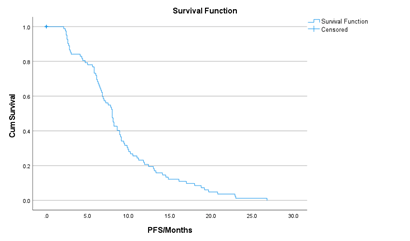
ORR was achieved in 43 (48.8%) patients, including 5 (5.6%) patients with CR, and 38 (43.2%) patients with PR. Stable disease was seen in 30 (34.1%) patients while 15 (17.2%) patients had progressive disease. The median PFS for the whole population was 8.00 months (95% CI, 7.19 to 8.80) (Figure 1). In the multivariate analysis, PFS was significantly affected by smoking history (8.10 versus 6.50 months, in smokers versus non-smokers, respectively, p = 0.047), and PD-L1 status (12.40 versus 8.00 versus 5.80 months, in TPS ≥ 50% versus TPS 1-49% versus TPS < 1%, respectively, p < 0.001). It was not affected by age, gender, histology, or pembrolizumab dose. The median PFS for the low fixed dose pembrolizumab was 8.00 months (95%CI, 7.21-8.98), and for the standard dose pembrolizumab was 8.00 months (95% CI, 6.798-9.202) (p= 0.73).
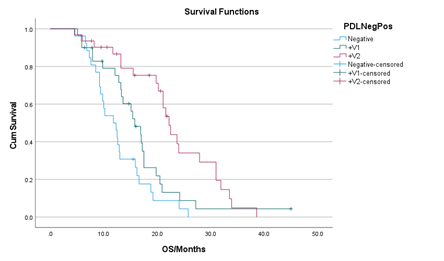
The median OS for the whole population was 17.48 months (95 CI, 15.53 to 19.44) (Figure 2). In the multivariate analysis, median OS was not affected by age, gender, histology, smoking status, or pembrolizumab dose. The median OS for the low fixed dose pembrolizumab was 17.02 months (95% CI, 17.02-19.41), and for the standard dose pembrolizumab was 17.60 months (95% CI, 15.15-20.18) (p=0.66) (Figure 3). Median OS was significantly affected by PD-L1 status only (Figure 4). The median OS for the high TPS group (≥ 50%) was 22.70 months (95% CI, 19.27 to 26.17); low TPS group (1-49%) was 16.47 months (95% CI, 13.29 to 19.66); and the negative TPS group (< 1%) was 12.71 months (95% CI, 10.58 to 14.83) (p<0.001). The median OS for all PD-L1 positive patients (TPS ≥ 1%) was 19.80 months (95% CI, 16.26 to 23.34), significantly higher than the median OS of PD-L1 negative patients (11.80 months (95% CI, 8.68 to 14.92), p < 0.001) (Figure 5).
Figure 1: Kaplan-Meier curve showing median Progression Free Survival (PFS) of the whole group (88 patients).
Figure 2: Kaplan-Meier curve showing median overall survival (OS) in the whole group (88 patients).
Figure 3: Kaplan-Meier curve of OS stratified by pembrolizumab dose. The median OS for the low fixed dose pembrolizumab (100) was 17.02 months (95% CI, 17.02-19.41), and for the standard dose pembrolizumab (200) was 17.60 months (95% CI, 15.15-20.18) (p=0.66).
Figure 4: Kaplan-Meier curve of OS stratified by PD-L1 TPS. The median OS for PD-L1 TPS ≥ 50% (+V2) was 22.7 months (95% CI, 19.27 to 26.17); PD-L1 TPS 1-49% (+V1) was 16.47 months (95% CI, 13.29 to 19.66); and PD-L1 TPS < 1% (Negative) was 12.71 months (95% CI, 10.58 to 14.83) (p<0.001).
Discussion
This study revealed that the addition of pembrolizumab to first line treatment protocols in advanced NSCLC in this Jordanian cohort of patients is of high clinical importance. It showed significant improvement of ORR, PFS, and OS over historical controls treated with chemotherapy alone, regardless of patient age, gender, smoking status, histology or pembrolizumab dose. The outcome was clinically significant across all PD-L1 TPS scores; but patients with higher PD-L1 TPS have better outcomes than patients with lower PD-L1 TPS, consistent with previous studies [5-11].
The efficacy of pembrolizumab plus chemotherapy and pembrolizumab monotherapy as first-line treatment in NSCLC was previously reported. The phase 3 KEYNOTE-189 trial enrolled non-squamous NSCLC patients, and the phase 3 KEYNOTE-407 trial enrolled squamous NSCLC patients for evaluation of first line pembrolizumab plus chemotherapy [7, 8]. In the KEYNOTE-189 trial, the ORR was 48.3%, median OS was 22.0 months, and subgroup analysis of patients with PD-L1 TPS ≥50% showed an ORR of 62.1% and median OS of 27.7 months in patients treated with pembrolizumab plus chemotherapy. In the KEYNOTE-407 trial, the ORR was 62.6%, median OS was 17.1 months, and subgroup analysis of patients with PD-L1 TPS ≥1% showed an ORR of 59.1% and median OS of 18.9 months in patients treated with pembrolizumab plus chemotherapy. The phase 3 KEYNOTE-024 trial enrolled patients with PD-L1 TPS ≥50%, and the phase 3 KEYNOTE-042 trial enrolled patients with PD-L1 TPS ≥1% to evaluate first line pembrolizumab monotherapy [5, 6]. In the KEYNOTE-024 trial, the ORR was 45.5% and median OS was 30.0 months in patients treated with pembrolizumab monotherapy. In the KEYNOTE-042 trial, the ORR was 27%, median OS was 16.9 months, and subgroup analysis of the patients with PD-L1 TPS ≥50% showed an ORR of 39% and median OS of 20.0 months in patients treated with pembrolizumab monotherapy.
In contrast, several studies reported the efficacy of first line pembrolizumab monotherapy for NSCLC patients with PD-L1 TPS ≥50% in the clinical setting. Tambo et al. studied 95 patients and showed an ORR of 40.0% and OS of NR [17]. Amrane et al. studied 108 patients and showed that the ORR was 57.3% and median OS was 15.2 months [18]. Aguilar et al. investigated 187 patients and reported an ORR of 44.4% and median OS of NR [19]. Tamiya et al. studied 213 patients and found an ORR of 51.2% and median OS of 17.8 months [20]. Cortellini et al. studied 1010 patients and reported an ORR of 48.9% and median OS of 27.4 months [21]. The present study revealed that the ORR and median OS in this Jordanian group was similar to those of the previous clinical trials and retrospective studies. This confirms the added benefit of pembrolizumab in this lung cancer population with different background and genetic make-up than the western population.
The incidence of lung cancer in Jordan has been on the rise for the last few years, especially among young patients (mean age of 63.8 years) [22]. Unfortunately, since the introduction of pembrolizumab in Jordan in 2016; most Jordanians with advanced NSCLC did not have access to this drug because of the high cost and lack of insurance coverage. Many other low-income countries in the world have similar problem. This retrospective study showed that a lower fixed dose of pembrolizumab (100mg every 3 weeks) is feasible and has no difference in ORR, PFS, or OS in a Jordanian cohort, with significant cost savings and wider patient accessibility. A further randomized controlled trial should be carried out to confirm this finding.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 19, Apr 2022Accepted: Wed 04, May 2022
Published: Wed 18, May 2022
Copyright
© 2023 Salah Abbasi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2022.02.01
Author Info
Layla Abbasi Adnan Almallah Nedal F Al-Rawashdeh Salah Abbasi
Corresponding Author
Salah AbbasiDepartment of Medicine, Al-Khalidi Medical Center, Amman, Jordan
Figures & Tables


References
1. Borghaei H,
Paz-Ares L, Horn L, Spigel DR, Steins M et al. (2015) Nivolumab versus Docetaxel
in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373:
1627-1639. [Crossref]
2. Brahmer J, Reckamp
KL, Baas P, Crinò L, Eberhardt WEE et al. (2015) Nivolumab versus Docetaxel in
Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 373:
123-135. [Crossref]
3. Herbst RS, Baas P,
Kim DW, Felip E, Pérez-Gracia JL et al. (2016) Pembrolizumab versus docetaxel
for previously treated, PD-L1-positive, advanced non-small-cell lung cancer
(KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540-1550. [Crossref]
4. Rittmeyer A,
Barlesi F, Waterkamp D, Park K, Ciardiello F et al. (2017) Atezolizumab versus
docetaxel in patients with previously treated non-small-cell lung cancer (OAK):
a phase 3, open-label, multicentre randomised controlled trial. Lancet
389: 255-265. [Crossref]
5. Mok TSK, Wu YL,
Kudaba I, Kowalski DM, Cho BC et al. (2019) Pembrolizumab versus chemotherapy
for previously untreated, PD-L1-expressing, locally advanced or metastatic
non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled,
phase 3 trial. Lancet 393: 1819-1830. [Crossref]
6. Reck M,
Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T et al. (2019) Updated Analysis
of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced
Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater.
J Clin Oncol 37: 537-546. [Crossref]
7. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E et al. (2020) Updated
Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and
Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung
Cancer. J Clin Oncol 38: 1505-1517. [Crossref]
8. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Parra HS et al. (2020) A
Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in
Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of
KEYNOTE-407. J Thorac Oncol 15: 1657-1669. [Crossref]
9. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D et al. (2018) Atezolizumab
for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med
378: 2288-2301. [Crossref]
10. West H, McCleod M,
Hussein M, Morabito A, Rittmeyer A et al. (2019) Atezolizumab in combination
with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy
alone as first-line treatment for metastatic non-squamous non-small-cell lung
cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet
Oncol 20: 924-937. [Crossref]
11. Jotte R, Cappuzzo
F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D et al. (2020) Atezolizumab
in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC
(IMpower131): results from a randomized phase III trial. J Thorac Oncol
15: 1351-1360. [Crossref]
12. Nimri O, Arqoub K, Tan’ni N (2016) Jordan Cancer Registry. Ministry of Health.
Cancer Incidence in Jordan-2016.
13. Low JL, Huang Y,
Sooi K, Ang Y, Chan ZY et al. (2021) Low dose pembrolizumab in the treatment of
advanced non-small cell lung cancer. Int J Cancer 149: 169-176. [Crossref]
14. Low JL, Sooi K,
Huang Y, Chan GHJ, Ang Y et al. (2020) Cost and efficacy of low dose
pembrolizumab in the treatment of non-small cell lung cancer patients in Asia. J
Clin Oncol 38: e19385.
15. Roach C, Zhang N,
Corigliano E, Jansson M, Toland G et al. (2016) Development of a Companion
Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in
Non-Small-cell Lung Cancer. Appl Immunohistochem Mol Morphol 24:
392-397. [Crossref]
16. Eisenhauer EA,
Therasse P, Bogaerts J, Schwartz LH, Sargent D et al. (2009) New response
evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur
J Cancer 45: 228-247. [Crossref]
17. Tambo Y, Sone T,
Shibata K, Nishi K, Shirasaki H et al. (2020) Real-World Efficacy of First-Line
Pembrolizumab in Patients With Advanced or Recurrent Non-Small-Cell Lung Cancer
and High PD-L1 Tumor Expression. Clin Lung Cancer 21: e366-e379. [Crossref]
18. Amrane K, Geier M, Corre R, Léna H, Léveiller G et al. (2020) First line
pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a
multicenter real-life cohort: the PEMBREIZH study. Cancer Med 9:
2309-2316. [Crossref]
19. Aguilar EJ,
Ricciuti B, Gainor JF, Kehl KL, Kravets S et al. (2019) Outcomes to first line
pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1
expression. Ann Oncol 30: 1653-1659. [Crossref]
20. Tamiya M, Tamiya A,
Hosoya K, Taniguchi Y, Yokoyama T et al. (2019) Efficacy and safety of
pembrolizumab as first-line therapy in advanced non-small cell lung cancer with
at least 50% PD-L1 positivity: a multicenter retrospective cohort study (HOPE-001).
Invest New Drugs 37: 1266-1273. [Crossref]
21. Cortellini A, Friedlaender A, Banna GL, Porzio G, Bersanelli M et al. (2020) Immune-related Adverse Events of Pembrolizumab in a Large Real-world Cohort of Patients With NSCLC With a PD-L1 Expression ≥ 50% and Their Relationship With Clinical Outcomes. Clin Lung Cancer 21: 498-508.e2. [Crossref]
22. ALQudah MA, ALFaqih MA, Hamouri S, Al-Shaikh AF, Haddad HK et al. (2021) Epidemiology and histopathological classification of lung cancer: A study from Jordan, retrospective observational study. Ann Med Surg (Lond) 65: 102330. [Crossref]
