Efficacy and Quality of Life Assessment of ‘Kapiva Dia Free Juice’ Treatment as an Adjuvant for Type 2 Diabetes
A B S T R A C T
Background: Diabetes mellitus (DM) is characterized by chronic hyperglycemia, defects in insulin secretion, action, or both, and is fuelled by the obesity epidemic. Conventional medication offers side effects and insufficient glycaemic management in chronic use. Safe and effective adjuvants from the natural source need to be studied clinically.
Methods: 30 patients of diabetes mellitus were enrolled in the study for 3 months. The interventional product kapiva dia free juice was administered twice daily along with the conventional antidiabetics. Fasting and post prandial blood glucose were measured every month while HbA1c, serum C-peptide level, BMI, biochemical and hematological investigations on baseline and day 90. Data was analysed using SPSS (version 19).
Result: HbA1c was reduced significantly by 11.11% after 90 days of treatment. The percentage of significant reduction in fasting and post prandial blood glucose was 30.05% and 33.11% respectively. BMI was significantly reduced by 2.92% with average weight reduction of 2.17 kg. Insulin resistance was also reduced significantly which was evident by lowered C-Peptide by 28.81%. All the biochemical and hematological parameters were found within normal range and no adverse events were reported related to the investigational product.
Conclusion: Kapiva dia free juice is an effective adjuvant treatment in diabetes mellitus which can improve glycemic control, reduce BMI and insulin resistance. This reduces cardiac risk and improves antidiabetic medication effectiveness. All hematological parameters were well within limits in the study which indicates the safety of the product.
Keywords
Diabetes mellitus, adjuvant, herbal, HbA1c, insulin resistance
Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by chronic hyperglycemia resulting from defects in insulin secretion, action, or both [1]. Diabetes prevalence has been constantly growing, and fuelled by rising obesity rates due to sedentary lifestyles & poor dietary patterns, ageing population, and a rise in high-risk ethnic groups [2, 3]. Diabetes can cause life-threatening, disabling, and costly complications, reducing life expectancy [4]. Diabetes prevalence in people of age below 20 years is currently highest in India, followed by Brazil and the United States. As of 2019, approximately 77 million adult diabetics were in India and will have approximately 80 million people with diabetes by 2030 [5]. According to WHO approximately 400 million people globally have diabetes, majorly in low-and middle-income countries and 1.5 million deaths are directly attributed to diabetes each year. There is an urgent need for intervention to manage diabetes and long-term complication prevention considering diabetes incidence and mortality [6].
Type 2 diabetes mellitus (T2DM) therapy includes lifestyle changes and treatment with oral hypoglycemic agents, alone or in combination to achieve glycemic control [7]. Since, almost three decades, the pharmacological therapy of T2DM has been focused on addressing hyperglycemia with sulphonylureas, biguanides, alpha-glycosidase inhibitors, and insulin. Worldwide studies have shown that hyperglycemia is a symptom of metabolic disorders like diabetes. In recent years the treatment goal is focused on rectifying the metabolic state and lowering the risk of micro and macrovascular complications as well [8].
Numerous investigations have revealed that over time oral hypoglycemics prove insufficient to achieve recommended glycaemic targets, necessitating insulin supplementation [9]. Insulin doses are also observed to be increasing with poor glycaemic control over period of years in patients of diabetes mellitus [10, 11]. There are many short term and long term side effects of oral antihyperglycemics as well as insulin supplements. The use of sulfonylureas is primarily associated with hypoglycemia, weight gain and gastrointestinal discomfort is one of the most frequent side effects of glucagon-like peptide-1 agonists etc. which affect patient compliance as well [12].
Therefore, safe and effective herbal adjuvant with fewer adverse effects can be sustainable approach. This can be successfully coupled with lifestyle changes, nutritional approaches and behavioural therapy for holistic goals. There are many research attempts proving the effectiveness of phytochemicals in obesity and diabetes as a monotherapy as well as adjuvant to the conventional [13]. There are many herbs mentioned in ayurveda as well as researched phytoconstituents being helpful in improving insulin sensitivity, blood glucose control, and lowering HbA1C [14, 15].
When incorporating herbal adjuvant to the anti-diabetic regimen, clinical evidence is indispensable. The current clinical study seeks to produce evidence about the efficacy and safety of kapiva dia free juice with anti-hyperglycemic potential as an adjuvant therapy in T2DM patients to improve prognosis and quality of life. Kapiva dia free juice is prepared using a combination of two ayurvedic preparation methods - swarasa and kwatha. Fresh juice of certain ingredients such as amla is added to the decoction of other ingredients. A combination of 11 ayurvedic herbs are added in an easy to consume juice form. All herbs are potent antihyperglycemic agents and help in achieving control on blood glucose levels naturally. These herbs additionally improve metabolism and help in having a sustained effect on the body.
Materials and Methods
I Study Objectives
Primary objectives of the research were to evaluate changes in glycosylated haemoglobin (HbA1c), fasting, post prandial blood sugar, serum C-peptide levels in diabetic subjects when kapiva dia free juice was used as an adjuvant to conventional medication for three months. Secondary objective of the research was evaluation changes in BMI in T2DM patients along with the assessment of safety.
II Inclusion Criteria
Patients aged 18 years and above of both the sex having HbA1c levels 6.5% and above, and body mass index (BMI) > 27 kg/m2 and < 45 kg/m2 were considered for the study. Patients with a diagnosis of T2DM for more than a year prior to signing up for clinical trial, those who were under the treatment of oral antidiabetic drugs were enrolled into the study. Patients on the stable prescription for the last three months were included in the study. However, the dosage modification after starting adjuvant intervention of kapiva dia free juice was allowed.
III Exclusion Criteria
Pregnant and lactating women were not allowed to take part in the study. Patients on insulin therapy and/or hyperosmolar hyperglycemic state were excluded from the study. Patients having a history of chronic illness, type 1 diabetes or ketoacidosis and secondary diabetes were excluded from the study. Myocardial infarction within the previous 6 months, have any other condition (including known drug or alcohol abuse, or psychiatric disorder including eating disorder) that precludes the patient from following and completing the protocol, blood donation within 2 months prior to the screening visit or plans to donate blood or blood products during the study were excluded from the study. Any medical condition of the patient from investigators discretion find patient not suitable for the study were excluded. Patients undergoing major or minor surgery were not considered in the study.
IV Methodology
This was an open label, single-arm clinical study. A total of 31 patients screened and 30 were enrolled in the study for a period of 3 months (Figure 1). Single research site was taken and the study was initiated after an approval from ACE independent ethics committee (IEC) followed by CTRI registration CTRI/2022/04/041781 [Registered on: 11/04/2022]. The study was conducted according to the principles of the declaration of Helsinki. Written informed consent was obtained from the patients for participation in the study during the screening visit. The demographic details of the patient were noted. Patients received a clinical examination, including neurological, cardiac, pulmonary, gastrointestinal, and genitourinary systems examination. Blood samples of all eligible patient were collected for biochemical and hematological investigations. Patient meeting all inclusion requirements and not exhibiting any exclusion criteria, were admitted to the study.
Figure 1: CONSORT flow of the study.
Patients were provided with an interventional product - kapiva dia free juice to be consumed for 90 days. Any adverse events that occurred throughout the screening period were documented. In case of any comorbidity or concurrent illness, the condition and medication were recorded. Vitals were recorded. Patients were screened for any allergies to the ingredients of the kapiva dia free juice. All patients were on their regular medication of T2DM as the standard of care until the investigator modified the doses.
The investigator evaluated patients' drug compliance on every visit. Patients were considered dropped out if they regularly missed dosage for more than three days in a row or more than six doses overall over the 30 days. Patients were assessed for any adverse events during the entire study period.
The patients were asked to follow up every month for 3 months. On each visit day, the patient underwent clinical examination, symptoms, screening for any adverse event, and blood sample collection for assessment of blood sugar and related parameters. Assessment of HbA1c, BMI, hematological and biochemical parameters were done at screening and day 90. The fasting and post prandial blood glucose were measured on every visit. The following visit schedule was followed:
Study Visits
Screening visit-1/ (Day -3 to Day 0)- Informed consent process
Visit 2/Day 1 - Randomization visit
Visit 3/Day 15 -Telephonic follow up visit
Visit 4/Day 30 (+2 days window period)- Site visit
Visit 5/Day 45 - Telephonic follow up visit
Visit 6/Day 60 (+2 days window period)- Site visit
Visit 7/Day 75 - Telephonic follow up visit
Visit 8/Day 90 (+2 days window period)- End of the study visit
The patients were asked to contact the investigator for any adverse event between the visits. After the completion of 90 days, the patients were asked to stop the study medication and advised to follow the investigator’s instructions for further treatment.
V Intervention and Dosage
Patients were instructed to consume 30 ml kapiva dia free juice in 200 ml water in morning and evening one hour prior to having meal for 90 days. Following are the key ingredients of kapiva dia free juice- Amla (Phyllanthus emblica), Karela (Momordica charantia), Jamun (Syzygium cumini), Giloy (Tinospora cordifolia), Tulsi (Ocimum tenuiflorum), Neem (Azadirachta indica), Belpatra (Aegle marmelos), Methi (Trigonella foenum-graecum), Kutki (Picrorhiza kurroa), Vijayasar (Pterocarpus marsupium), Gudmar (Gymnema sylvestre). All the ingredients are mentioned for their beneficial activity in the management of diabetes and related complications in ancient ayurvedic and modern research literature.
VI Sample Size
The study was exploratory and proof of concept study. Since this is a supplement adjuvant therapy, it is not placebo-controlled. The sample size was considered to be thirty complete patients based on clinical and research judgment of the investigators.
Results
Amongst 30 subjects the male-to-female ratio was 13:17. All the patients had type 2 diabetes mellitus and were taking the following medications as standard of care (alone or in combination): Metformin, sitagliptin, glimepiride, dapagliflozin, vildagliptin, pioglitazone, teneligliptin, glibenclamide, voglibose. Patients also had comorbidities and were on concomitant medications for the same (Table 1).
Table 1: Comorbidity and concomitant medication.
|
Concomitant indication |
No. of Patients |
Concomitant Medication |
|
Hypertension |
12 |
Amlodipine, Telmisartan |
|
Hypothyroidism |
2 |
Thyroxine |
|
General wellbeing |
4 |
A2Z Gold (Multivitamin), Vitamin + Zinc Sulphate,
Renomega forte |
|
Dyslipidaemia |
2 |
Rosuvastatin |
|
Dyspnoea |
1 |
Levosalbutamol + Ipratropium |
The mean score of HbA1c was 9.36 % and 8.32% at baseline and day 90 respectively. There was a significant reduction in HbA1C (11.11%) from baseline to day 90 (Table 2). There was a statistically significant reduction of 2.92 % (2.17 kg in 3 months) in BMI after treatment (0.83 kg/m2). Data is represented in (Table 2).
Table 2: Assessment
of changes in HbA1C and BMI.
|
TIMELINE |
MEAN |
SD |
p-value |
|
Changes in Glycosylated haemoglobin levels (HbA1c %)
* |
|||
|
Baseline |
9.36 |
1.86 |
0.01 |
|
Day 90 |
8.32 |
1.62 |
|
|
Changes in BMI (kg/m2)* |
|||
|
Baseline |
28.37 |
1.17 |
0.01 |
|
Day 90 |
27.54 |
1.16 |
|
|
Changes in Fasting blood glucose (mg/ dl) # |
|||
|
Baseline |
211.3 |
63.52 |
0.01 |
|
Day 90 |
147.8 |
23.9 |
|
|
Changes in Post-prandial blood glucose (mg/dl) # |
|||
|
Baseline |
254.9 |
70.9 |
0.01 |
|
Day 90 |
170.5 |
27.2 |
|
|
Changes in C-peptide (ng/ml) # |
|||
|
Baseline |
4.13 |
1.98 |
0.01 |
|
Day 90 |
2.94 |
0.77 |
|
*-Data was analysed by paired student t test. Significant
at p<0.05. #- Data was analysed by Wilcoxon t test. Significant at p<0.05.
SD- Standard deviation.
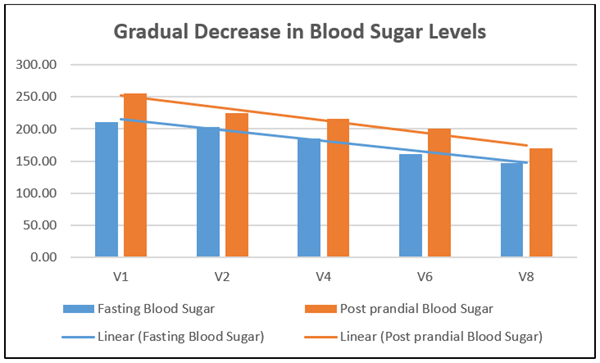
There was a significant reduction (30.05%) in fasting blood sugar after treatment with kapiva dia free juice for 90 days. The mean value was 211.3 mg/dl at baseline and 147.8 mg/dl at day 90 (with a mean value difference of 63.5 mg/dl). There was a significant reduction in post prandial blood glucose (33.11%) after treatment for 90 days. The mean post prandial blood sugar was 254.9 mg/dl at baseline and 170.5 mg/dl at day 90 (mean difference of 84.4 mg/dl). There was a gradual reduction in fasting and post prandial blood sugar levels after treatment with kapiva dia free juice (Figure 2). Visible fasting and post prandial blood sugar reduction were seen in an average of 6.13 and 6.23 weeks respectively. There was a significant reduction in C-peptide (28.81%) after 90 days of the treatment. C-peptide is used to measure the insulin secretion of an individual (Table 2).
Figure 2: Gradual decrease in blood sugar levels.
Adverse events were recorded in eleven patients. All of the adverse events were mild in nature. None of the adverse event was related to the consumption of the kapiva dia free juice. Fever, cough, body pain, cold, stomach burning, runny nose, loose motion, dizziness were the adverse events reported during the study duration. All AEs were resolved with rescue medications such as paracetamol, levocetirizine, herbal cough syrup, loperamide and changes in dietary habits within 24 hours of occurrence.
Assessment of haemoglobin, total and differential WBC count, biochemistry evaluations like SGOT, SGPT, and serum creatinine were performed on screening visit and day 90. There were no clinically significant changes observed. All parameters remained within a normal range throughout the study period. There was no protocol deviation observed throughout the study and there was 90% compliance with the test product consumption was accomplished by all the patients.
Discussion
After 90 days of treatment, the patients showed a substantial improvement in all key biochemical markers of T2DM when compared to the baseline. HbA1c, fasting and post prandial blood glucose were reduced significantly as compared to baseline. C-peptide was also reduced significantly indicating that there was reduction in insulin resistance. Significant reduction in BMI after the 90 days of treatment was observed which can be linked to reduced risk factors for diabetes-related cardiovascular complications, which may in long run ascertain prevention of progression and thus reattained quality of life of patients. There have been no adverse events related to investigational product, and the results of the examination of the laboratory variables support to the safety of the kapiva dia free juice.
For the optimum management of T2DM and to reduce the issues brought on by hyperglycemia, a comprehensive and coordinated approach is required. The holistic approach of diabetes management could be incorporating safer and effective herbal intervention along with diet, and exercise [16]. The American Diabetes Association recommends utilizing HbA1c rather than fasting plasma glucose as a reliable predictor of chronic glycemia and a significant link with the risk of long-term complications [17]. HbA1c is an indicator of dyslipidemia as well as cardiovascular risk [18]. The major strength of the intervention is that it could help lower HbA1c and thus with regular use it can lead to reduction in existing oral hypoglycemic agent doses. This essentially will attain the aim of adjuvant treatment of kapiva dia free juice by improving the biomarker status with reduced requirement of conventional medication and improved prognosis of disease. By virtue of the synergistic activity of the herbal ingredients used in the formulation we can expect the results attained.
Emblica officinalis (Amla) as a constituent in the juice has been reported to possess bioactive compounds like tannins, flavonoids which are confirmed to have diverse pharmacological activities such as antihyperlipidemic, hypoglycaemic, antioxidant, wound healing activities, etc. Study conducted by Akhtar MS et al. reported that Emblica officinalis improved fasting and post prandial blood glucose and reduced body weight which was similar to our study [19, 20]. Bitter melon or karela contains lectin, acting like insulin, on peripheral tissues improving glucose mobilization along with suppressing appetite [21, 22].
Jamun (Syzygium cumini) possesses alkaloids, tannins, and phenols contributing as a rich source of nutrition and medicine [23]. In India decoction of kernels of Eugenia jambolana is used as household remedy for diabetes. This also forms a major constituent of many herbal formulations for diabetes owing to its anti hyperglycemic potential [24]. Azadirachta indica (Neem) is a medicinal plant, used in ayurveda for treating diabetes mellitus. Investigations reported that different extracts and phytochemicals of the same exert antidiabetic activity by affecting blood glucose level, lipid profile, oxidative stress, carbohydrate digestion enzymes, diabetic complications, glucose tolerance, and uptake of glucose [25, 26].
Fenugreek extract has a preventative effect on dyslipidaemia and fat accumulation by inhibiting poor lipid digestion and absorption, improving glucose and lipid metabolism, improving insulin sensitivity, increasing antioxidant defence, and downregulating lipogenic enzymes. Studies have demonstrated that heteropolysaccharide galactomannan from fenugreek seeds aid in an effective weight loss [27-29]. Study conducted by Sankhla A et al. concluded that bael patra extract effectively lowered the serum and urine glucose levels in diabetic patients which was similar to our study [30].
The leaves of tulsi are enriched with antioxidants and essential oils like eugenol, methyl eugenol, and caryophyllene. These compounds aid in the secretion of insulin and upsurge the body’s sensitivity to insulin. The blend of these powerful medicinal plants as kapiva dia free juice helped in achieving desired glycemic control and reducing body weight to improve quality life. The antidiabetic effect of leaf extract from Gymnema sylvestre (GS4) in controlling hyperglycemia was investigated in twenty-two T2DM patients as a supplement to the conventional oral drugs. During GS4 supplementation, the patients showed a significant reduction in blood glucose, HbA1C and glycosylated plasma proteins, and conventional drug dosage could be decreased. Five of the twenty-two diabetic patients were able to discontinue their conventional drug and maintain their blood glucose homeostasis with GS4 alone. These data suggest that the beta cells may be regenerated/repaired in type 2 diabetic patients on GS4 supplementation. This is supported by the appearance of raised insulin levels in the serum of patients after GS4 supplementation [31].
A flexible dose 12-week open trial was undertaken in India to evaluate the efficacy of an ayurvedic drug Vijayasar (Pterocarpus marsupium) in the treatment of newly diagnosed or untreated non-insulin dependent diabetes mellitus. Control of blood glucose (both fasting and postprandial levels) had been attained in 69% patients studied, and the dose on which control was attained was 2 g of the extract in about 73 per cent of the patients, 3 g in about 16 per cent and 4 g in 10 per cent of the patients. Both the fasting and postprandial blood glucose levels as well as HbA1c decreased significantly [32]. Investigation done by Deep HS et al. correlated poor glycemic control with increased insulin resistance (high c peptide levels). In our study, C-peptide levels were reduced after 90 days of treatment suggesting reduced insulin resistance in study patients [33].
There are many herbal remedies suggested for diabetes and diabetic complications in many traditional reference books and they are being practiced to prescribed. Though it’s a well-known fact that single herb or polyherbal formulations are effective in improving prognosis in diabetes mellitus. Still, there are very few clinical trials that provide scientific validation of their use as an adjuvant along with conventional medication. The strength of the study is that the parameters considered for evaluation provide immediate and long-term approach to manage diabetes. Reduction in fasting and post meal glucose is most desired immediate biomarker for diabetes while improved BMI, HbA1c and C-peptide could be linked to long term weight management and improved prognosis of diabetes. It can be concluded that kapiva dia free juice has potential to improve quality of life of T2DM patients. Beneficial effects of herbal ingredients from the intervention got translated in the clinical trial. There were no clinically significant changes observed in hematological and biochemical parameters proving the safety of kapiva dia free juice. The future scope of the work would be to evaluate effectiveness of the kapiva dia free juice in larger subject population with long term treatment duration to look into reduction in doses of conventional antidiabetic medication.
Conclusion
The kapiva dia free juice is a good candidate as an effective adjuvant treatment in diabetes mellitus because it can offer better glycemic control, reduced BMI and insulin resistance leading to reduced cardiac risk and improved effectiveness of antidiabetic medications. Based on biochemical, hematological data and absence of any drug related adverse events, it can be concluded that the juice is safe to consume at any given dose.
Acknowledgments
The authors would like to acknowledge the research team and the back-office team involved in the research work. We would like to acknowledge the support of Trialguna Pvt Ltd, Bangalore, as a clinical research organization for this trial.
Funding
Investigational product and testing expenses borne in the trial were supported by Adret Retail Pvt Ltd. (Kapiva Ayurveda), India.
Conflicts of Interest
Dr. Kriti Soni and Dr. Anusha Rao are part of R&D team at Adret Retail Pvt Ltd (Kapiva Ayurveda). Another author declares no conflict of interest.
Statement of Ethics
All aspects of this study involving human subjects were performed in an ethical manner, in compliance with the Helsinki Declaration and approved by the ACE Independent ethics committee.
Author Contributions
Dr. Kriti Soni: Study protocol creation, study document finalization, data analysis, and report and manuscript writing. Dr. Rajendra Narayan Sharma: Subject visits and data collection, Adverse event analysis etc. Dr. Anusha Rao: Study document development, data analysis, and report and manuscript writing.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 25, Apr 2023Accepted: Mon 22, May 2023
Published: Tue 30, May 2023
Copyright
© 2023 Kriti Soni. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JDMC.2023.01.01
Author Info
Kriti Soni Rajendra Narayan Sharma Anusha Rao
Corresponding Author
Kriti SoniHead, R&D Kapiva Ayurveda, Vaishnavi Tech Park, Ambalipura Village, Varthur Hobli, Varthur, Bengaluru, Karnataka, India
Figures & Tables
Table 1: Comorbidity and concomitant medication.
|
Concomitant indication |
No. of Patients |
Concomitant Medication |
|
Hypertension |
12 |
Amlodipine, Telmisartan |
|
Hypothyroidism |
2 |
Thyroxine |
|
General wellbeing |
4 |
A2Z Gold (Multivitamin), Vitamin + Zinc Sulphate,
Renomega forte |
|
Dyslipidaemia |
2 |
Rosuvastatin |
|
Dyspnoea |
1 |
Levosalbutamol + Ipratropium |
Table 2: Assessment
of changes in HbA1C and BMI.
|
TIMELINE |
MEAN |
SD |
p-value |
|
Changes in Glycosylated haemoglobin levels (HbA1c %)
* |
|||
|
Baseline |
9.36 |
1.86 |
0.01 |
|
Day 90 |
8.32 |
1.62 |
|
|
Changes in BMI (kg/m2)* |
|||
|
Baseline |
28.37 |
1.17 |
0.01 |
|
Day 90 |
27.54 |
1.16 |
|
|
Changes in Fasting blood glucose (mg/ dl) # |
|||
|
Baseline |
211.3 |
63.52 |
0.01 |
|
Day 90 |
147.8 |
23.9 |
|
|
Changes in Post-prandial blood glucose (mg/dl) # |
|||
|
Baseline |
254.9 |
70.9 |
0.01 |
|
Day 90 |
170.5 |
27.2 |
|
|
Changes in C-peptide (ng/ml) # |
|||
|
Baseline |
4.13 |
1.98 |
0.01 |
|
Day 90 |
2.94 |
0.77 |
|
*-Data was analysed by paired student t test. Significant
at p<0.05. #- Data was analysed by Wilcoxon t test. Significant at p<0.05.
SD- Standard deviation.
References
1. Kharroubi AT, Darwish HM (2015) Diabetes mellitus: The
epidemic of the century. World J Diabetes 6: 850-867. [Crossref]
2. World Health Organization (2022) Diabetes: World Health
Organization.
3. DeFronzo RA, Bonadonna RC, Ferrannini E (1992) Pathogenesis
of NIDDM. A balanced overview. Diabetes Care 15: 318-368. [Crossref]
4. Heald AH, Stedman M, Davies M, Livingston M, Alshames R
et al. (2020) Estimating life years lost to diabetes: outcomes from analysis of
National Diabetes Audit and Office of National Statistics data. Cardiovasc Endocrinol
Metab 9: 183-185. [Crossref]
5. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K
et al. (2022) IDF Diabetes Atlas: Global, regional, and country-level diabetes prevalence
estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:
109-119. [Crossref]
6. Jha RP, Shri N, Patel P, Dhamnetiya D, Bhattacharyya K
et al. (2021) Trends in the diabetes incidence and mortality in India from 1990
to 2019: a joinpoint and age-period-cohort analysis. J Diabetes Metab Disord
20: 1725-1740. [Crossref]
7. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden
ZT et al. (2016) Consensus statement by the american association of clinical endocrinologists
and american college of endocrinology on the comprehensive type 2 diabetes management
algorithm--2016 executive summary. Endocr Pract 22: 84-113. [Crossref]
8. Southwell A, Eckland D (1998) Limitations of treatments
available for the management of type 2 diabetes: results from an international survey
of physicians. Practical Diabetes International 15: 112-116.
9. Downie M, Kilov G, Wong J (2016) Initiation and Intensification
Strategies in Type 2 Diabetes Management: A Comparison of Basal Plus and Premix
Regimens. Diabetes Ther 7: 641-657. [Crossref]
10. Muliyil DE, Vellaiputhiyavan K, Alex R, Mohan VR (2017)
Compliance to treatment among type 2 diabetics receiving care at peripheral mobile
clinics in a rural block of Vellore District, Southern India. J Family Med Prim
Care 6: 330-335. [Crossref]
11. Mukherjee S, Sharmasarkar B, Das KK, Bhattacharyya A, Deb
A (2013) Compliance to anti-diabetic drugs:observations from the diabetic clinic
of a medical college in Kolkata, India. J Clin Diagn Res 7: 661-665. [Crossref]
12. Valerón PF, de Pablos Velasco PL (2013) Limitations of
insulin-dependent drugs in the treatment of type 2 diabetes mellitus. Med Clin
(Barc) 141 Suppl 2: 20-25. [Crossref]
13. Fernandes I, Oliveira J, Pinho A, Carvalho E (2022) The
Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites
12: 184. [Crossref]
14. Alinejad Mofrad S, Foadoddini M, Saadatjoo SA, Shayesteh
M (2015) Improvement of glucose and lipid profile status with Aloe vera in pre-diabetic
subjects: a randomized controlled-trial. J Diabetes Metab Disord 14: 22.
[Crossref]
15. Leone A, Fico G, Bertoli S, Battezzati A (2022) Editorial:
Plant-Based Products, Phytochemicals and Glycemic Control. Front Endocrinol (Lausanne)
13: 906690. [Crossref]
16. George CM, Brujin LL, Will K, Howard Thompson A (2015)
Management of Blood Glucose with Noninsulin Therapies in Type 2 Diabetes. Am
Fam Physician 92: 27-34. [Crossref]
17. Gillett MJ (2009) International Expert Committee report
on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32(7):
1327-1334. Clin Biochem Rev 30: 197-200. [Crossref]
18. Khan HA, Sobki SH, Khan SA (2007) Association between glycaemic
control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia.
Clin Exp Med 7: 24-29. [Crossref]
19. Hasan MR, Islam MN, Islam MR (2016) Phytochemistry, pharmacological
activities and traditional uses of Emblica officinalis: A review. International
Current Pharmaceutical Journal 5: 14-21.
20. Akhtar MS, Ramzan A, Ali A, Ahmad M (2011) Effect of Amla
fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal
subjects and type 2 diabetic patients. Int J Food Sci Nutr 62: 609-616. [Crossref]
21. Selvakumar G, Shathirapathiy G, Jainraj R, Yuvaraj Paul
P (2017) Immediate effect of bitter gourd, ash gourd, Knol-khol juices on blood
sugar levels of patients with type 2 diabetes mellitus: A pilot study. J Tradit
Complement Med 7: 526-531. [Crossref]
22. Gupta M, Sharma S, Gautam AK, Bhadauria R (2011) Momordica
charantia Linn.(Karela): Nature’s silent healer. International Journal of Pharmaceutical
Sciences Review and Research 11: 32-37.
23. Arya SS, Pegu K, Sadawarte PD (2017) Bioactive Compounds
and Health Benefits of Jamun (Syzygium cumini).
Bioactive Molecules in Food 1-20
24. Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TPA
(2007) Indian herbs and herbal drugs used for the treatment of diabetes. J Clin
Biochem Nutr 40: 163-173. [Crossref]
25. Satyanarayana K, Sravanthi K, Shaker IA, Ponnulakshmi R
(2015) Molecular approach to identify antidiabetic potential of Azadirachta indica.
J Ayurveda Integr Med 6: 165-174. [Crossref]
26. Patil SM, Shirahatti PS, Ramu R (2022) Azadirachta indica
A. Juss (neem) against diabetes mellitus: a critical review on its phytochemistry,
pharmacology, and toxicology. J Pharm Pharmacol 74: 681-710. [Crossref]
27. Gaddam A, Galla C, Thummisetti S, Marikanty RK, Palanisamy
UD et al. (2015) Role of Fenugreek in the prevention of type 2 diabetes mellitus
in prediabetes. J Diabetes Metab Disord 14:74. [Crossref]
28. Kandhare AD, Bandyopadhyay D, Thakurdesai PA (2018) Low
molecular weight galactomannans-based standardized fenugreek seed extract ameliorates
high-fat diet-induced obesity in mice via modulation of FASn, IL-6, leptin, and
TRIP-Br2. RSC Adv 8: 32401-32416. [Crossref]
29. Kumar P, Bhandari U, Jamadagni S (2014) Fenugreek seed
extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced
obese rats. Biomed Res Int 2014: 606021. [Crossref]
30. Sankhla A, Sharma S and Sharma N (2009) Hypoglycemic effect
of bael patra (aegle marmelos) in niddm patients. Asian Journal of Dairy and
Food Research 28: 233-236.
31. Baskaran K, Ahamath BK, Shanmugasundaram KR, Shanmugasundaram
ERB (1990) Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent
diabetes mellitus patients. J Ethnopharmacol 30: 295-305. [Crossref]
32. (1998) Flexible dose open trial of Vijayasar in cases of newly-diagnosed non-insulin-dependent diabetes mellitus. Indian Council of Medical Research (ICMR), Collaborating Centres, New Delhi. Indian J Med Res 108: 24-29. [Crossref]
33. Deep HS, Singh BP, Singh SP (2017) Evaluation of serum c-peptide levels in type 2 diabetics in Punjabi population. Int J Adv Med 4: 1026-1030.
