Effects of Low Dose Fractional Radiation on Chemosensitivity of Gemcitabine-Resistant Human Pancreatic Cancer SW1900/GZ Cell
A B S T R A C T
Objective: To investigate whether low-dose fractionated radiation (LDFRT) could enhance gemcitabine sensitivity in drug-resistant human pancreatic cancer SW1900/GZ cell, and to further explore the underlying mechanism.
Methods: Gemcitabine-resistant human pancreatic cancer SW1900 cell line (SW1900/GZ) was induced by high concentration gemcitabine intermittent shock in vitro. The cell counting kit 8 (CCK8) was used to determine SW1900/GZ cell lines. SW1900/GZ cells were divided into six groups as follows: control, LDFRT, high dose radiation (HDRT), gemcitabine (GEM), low dose fractional radiation plus gemcitabine (LDFRT+ GEM) and high dose radiation plus gemcitabine (HDRT+ GEM) groups. The rate of apoptosis was determined by flow cytometry (FCM). Protein levels of multidrug resistance gene (MDR) and multidrug resistance-related protein gene (MRP) were examined by Western blotting.
Results: The results of CCK8 test showed that the half-maximal inhibitory concentration (IC50) of non-drug-resistant cell line SW1900 and drug-resistant cell line SW1990/GZ were 230.4ng/ml and 856.6ug/ml respectively. The IC50 of SW1990/GZ was 3700 times more than the former. LDFRT significantly promoted apoptosis in SW1900 cells. Moreover, in the LDFRT group, protein levels of MDR and MRP were markedly decreased.
Conclusion: This study established an effective gemcitabine-resistant cell line SW1900 of human pancreatic cancer (SW1900/GZ cell line). LDFRT sensitizes resistant SW1900/GZ pancreatic cancer cell to gemcitabine through down-regulation the expression of MDR and MPR proteins.
Keywords
Pancreatic cancer, drug resistance, gemcitabine, low dose fractionated radiation (LDFRT)
Introduction
Pancreatic cancer is the sixth leading cause of cancer-associated deaths in China with about 90,100 new cases and 79,400 deaths by the end of 2015 [1, 2]. In the United States, it is estimated that pancreatic cancer will become the second leading cause of cancer-related deaths by 2030 [3]. Cancer of the pancreas remains one of the most deadly common cancer types: the Mortality/Incidence ratio is 98% [4]. The overall five-year survival rate is about 6% with a range from 2-9%, partly reflecting varying data quality worldwide. The survival rates vary very small between developed and developing countries [5]. Radical resection is still the sole curative option, but about 80% of pancreatic cancers are diagnosed at locally advanced or/and metastatic stages, and curative surgical resection is not possible [2, 6]. Therefore, chemotherapy remains the main treatment for most patients with pancreatic cancer to alleviate the symptom and prolong the survival [2].
Gemcitabine (GEM, 2′, 2′-difluoro-2′-deoxycytidine, dFdC), a deoxycytidine analog that inhibits DNA replication and arrests tumor growth, has been currently recommended as the first-line chemotherapeutic drug for treatment of pancreatic cancer. However, it merely extends the median survival for several months [2, 7]. The development of chemoresistance is a main reason leading to chemotherapy failure in pancreatic cancer. The high rate of chemoresistance reduces the effectiveness of its clinical treatment [8]. Thus, it is necessary to find potential adjuvants to reverse the resistance in GEM-resistant pancreatic cancer. Joiner and colleagues revolutionized thinking about low doses of radiation therapy (RT) (<100cGy) by demonstrating an initial phase of hyper-radiosensitivity (HRS) using doses from 0 to 80cGy [9]. Marples B et al. reported that low-dose RT delivered in ultrafractionated form was acting synergistically with chemotherapy in vitro [10]. Preclinical data showed that the maximal HRS phenomenon occurs at a dose between 50 and 80cGy and that four low-dose fractions provide optimal cell killing in vitro when combined with chemotherapy [11, 12]. Arnold SM et al. reported the Phase II results of this new treatment paradigm [12]. Forty patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN) received paclitaxel (225 mg/m2), carboplatin (area under the curve of 6), and four 80cGy fractions of radiotherapy (two each on Days 1 and 2). LDFRT combined with paclitaxel and carboplatin was found to be effective with a similar toxicity profile to chemotherapy alone. A response rate of 90% at the primary site and a nodal response rate of 69% were noted in this study [12].
However, these studies mainly focused on head and neck cancer. It remains unclear whether LDFRT would improve the chemosensitivity of pancreatic cancer. Therefore, we constructed a Gemcitabine-resistant human pancreatic cancer SW1900 cell line (SW1900/GZ) to explore whether LDFRT would improve the chemosensitivity of pancreatic cancer and sought to provide a new treatment paradigm for the treatment of gemcitabine-resistant pancreatic cancer.
Materials and Methods
I Materials and Cell Culture
Human pancreatic cancer SW1900 cell line was purchased from the cell bank of the Typical Culture Preservation Committee of the Chinese Academy of Sciences (Beijing, China). Cells were cultivated in RPMI-1640 medium with 10% antibiotics (100μg/mL streptomycin and 100 U/mL penicillin) and 10% fetal bovine serum (FBS) (Gibco Co, Australia) in a humidified 5% CO2 incubator at 37℃. The culture medium was changed every 2-3 days according to the cellular quorum. GEM was provided by Eli Lilly and Company, US. Cell Counting Kit and Annexin V-FITC/PI Apoptosis Detection Kit were purchased from Dojindo (Dojindo Laboratories, Japan). Antibodies against MPR, MDR and GADPH were purchased from Bioss (Bioss Biotech Co. Ltd., China).
II Screening of Drug-Resistant Cell Lines
When SW1990 cells grew well and entered logarithmic growth phase, they were transferred into 25 cm2 plastic culture flasks according to 2×106/flask with 20 ng/ml GEM medium at initial concentration. Most of the cells died at 37℃ and 5% CO2 culture box. After entering logarithmic growth phase, the cells were digested and passaged. According to the growth status of cells, the cells were exposed to double dose or equal dose GEM for further culture. The tolerance of SW1990 cells to GEM was gradually improved by combined induction of high concentration GEM intermittent shock according to specific conditions. The concentration of GEM in the final culture medium reached 100ug/ml. The effective GEM-resistant cell line SW1900, the SW1900/GZ cell line, were established. The cells in logarithmic growth phase were cultured for 5 generations for use.
III Detection of Half Maximal Inhibitory Concentration (IC50) in GEM-Resistant and Non-Drug-Resistant Cell Lines
The logarithmic growth phase GEM-resistant cell line SW1990/GZ and non-drug-resistant cell line SW1990 were diluted into 5×104/ml, and 100 UL were inoculated into 96-well plates. The two groups of cells were divided into six groups: 0 ng/ml, 10 ng/ml, 100 ng/ml, 500 ng/ml, 1000 ng/ml and 1500 ng/ml for non-drug-resistant cell lines, and 0 ug/ml, 50 ug/ml, 100 ug/ml, 300 ug/ml, 500 ug/ml and 1000 ug/ml for GEM-resistant cell lines. CCK8 was used to detect the number of living cells and IC50 was calculated.
IV Radiation Conditions
The ELEKTA Synergy linear accelerator and 6 MV X-ray were used to radiate the cells. The area of the radiation field was 16 × 18 cm, and the source skin distance (SSD) of the cells was 100 cm. The dosage rate was 600cGy/min. The irradiation dose was adjusted by multi-channel ionization chamber, and the attenuation of culture dishes was corrected to ensure that cells received uniform and accurate radiation. The SW1900/GZ cells were randomly divided into six groups as follows: control, low dose fractional radiation (LDFRT), high dose radiation (HDRT), gemcitabine (GEM), low dose fractional radiation plus gemcitabine (LDFRT+ GEM) and high dose radiation plus gemcitabine (HDRT+ GEM) groups. Cells in the LDFRT and LDFRT+ GEM group were exposed to 40cGy radiation each time (total dose 1.6Gy in four fractions, once a day for 4 consecutive days). Cells in the HDRT and HDRT+ GEM groups were subjected to 2Gy radiation at one time, whereas the control group, the GEM group, was given no radiation. The concentration of GEM was 200ug/ml.
V Cell Apoptosis Analysis
Annexin V/Propidium iodide (PI) staining for flow cytometry (FCM) was used to quantify apoptosis. Cells from the six groups were incubated in 6-well plates at a density of 5×105 cells/well, and cultured with the concentrations of GEM (200 μg/mL) for 48 h. The cells were then harvested, centrifuged for 10 min at 1500 rpm, and washed twice with ice-cold PBS. The cells were then resuspended in 500μL of binding buffer and sequentially stained with 5 µL of Annexin V-FITC and 5 µL of PI solution for 15 min in the dark, as per the manufacturer’s instructions. Apoptosis rate was determined by flow cytometry (BD FACSCalibur system). The percentages of early apoptotic cells (high FITC and low PI signal) and the late apoptotic cells (high FITC and high PI signal) were plotted and analysed using FlowJo software.
VI Western Blot Analysis
SW1900/GZ cells were washed twice with ice-cold PBS and lysed in radioimmunoprecipitation (RIPA) buffer (Beyotime Institute of Biotechnology, China) for protein extraction followed by centrifugation at 14000 ×g for 15 min at 4°C. The protein concentration was assayed using the BCA Protein assay kit (Beyotime Institute of Biotechnology Jiangsu, China), and the samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% acrylamide gels followed by transfer to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in Tris-buffered saline with Tween (TBST) (in mmol/L: NaCl 150, Tris-HCl 20, pH 7.5, 0.05% Tween 20) containing 10% skim milk for 2h at room temperature. The membranes were then incubated overnight at 4°C with the relevant primary antibodies (against MPR, MDR, and GAPDH) (1:000), followed by appropriate secondary antibodies conjugated with horseradish peroxidase (1:10000). The proteins were visualized using the enhanced chemiluminescence (ECL) Western blotting system, and ImageJ software was used to analyse the bands.
VII Statistical Analysis
Statistical analysis was performed using SPSS 21.0 software. All the values from the groups were each expressed as mean ± standard deviation (X±SD). Statistical differences between two groups were compared using the Student’s t -test and among multiple groups by one-way analysis of variance (ANOVA). Values of P <0.05 were considered statistically significant.
Results
I Construction of GEM-Resistant Pancreatic Cancer Cell Line SW1990/GZ
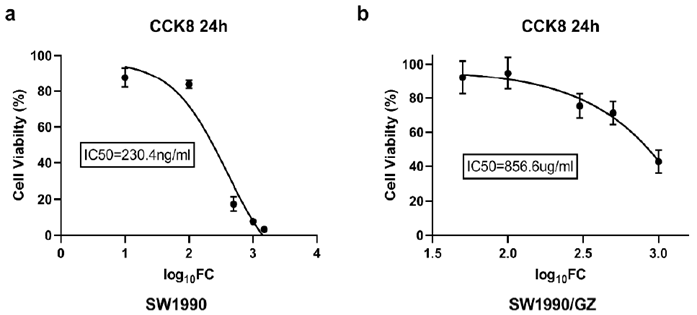
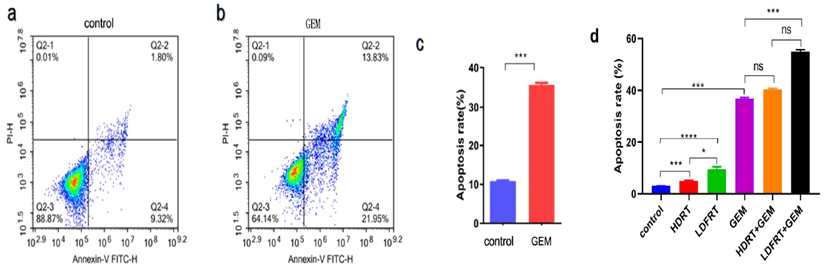
After 24 hours treatment with different concentration of GEM to SW1990 cells, CCK8 was used to detect the number of living cells and IC50 was calculated. The results showed that the IC50 of non-drug-resistant cell lines was 230.4ng/ml (Figure 1a), the IC50 of GEM-resistant cell lines was 856.6ug/ml (Figure 1b), and the IC50 of GEM-resistant cell lines was 3700 times of that of non-drug-resistant cell lines. In order to explore the enhancement of chemosensitivity by LDFRT, we chose the concentration of GEM of 200 ug/ml to detect apoptosis by FCM. Figures 2a & 2b were apoptotic maps detected by FCM in control group and GEM group. The difference was statistically significant (p < 0.001), as shown in (Figure 2c). The concentration of GEM 200ug/ml was determined as next step experiment.
Figure 1: IC50 of SW1990 and SW1990/GZ pancreatic cancer cells. IC50 was the half lethal dose of the drug.
Figure 2: FCM analysis of apoptosis rate of SW1990/GZ cells under different treatment conditions and their statistical charts: a) FCM of apoptotic cells in control group; b) FCM of apoptotic cells in GEM (200ug/ml) group; c) statistical charts of apoptotic rates in control group and GEM group; d) apoptotic rates and their statistical charts in different treatment groups. There was no statistical difference in ns, * P < 0.05, *** P < 0.001.
II LDFRT Enhances Susceptibility to GEM in Resistant Pancreatic Cancer Cell SW1990/GZ
To investigate the effect of LDFRT on GEM susceptibility, pancreatic cancer cells SW1990/GZ were exposed to the six groups: control, LDFRT, HDRT, GEM, LDFRT+ GEM and HDRT+ GEM. FCM was used to detect apoptosis. The specific results were as follows: 1. the apoptotic rate of LDFRT group was higher than that of HDRT and control groups (p < 0.05); the apoptotic rate of LDFRT+ GEM group was higher than that of GEM and HDRT+ GEM groups (p < 0.01). The differences were considered statistically significant. However, there was no significant difference in the apoptotic rate between HDRT+ GEM group and GEM group (p > 0.05). The results showed that LDFRT enhances susceptibility to GEM in resistant pancreatic cancer cell SW1990/GZ and reverse the resistance to GEM, as shown in (Figure 2d).
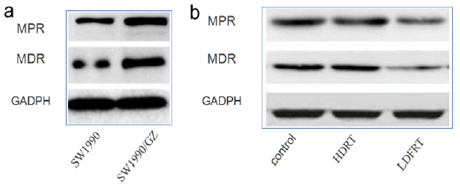
III LDFRT Enhances Susceptibility to GEM in Resistant Pancreatic Cancer Cells SW1990/GZ Via Downregulation of MPR, MDR
Previous studies had shown that LDFRT enhances susceptibility to GEM-resistant pancreatic cancer cells SW1990/GZ. We continued to explore the mechanism of LDFRT effects. Western blot was used to detect the expression of drug resistance-related proteins. The results showed that the expression of multidrug resistance gene (MDR) and multidrug resistance-related protein gene (MRP) in the cell line SW1990/GZ was significantly up-regulated compared with the non-drug-resistant cell line, as shown in (Figure 3a). The expression of MDR and MPR protein decreased significantly in the LDFRT group, but did not change significantly in the HDRT group (Figure 3b). The results showed that the down-regulation of MDR and MPR protein expression might enhances susceptibility to GEM in resistant pancreatic cancer cells SW1990/GZ by LDFRT.
Figure 3: Western blot was used to detect the protein expression changes of MDR and MPR related genes: a) the difference of MDR and MPR expression between drug-resistant cell line SW1990/GZ and Non-drug-resistant cell line SW1990; b) the change of MDR and MPR expression in drug-resistant cell line SW1990/GZ under different treatment conditions.
Discussion
Pancreatic cancer is one of the most aggressive human cancers with fourth highest mortality rate among all tumors [13]. Although the outcomes of surgery and chemotherapy for pancreatic cancer have improved significantly, the 5-year overall survival rate remains less than 7% [14]. Pancreatic cancer lacks early symptoms; therefore, most patients present with metastasis. For many cases treated with surgery, the tumor recurs within 1-2 years and develops metastasis [15]. For these patients, GEM-based chemotherapy is the priority treatment; however, the cancer cells tend to develop chemoresistance, resulting in poor efficacy of GEM. There is an urgent need for strategies to enhance chemosensitivity to GEM, which would improve the outcome of chemotherapy.
In this study, through high concentration GEM intermittent shocks, we established GEM-resistant cells of human pancreatic cancer SW1990/GZ, whose IC50 was 3700 times of that of non-drug-resistant cell SW1990, to investigate whether LDFRT could enhance GEM sensitivity in SW1900/GZ cell, and to further explore the underlying mechanism. Studies have also shown that HRS occurs after LDFRT in vitro [9, 10]. Kunos et al. reported, when combined with LDFRT, the therapeutic effect of chemotherapy could be enhanced, and demonstrated that LDFRT was well tolerated and improved the chemosensitivity of docetaxel in patients with recurrent ovarian cancer, without increasing the side effects of docetaxel [16]. Yu HS and his team found in their studies that LDFRT stimulated the growth of normal cells but not tumor cells in vitro and in vivo, and could reverse cisplatin resistance in ovarian cancer by suppressing DNA damage repair and promoting apoptosis through the ERCC1 and Bcl-2 gene [17]. Recently their studies had further pointed out that the PI3K/AKT/GSK-3β pathway plays an important role in cisplatin-resistance in patients with ovarian cancer, and LDFRT may enhance the cellular susceptibility to cisplatin by suppressing this pathway [18].
Our study found that the apoptotic rate of SW1990/GZ in LDFRT group was significantly higher than that of HDRT group. This study shows that there is HRS phenomenon induced by LDFRT, which is consistent with many previous studies [8-10]. This suggests that LDFRT enhances susceptibility to GEM in resistant pancreatic cancer cell SW1990/GZ and reverse the resistance to GEM. GEM is the preferred drug for pancreatic cancer, but the incidence of drug resistance is high. Although the mechanism of drug resistance in vivo has not been clearly defined, multidrug resistance (MDR), either inherent or acquired, is a serious problem in chemotherapy and is one of the main reasons for a poor outcome [19]. There are many different mechanisms accounting for drug resistance but MDR protein family plays an essential role [20]. Yu P et al. considered that the existence of MDR in high-risk patients leads to unsatisfactory outcome of chemotherapy. Inhibition of MDR proteins or blocking drug resistance pathways may improve the chemotherapy efficacy, and cancer prognosis [21]. Zhao YP et al. analysed the gene expression profiles of multidrug-resistant pancreatic cancer cell lines and found that the expression of MDR gene was significantly up-regulated [22]. This result is consistent with our study. Our study showed that the expression of MDR and MRP in the cell SW1990/GZ was significantly up-regulated compared with the non-drug-resistant cell SW1990.
Interestingly, our study also found that LDFRT could significantly reduce the down-regulation of MDR and MPR protein, while HDRT not down-regulate the expression of MDR and MPR protein. Compared with control group, LDFRT significantly increased apoptosis of pancreatic cancer SW1990/GZ cells, while HDRT did not significantly increase apoptosis. This suggests that LDFRT can increase the chemosensitivity of GEM and reverse the drug resistance of GEM. The mechanism of reversing drug resistance may be that LDFRT can effectively reduce the expression of drug resistance-related proteins, such as MDR and MPR. This study provides a new strategy for reversing chemoresistance in pancreatic cancer and has potential for clinical transformation. Whether LDFRT can effectively reverse drug resistance needs to be confirmed by clinical trials. So, we look forward to the development of phase II clinical trials.
Acknowledgement
The work was supported by Hainan Provincial Natural Science and Technology Foundation of China (grant # 20168303).
Competing Interests
None.
Data Availability
Available upon request.
Abbreviations
LDFRT: Low-Dose Fractionated Radiation
SW1900/GZ: Gemcitabine-Resistant Human Pancreatic Cancer SW1900 Cell Line
CCK8: Cell Counting Kit 8
HDRT: High Dose Radiation
GEM: Gemcitabine
FCM: Flow Cytometry
MDR: Multidrug Resistance Gene
MRP: Multidrug Resistance-Related Protein Gene
RT: Radiation Therapy
HRS: Hyper-Radiosensitivity
FBS: Fetal Bovine Serum
SSD: Source Skin Distance
PI: Propidium Iodide
IC50: Half Maximal Inhibitory Concentration
RIPA: Radioimmunoprecipitation
SDS-PAGE: Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
PVDF: Polyvinylidene Difluoride
TBST: Tris Buffered Saline with Tween
ECL: Enhanced Chemiluminescence
ANOVA: One-Way Analysis Of Variance
Article Info
Article Type
Research ArticlePublication history
Received: Tue 05, Jan 2021Accepted: Wed 27, Jan 2021
Published: Thu 11, Mar 2021
Copyright
© 2023 Danni XU. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RCO.2021.01.01
Author Info
Yanda LU Fengxiang Han Chunxiang Luo Fen Huang Donghua Xie Huamao Sun Weisi Chen Danni XU
Corresponding Author
Danni XUDepartment of Oncology, The First Affiliated Hospital of Hainan Medical University, Haikou, Hainan, PR China
Figures & Tables
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H et al. (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66: 115-132. [Crossref]
2. Dai JT, Zhang Y, Li HC, Deng YH, Elzatahry AA, Alghamdi A et al. (2017) Enhancement of gemcitabine against pancreatic cancer by loading in mesoporous silica vesicles. Chinese Chemical Letters. Chin Chem Lett 28: 531-536.
3. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM et al. (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74: 2913-2921. [Crossref]
4. Ilic M, Ilic I (2016) Epidemiology of pancreatic cancer. World J Gastroenterol 22: 9694-9705. [Crossref]
5. Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM et al. (2003) EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol 14: v61-v118. [Crossref]
6. Peixoto RD, Ho M, Renouf DJ, Lim HJ, Gill S et al. (2017) Eligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Am J Clin Oncol 40: 507-511. [Crossref]
7. Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T et al. (2014) Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev 40: 118-128. [Crossref]
8. Zhou Y, Zhou Y, Yang M, Wang K, Liu Y et al. (2019) Digoxin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine via inhibiting Nrf2 signaling pathway. Redox Biol 22: 101131. [Crossref]
9. Joiner MC, Marples B, Lambin P, Short SC, Turesson I (2001) Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys 49: 379-389. [Crossref]
10. Marples B, Cann NE, Mitchell CR, Johnston PJ, Joiner MC et al. (2002) Evidence for the involvement of DNA-dependent protein kinase in the phenomena of low dose hyper-radiosensitivity and increased radioresistance. Int J Radiat Biol 78: 1139-1147. [Crossref]
11. Dey S, Spring PM, Arnold S, Valentino J, Chendil D et al. (2003) Low dose fractionated radiation potentiates the effects of paclitaxel in wild-type and mutant p53 head and neck tumor cell lines. Clin Cancer Res 9: 1557-1565. [Crossref]
12. Arnold SM, Regine WF, Ahmed MM, Valentino J, Spring P et al. (2004) Low-dose fractionated radiation as a chemopotentiator of neoadjuvant paclitaxel and carboplatin for locally advanced squamous cell carcinoma of the head and neck: results of a new treatment paradigm. Int J Radiat Oncol Biol Phys 58: 1411-1417. [Crossref]
13. Kamisawa T, Wood LD, Itoi T, Takaori K (2016) Pancreatic cancer. Lancet 388: 73-85. [Crossref]
14. Stathis A, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 7: 163-172. [Crossref]
15. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M (2011) Pancreatic cancer. Lancet 378: 607-620. [Crossref]
16. Kunos CA, Sill MW, Buekers TE, Walker JL, Schilder JM et al. (2011) Low-dose abdominal radiation as a docetaxel chemosensitizer for recurrent epithelial ovarian cancer: a phase I study of the Gynecologic Oncology Group. Gynecol Oncol 120: 224-228. [Crossref]
17. Liu XR, Yu HS (2016) Low dose radiation enhances susceptibility to cisplatin in resistant ovarian cancer cells via down regulation of ERCC1 and Bcl-2. Oncol Transl Med 2: 84-89.
18. Jia XM, Ming J, Nie XF, Liang DH, Jiang T et al. (2017) Low-dose fractionated radiation reverses cisplatin resistance in ovarian cancer cells via PI3K/AKT/GSK-3βsignaling. Oncol Translat Med 3: 203-209.
19. Xu HW, Xu L, Hao JH, Qin CY, Liu H (2010) Expression of P-glycoprotein and multidrug resistance-associated protein is associated with multidrug resistance in gastric cancer. J Int Med Res 38: 34-42. [Crossref]
20. Hu WQ, Peng CW, Li Y (2009) The expression and significance of P-glycoprotein, lung resistance protein and multidrug resistance-associated protein in gastric cancer. J Exp Clin Cancer Res 28: 144. [Crossref]
21. Yu P, Du Y, Yang L, Fan S, Wu J et al. (2014) Significance of multidrug resistance gene-related proteins in the postoperative chemotherapy of gastric cancer. Int J Clin Exp Pathol 7: 7945-7950. [Crossref]
22. Zhao YP, Chen G, Feng B, Zhang TP, Ma EL et al. (2007) Microarray analysis of gene expression profile of multidrug resistance in pancreatic cancer. Chin Med J (Engl) 120: 1743-1752. [Crossref]
