Effects of Brief and Intensive Therapy for Dysphagia in Patients with Head and Neck Cancer during Radio-Chemotherapy: A Pilot Study
A B S T R A C T
Objective: To verify the effectiveness of a Brief and Intensive Dysphagia Therapy programme, in patients with head and neck cancer, during radio-chemotherapy.
Study Design: This is a randomized clinical trial - pilot project with a random sample consisting of 11 patients divided into two groups: 7 in the intervention group, who received brief and intensive therapy and 4 in the control group, with weekly therapy. The assessment instruments used were tongue pressure measurement, mouth-opening measures, oral intake scale and quality of life questionnaire. Patients were evaluated before radio-chemotherapy, after 15 days of speech therapy and after radio-chemotherapy.
Results: The intervention group presented higher values of lingual apex pressure (p = 0.00), mouth opening maintenance from the second evaluation (35.14 ± 16.82 mm) until the end of the radio-chemotherapy (35.29 p± 5.93 mm), greater oral intake functionality (p = 0.00) and improvement in overall aspects of quality of life questionnaire (p = 0.05) after completion of brief and intensive therapy and radio-chemotherapy.
Conclusion: Brief and intensive therapy presented superior results in the maintenance and/or rehabilitation of the swallowing mechanism in patients with head and neck cancer undergoing radio-chemotherapy.
Keywords
Speech-Language pathology, deglutition disorders, myofunctional therapy, radiotherapy, rehabilitation
Introduction
Treatment for head and neck cancer (HNC) may include, alone or in combination, surgery, radiotherapy and chemotherapy [1]. Considering the organ preservation protocol, radio-chemotherapy (RCT) has been a widely used approach [2, 3]. However, this combination therapy presents a potential risk for deglutition disorders since even with organ preservation, functions may remain impaired [4, 5].
According to the literature, dysphagia in patients undergoing this treatment modality can be attributed to abnormal motility of the oral cavity, oropharynx, and larynx muscles, as well as edema and loss of sensation [6]. In addition, changes such as mucositis, odynophagia and xerostomia also contribute to the potential effects of this disorder [7].
The deglutition process, when impaired, has a great impact on the quality of life of individuals [8]. Moreover, these changes predispose the development of respiratory-related diseases, such as aspiration pneumonia, which in turn may aggravate the clinical status of patients [9].
The therapeutic techniques commonly used for deglutition rehabilitation are direct and indirect therapies, which include features such as compensatory maneuvers and oral sensorimotor stimulation, usually performed weekly [10, 11]. Recently, Brief and Intensive Therapy (BIT) has been explored as a therapeutic approach based, as well as other modalities, preserved physiological abilities to improve aspects of deglutition mechanism strength, mobility and coordination. It is noteworthy that the difference in this therapy is related to the time-frequency of care and not to the technical procedures addressed [12]. However, there are no reports described in the literature demonstrating the efficacy of this therapy during RCT. Thus, the aim of this study was to verify the effectiveness of a pilot project of BIT for dysphagia in patients with HNC during RCT compared to weekly therapy.
Materials and Methods
I Patients
The study participants were individuals undergoing RCT at a cancer referral hospital between April and July 2018. The study included adults over 18 years old, of both sexes, diagnosed with HNC with an indication for RCT. Exclusion criteria were individuals who underwent previous speech therapy for 3 months and patients with an indication for adjuvant, neoadjuvant, or palliative radiotherapy treatment.
II Ethical Approval and Consent to Participate
This study was preceded by the approval of the Research Ethics Committee Irmandade Santa Casa de Misericórdia from Porto Alegre (Nº. 2.539.241) and registered in the Clinical Trails (NCT 03755921). Written informed consent was obtained from each subject before the start of the study.
III Study Design
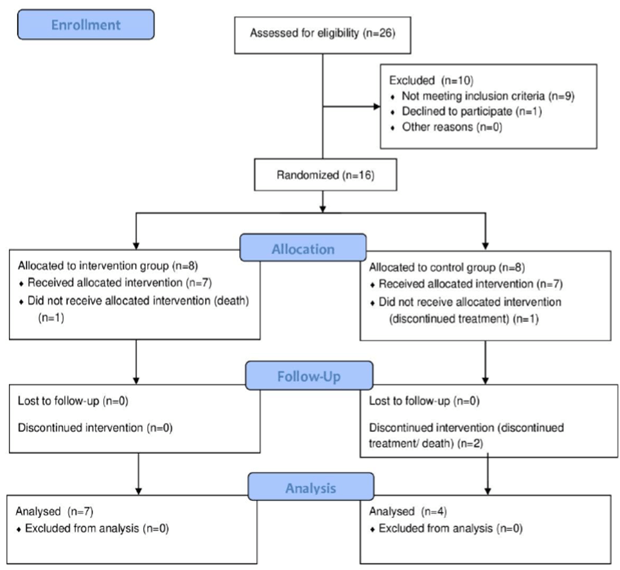
Randomization was performed through the website (Link), separating individuals into groups. The random sample consisted of 11 individuals, divided into 2 groups: intervention Group (IG) n = 7, received BIT and Control Group (CG) n = 4, with weekly therapy (Figure 1).
Figure 1: Consolidated Standards of Reporting Trials flow diagram that displays enrollment, allocation, follow-up, and analysis data.
The three evaluation moments were pre-RCT (one hour before the first sessions), after 15 days of RCT and speech therapy and post-RCT (one hour after the last sessions), being performed by the same evaluator, who participated in the blinded procedures regarding participants' therapy models. Both groups were treated by different therapists.
IV Therapeutic Programme
IG participants underwent a deglutition rehabilitation programme from a BIT programme model, adapted for patients with HNC, who had speech therapy five days a week (Monday to Friday), with 40 minutes daily for three weeks, totaling 15 sessions [12]. Although the BIT sessions occurred once a day, IG patients were oriented to perform the exercises at home three more times a day.
The participants of the CG received speech therapy once a week, also lasting 40 minutes, totaling three sessions in the same period as the BIT group. However, the CG participants were instructed to perform the exercises at home four times a day to receive the same number of daily exercises as the IG. It is noteworthy that patients in two groups receive a daily exercise control diary.
Both groups received care individually, with cervical relaxation techniques, postural maneuvers and lower airway cleaning, changes in consistency and food volumes, as well as strength exercises and mobility of articulatory structures, according to their needs. It is noteworthy that the main difference between the groups refers to the temporal frequency of the therapy performed and not to the technical procedures addressed.
V Measurements
The evaluation procedures to compare the effects of therapeutic models on groups were applied through the following instruments:
i Iowa Oral Performance Instrument (IOPI)
Instrument that allows to evaluate the lingual pressure by inserting a bulb in the oral cavity. The patient should raise the back and / or the anterior portion of the tongue against this bulb, pressing it as much as possible. The pressure transducer circuits of the device detect bulb compression and indicate the peak pressure [13]. According to the equipment manufacturer's guidelines, 3 lingual pressure measurements were evaluated, considering the one with the highest value.
ii Mouth Opening
The mouth opening measurements were performed using a kingtools analog steel caliper (150 mm). Participants were instructed to perform maximum mouth opening at the painless limit. The maximum interincisal distance was considered. If the participant had no dentition, the midline of the face was considered, considering the distances between the alveolar ridge or lips.
iii Clinical Swallowing Assessment and Functional Oral Intake Scale (FOIS)
The clinical swallowing assessment involves two stages, according to the protocol used in the clinical practice of the service. In the structural evaluation, morphological aspects, strength, mobility, coordination, and sensitivity of the phonoarticulatory structures and the presence of the swallowing reflex trigger were observed. Functional assessment was only performed when the patient had clinical oral diet conditions, using different food consistencies, according to the patient's performance in each tested consistency and with the ability to protect the lower airways. After the evaluation, the results were demonstrated by FOIS.
The FOIS is a scale composed of a 7-level scale, which grades the oral intake of individuals. At level 1, the patient is exclusively using an alternative feeding route, with no oral conditions at all. At levels 2 and 3, it still depends on the alternative route of food but is already able to eat some food and / or a consistency. Levels 4, 5 and 6 have full oral conditions in one or more consistencies, with or without compensations, but with some restrictions. At level 7, the patient has total oral conditions without restrictions [14].
iv M. D. Anderson Dysphagia Inventory (MDADI)
A 20-item questionnaire involving global, physical, functional and emotional domains aimed at assessing the impact of dysphagia on the quality of life of patients undergoing treatment for HNC [15].
VI Perceptual Analysis
The collected data were analysed using the statistical programme SPSS 23.0 (Statistical Package for Social Sciences for Windows). Results were expressed as mean or median, standard deviation or error and the 25th and 75th percentiles, depending on the nature of the variable. The effect of the intervention was analysed via Generalized Estimation Equation Models (GEE), considering the time factors (pre-RCT versus 15 days of RCT and speech therapy, versus post-RCT (IG versus CG) and the interaction times significant effects when p <0.05.
Results
Regarding the homogeneity of the groups, the mean age of the CG was 66.75 ± 14.19 years and in the IG 57.42 ± 20.18 years were not significantly different (p = 0.44). The sample also did not differ (p = 0.10) regarding the tumor location (mouth, oropharynx or larynx), in the distribution between groups. The other data of the sample characterization can be observed in (Table 1).
Table 1: Patient
characteristics (N = 11).
|
Variable |
|
% |
N |
|
Age
* |
|
60,82 |
18,09 |
|
Total
radiotherapy dose * |
GY |
67,64 |
3,98 |
|
Gender |
Male |
55 |
6 |
|
|
Female |
45 |
5 |
|
Site |
Month |
27 |
3 |
|
|
Larynx |
36 |
4 |
|
|
Oropharynx |
36 |
4 |
|
Tracheostomy |
Yes |
36,36 |
4 |
|
|
No |
63,64 |
7 |
|
Feeding |
Oral |
90,91 |
10 |
|
|
Enteral |
9,09 |
1 |
|
Dentition |
Normal |
27,27 |
3 |
|
|
Edentulous |
27,27 |
3 |
|
|
Dental prosthesis |
45,45 |
5 |
|
Food Consistency |
Normal |
72,73 |
8 |
|
|
Pasty |
18,18 |
2 |
|
|
Enteral
|
9,09 |
1 |
*Presented
in medium and standard deviation.
Regarding lingual apex pressure (Table 2), there is an interaction effect between the groups and the pressure assessment period (p = 0.00), indicating a significant decrease in CG at the end of treatment in relation to the end of BIT. From the end of BIT, IG, presented significantly higher-pressure values than CG.
Table 2: Characterization of apex and dorsum lingual pressure
measures, mouth opening and FOIS scale, at all moments of evaluation (N=11).
|
|
Pre-treatment |
After
15 days of speech therapy |
Post-tratament |
Time |
Group |
Time x group |
|
|
Lingual apex pressure (kPa) |
|||||||
|
IG |
36,67±5,30 |
37,17±5,50 † |
38,35±6,25 † |
0,00 |
0,00 |
0,00 |
|
|
CG |
24,25±7,25 |
23,50±3,51 |
11,20±1,86 ‡ |
|
|||
|
Lingual dorsum pressure (kPa) |
|||||||
|
IG § |
34,60±4,53 |
32,80±4,26 |
35,60±3,05 |
0,29 |
0,00 |
0,11 |
|
|
CG |
18,25±2,48 |
25,25±6,23 |
19,32±5,87 |
|
|||
|
Month Opening (mm) |
|||||||
|
GI |
37,00±6,08 |
35,14±5,88 ¶ |
35,28±5,93 |
0,00 |
0,36 |
0,05 |
|
|
GC |
46,75±2,27 |
44,00±3,50 |
35,87±1,69 ‡ ¶ |
|
|||
|
FOIS # |
|||||||
|
GI |
7 [4-6] |
7 [5-7] ∆ |
7 [5-7] † |
0,03 |
0,00 |
0,05 |
|
|
GC |
6,5 [5-7] |
4,5 [2-5] |
1 [1-3] ‡ ¶ |
|
|
|
|
IG= Intervention Group; CG= Control Group. FOIS=
Functional Oral Intake Scale. Data are means ± standard deviation; kPa=
Kilopascal; mm=millimeters; #Median [25%-75%]; †
differs significantly from the CG (time effect x group); ‡ differs
significantly after speech therapy (time effect x group); § differs
significantly from the CG (effect of group); ¶ differs significantly from the
first evaluation (time effect x group); Ø differs significantly from the first
evaluation (time effect x group).
There was no significant effect of interaction between the group and evaluation on the lingual dorsum (p = 0.11), but the pressure was significantly higher in IG than in CG, regardless of the moment of evaluation (p = 0.00). That is, in IG, the pressure of the lingual dorsum was maintained from the beginning to the end of the treatment higher than in the CG (Table 2).
According to the FOIS scale findings (Table 2), a significant interaction effect was also identified (p = 0.01). Now of the first evaluation, 50% of the individuals from the IG scored 7 points and, in the CG, the score was between 6 and 7 (6,5). From the second evaluation onwards, statistically significant differences were observed between the groups. The IG continued to score 7, while the CG was between 4 and 5 (4,5). After the end of radiotherapy, the IG remained with score 7, while the CG went to 1. That is, the FOIS of the CG was significantly worse after the end of radiotherapy compared to the other two previous evaluations.
Regarding food consistency, it was observed that at the beginning of radiotherapy, in IG 5 patients (71.4%) and in CG 2 patients (50.0%), they food with all consistencies. After completion of speech therapy, IG continued with 5 patients (71.4%) feeding on all consistencies, while in the CG, no patient (0.0%) continued with a normal diet (all consistencies). After the end of the radiotherapy treatment, the IG remained with 5 patients (71.4%), feeding with all consistencies; in the CG, only 1 patient (25.0%) was fed with a pasty consistency, while 3 others (75.0%) no longer used oral diet.
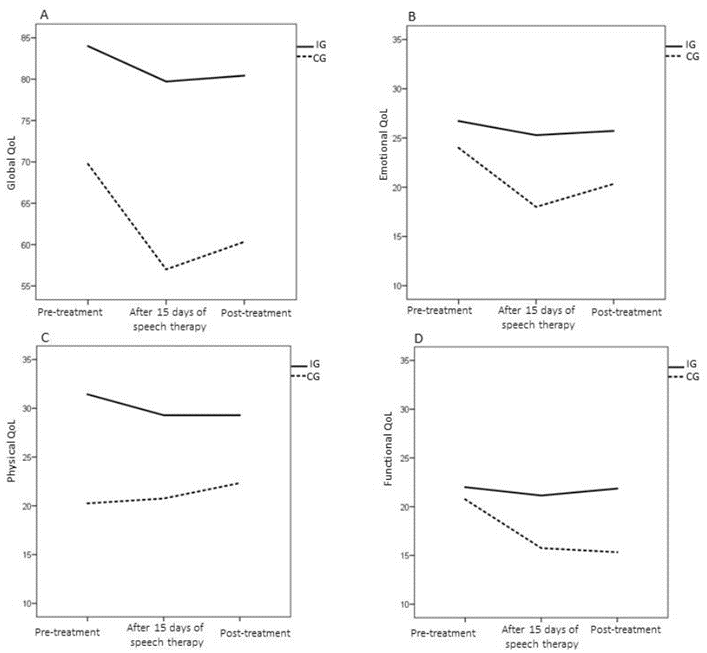
In the total analysis of the quality-of-life questionnaire (Figure 2), there was no time and group interaction effect (p = 0.55), but this was always higher in IG than in CG, regardless of the moment of evaluation (p = 0.01). Analyzing domains of the questionnaire, it appears that in the functional domain, there is an interaction effect (0.033). The CG individuals had worse scores in the last two evaluations compared to the first evaluation (20.75 ± 1.98). Better quality of life was observed in this domain in IG compared to CG after speech therapy (IG: 21.14 ± 1.34; CG: 15.75 ± 2.40), and after radiotherapy (IG: 21.85 ± 1, 62; CG: 12.89 ± 3.51).
Regarding the physical and emotional domains, the groups differ regardless of the moment of the evaluation (p = 0.00 and p = 0.02, respectively); in other words, the CG had lower quality of life in the physical domains (CG: 21 ± 1.26; IG: 30 ± 2.17) and emotional (CG: 20 ± 1.67; IG: 26 ± 1.81) than IG.
According to the global domain, an interaction effect was observed (p = 0.05). At the time of the first evaluation, both groups scored up to 5 (maximum scale value). In the second evaluation, it was found that 50% of IG continued to score 5, while 50% of CG scored up to 2. After the end of the radiotherapy, the IG and the CG kept score of 5 and 2. Respectively, that is, the CG significantly worsened in the second evaluation compared to the first.
Figure 2: Characterization of the results of the global, emotional, physical and functional domain of the MD Anderson quality of life questionnaire, in both groups, at all moments of evaluation.
Regarding mouth opening measures (Table 2), it appears that the groups differ throughout treatment (p = 0.05). In the inter-subject comparison, it is observed that the IG maintains the opening value from the second evaluation (35.14 ± 16.82 mm) until the end of the RCT (35.29 ± 5.93 mm). In the CG, there was a reduction in mouth opening at the third evaluation (35.88 ± 1.69mm) compared to the first (46.75 ± 7.25mm) and at the end of weekly therapy sessions (44.00 ± 8, 08 mm).
Discussion
This study showed the effects of BIT for dysphagia in patients with HNC during RCT compared to speech therapy with a weekly frequency. The sample consisted of predominantly male individuals (55%), corroborating another author, however, the percentage of women was high [16]. A significant increase in female incidence is described in the literature, possibly due to changes in habits, increased tobacco and alcohol consumption, and other environmental exposures [17]. Regarding the age group, the bibliographic data indicate a higher incidence in individuals over 50 years, in agreement with the findings of this study [18]. Regarding the primary anatomical site, there was a greater predominance of laryngeal and oropharyngeal cancer, followed by oral cavity, showing results like other studies conducted in the population with HNC [18, 19].
Although the proportion of the anatomical site is not identical in the participants, it should not be accepted that the amount of radiation received influenced the effects of radiotherapy since most individuals in this sample received the same radiation fraction of approximately 70 Gray.
Regarding the lingual apex pressure, it was found that the BIT was able to maintain and even improve the muscle strength of the anterior region of the tongue, despite the effects of RCT. Normal values, according to the literature, for individuals over 60 years old are from 37 kPa [20]. Participants in this group started treatment with a mean anterior lingual pressure below the normal range and, at the end, started to present mean values within the reference values for this population (Table 2). The group that received weekly therapy, on the other hand, maintained the values of anterior lingual pressure below normal always of evaluation (Table 2). Exercise execution is a determining factor for rehabilitation. In both groups, individuals were instructed to perform therapy also at home. It is believed that the IG obtained better results since at least once a day, the patients performed the exercises with the therapist's monitoring, besides being continually motivated about the importance of performing them.
In the BIT group, there was a higher-pressure value in the lingual apex region, to the detriment of the back, corroborating the literature [21]. This result is justified by the predominance of type II muscle fibers in the lingual apex, characterized by being able to generate a more intense force while maintaining it for a shorter time [22]. During deglutition, the tongue assumes the role of ejection of the food and for this, it projects against the anterior region of the palate making a plunger movement [23]. On the back of the tongue, there is a predominance of type I muscle fibers, more resistant to fatigue but capable of generating less force when compared to type II fibers. The combination of these types of muscle fibers contributes to the accuracy of speech and swallowing joint movements [22].
Just as tongue strength plays an important role during deglutition, proper oral opening is also crucial for the articulation of speech and eating. The reference value of this measurement in head and neck cancer patients is greater than or equal to 35mm [24]. The reduction in mandibular opening in this population also called radio-induced trismus, occurs due to fibrosis that occurs in the masticatory muscles [25].
During this research, mouth-opening measurements were expected to decrease throughout RCT, however, the group that underwent BIT kept mouth opening within reference values until the end of RCT. In turn, the CG showed a decline in these measures. Performing maneuvers for rehabilitation of trismus can cause painful sensation in patients, causing difficulties in adhesion and execution of movements. A study aimed at analyzing the effectiveness of a weekly oral myofunctional therapeutic programme for maxillary mandibular opening in patients with oral or oropharyngeal cancer undergoing radiotherapy observed increased maxillary mandibular opening after speech therapy [26]. However, in the present study, there was an improvement in mouth opening patterns in patients who underwent BIT even in the absence of specific exercises, since this was not the main complaint mentioned. In addition, individuals showed greater adherence to deglutition functional exercises, as they did not cause discomfort. Therefore, it is assumed that during the performance of swallowing functional exercises, mandibular movement occurs, even at a lower intensity, contributing to the maintenance of mouth-opening patterns. This data suggests that the therapy model employed here can minimize the effects of RCT on trismus and contribute to the rehabilitation of patients who do not support discomfort in direct intervention models. In addition, it is believed that functional activities such as swallowing and oral hygiene do not require maximum movement excursion measures. Therefore, even if patients were slightly below normal standards, it would still be possible to maintain its functionality.
Regarding the functionality of oral intake, the results showed that most IG individuals, who in the first evaluation were exclusively orally fed (except for a patient receiving an enteral diet), maintained a maximum FOIS score when RCT completion. However, most individuals from the CG, who at the first evaluation also fed exclusively orally, declined to exclusive enteral diet. The literature indicates that the most frequent dysphagia-related speech-language disorders in patients undergoing RCT are changes in the strength and mobility of speech-language structures and delayed swallowing reflex triggering associated with radiation fibrosis and changes in swallowing-related muscle innervation. [9]. It is noteworthy that even in the face of these changes, individuals undergoing BIT remained until the end of treatment with satisfactory swallowing functionality. This result directly impacts the quality of life of these individuals. According to a study conducted on patients with RCT, the first four weeks of treatment open a window of clinical and scientific opportunities to be performed, as most patients have not yet developed mucositis and pain [27]. It appears that this is the ideal time for the beginning of speech therapy stimulation. However, the fact that the CG presented a decline in oral intake may be related to not performing the number of exercises proposed daily at home, since they were attended by the therapist on a weekly basis. On the other hand, the data found in IG may be justified by the intensive exercise performed daily with the therapist in the first weeks of treatment, when the effects of RCT are still less intense. Thus, we realize the importance of referring to speech therapy in the period before the beginning of RCT, with the objective of adjusting strength and mobility of the stomatognathic system, aiming at the maintenance or minimal reduction of these parameters.
Regarding food consistency, in the first evaluation, individuals from both groups were fed a normal oral diet (except for one patient receiving exclusive enteral diet in the IG). During the treatment, some adjustments were necessary, especially for diet in the pasty consistency, due to physiological changes resulting RCT in the CG. In IG, only one patient needed consistency adaptation during and after RCT, while the others remained on a normal diet until the end of treatment. One study report that oral intake in HNC patients is a multidimensional issue, as functional outcome is not only affected by dysphagia but also by the dental condition of individuals [28]. In the present research, it was observed that the patients who did not have complete dentition used dental prosthesis, which contributed to the improvement of oral intake.
Studies describe that the effects of treating HNC have a negative impact on patients' quality of life [29]. The results of this research showed that individuals who underwent BIT had a higher quality of life than the CG, regarding the way deglutition difficulties affect their daily routine. It is also noteworthy that CG patients had a worse functional domain related to the impact of swallowing on daily activities in the last two evaluations. It is believed that these results may be related to the inability of the CG to feed orally since the individuals from the IG who had higher quality of life also had a maximum score on FOIS.
Conclusion
This pilot study showed that the BIT programme effectively contributed, in the studied sample, to the maintenance of swallowing aspects, demonstrated by lingual pressure parameters, mouth opening, oral intake and quality of life.
Thus, it was found that individuals undergoing this therapeutic programme had higher functional responses than participants who underwent weekly therapy. Thus, BIT contributed positively to the maintenance and/or rehabilitation of the deglutition mechanism in patients HNC cancer undergoing RCT.
Trial Registration
NCT 03755921
Conflicts of Interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 20, Sep 2022Accepted: Tue 11, Oct 2022
Published: Mon 24, Oct 2022
Copyright
© 2023 Elana de Menezes Rossetto. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2022.04.04
Author Info
Elana de Menezes Rossetto Luísa Bello Gabriel Bárbara Luísa Simonetti Vera Beatris Martins Monalise Costa Batista Berbert
Corresponding Author
Elana de Menezes RossettoDepartment of Speech Language Pathology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Rio Grande do Sul, Brazil
Figures & Tables
Table 1: Patient
characteristics (N = 11).
|
Variable |
|
% |
N |
|
Age
* |
|
60,82 |
18,09 |
|
Total
radiotherapy dose * |
GY |
67,64 |
3,98 |
|
Gender |
Male |
55 |
6 |
|
|
Female |
45 |
5 |
|
Site |
Month |
27 |
3 |
|
|
Larynx |
36 |
4 |
|
|
Oropharynx |
36 |
4 |
|
Tracheostomy |
Yes |
36,36 |
4 |
|
|
No |
63,64 |
7 |
|
Feeding |
Oral |
90,91 |
10 |
|
|
Enteral |
9,09 |
1 |
|
Dentition |
Normal |
27,27 |
3 |
|
|
Edentulous |
27,27 |
3 |
|
|
Dental prosthesis |
45,45 |
5 |
|
Food Consistency |
Normal |
72,73 |
8 |
|
|
Pasty |
18,18 |
2 |
|
|
Enteral
|
9,09 |
1 |
*Presented
in medium and standard deviation.
Table 2: Characterization of apex and dorsum lingual pressure
measures, mouth opening and FOIS scale, at all moments of evaluation (N=11).
|
|
Pre-treatment |
After
15 days of speech therapy |
Post-tratament |
Time |
Group |
Time x group |
|
|
Lingual apex pressure (kPa) |
|||||||
|
IG |
36,67±5,30 |
37,17±5,50 † |
38,35±6,25 † |
0,00 |
0,00 |
0,00 |
|
|
CG |
24,25±7,25 |
23,50±3,51 |
11,20±1,86 ‡ |
|
|||
|
Lingual dorsum pressure (kPa) |
|||||||
|
IG § |
34,60±4,53 |
32,80±4,26 |
35,60±3,05 |
0,29 |
0,00 |
0,11 |
|
|
CG |
18,25±2,48 |
25,25±6,23 |
19,32±5,87 |
|
|||
|
Month Opening (mm) |
|||||||
|
GI |
37,00±6,08 |
35,14±5,88 ¶ |
35,28±5,93 |
0,00 |
0,36 |
0,05 |
|
|
GC |
46,75±2,27 |
44,00±3,50 |
35,87±1,69 ‡ ¶ |
|
|||
|
FOIS # |
|||||||
|
GI |
7 [4-6] |
7 [5-7] ∆ |
7 [5-7] † |
0,03 |
0,00 |
0,05 |
|
|
GC |
6,5 [5-7] |
4,5 [2-5] |
1 [1-3] ‡ ¶ |
|
|
|
|
IG= Intervention Group; CG= Control Group. FOIS=
Functional Oral Intake Scale. Data are means ± standard deviation; kPa=
Kilopascal; mm=millimeters; #Median [25%-75%]; †
differs significantly from the CG (time effect x group); ‡ differs
significantly after speech therapy (time effect x group); § differs
significantly from the CG (effect of group); ¶ differs significantly from the
first evaluation (time effect x group); Ø differs significantly from the first
evaluation (time effect x group).
References
1.
Nör JE, Gutkind JS (2018) Head and Neck Cancer in the New Era
of Precision Medicine. J Dent Res 97: 601-602. [Crossref]
2.
Pederson AW, Salama JK, Witt ME, Stenson KM, Blair EA et al.
(2011) Concurrent chemotherapy and intensity-modulated radiotherapy for organ
preservation of locoregionally advanced oral cavity cancer. Am J Clin Oncol 34:
356-361. [Crossref]
3.
Čoček A, Ambruš M, Dohnalová A, Chovanec M, Kubecová M et al.
(2018) Locally advanced laryngeal cancer: Total laryngectomy or primary
non-surgical treatment? Oncol Lett 15: 6701-6708. [Crossref]
4.
Xinou
E, Chryssogonidis I, Fountzila AK, Mpoukla DP, Printza A (2018)
Longitudinal Evaluation of Swallowing with Videofluoroscopy in Patients with
Locally Advanced Head and Neck Cancer After Chemoradiation. Dysphagia 33:
691-706. [Crossref]
5.
Starmer HM, Tippett D, Webster K, Quon H, Jones B et al.
(2014) Swallowing outcomes in patients with oropharyngeal cancer undergoing
organ-preservation treatment. Head Neck 36: 1392-1397. [Crossref]
6.
Jacobi I, Navran A, van der Molen L, Heemsbergen WD, Hilgers
FJM (2016) Radiation dose to the tongue and velopharynx predicts
acoustic-articulatory changes after chemo-IMRT treatment for advanced head and
neck cancer. Eur Arch Otorhinolaryngology 273: 487-494. [Crossref]
7.
Bressan V, Stevanin S, Bianchi M, Aleo G, Bagnasco A et al.
(2016) The effects of swallowing disorders, dysgeusia, oral mucositis and
xerostomia on nutritional status, oral intake and weight loss in head and neck
cancer patients: A systematic review. Cancer Treat Rev 45: 105-119. [Crossref]
8.
Wicherek MD, Woźniak MB, Kazmierczak W, Kożuch KC, Dziobek K
et al. (2017) The quality of life and the occurrence of dysphagia in patients
with head and neck cancer following combined oncological treatment. Med Res
J 2: 13-19.
9.
Starmer HM (2014) Dysphagia in head and neck cancer. Curr
Opin Otolaryngol Head Neck Surg 22: 195-200. [Crossref]
10. Marchesan IQ, Furkim AM (2003)
Manobras Utilizadas Na Reabilitação Da Deglutição. Rio Janeiro
375-384.
11. da Silva RG (2007) A eficácia da
reabilitação em disfagia orofaríngea. Pró-Fono 19: 123-130. [Crossref]
12. Crary
MA, Carnaby GD, LaGorio LA, Carvajal PJ (2012) Functional and physiological
outcomes from an exercise-based dysphagia therapy: a pilot investigation of the
McNeill Dysphagia Therapy Program. Arch Phys Med Rehabil 93: 1173-1178.
[Crossref]
13. White
R, Cotton SM, Hind J, Robbins J, Perry A (2009) A comparison of the reliability
and stability of oro-lingual swallowing pressures in patients with head and
neck cancer and healthy adults. Dysphagia 24: 137-144. [Crossref]
14. Crary
MA, Mann GDC, Groher ME (2005) Initial psychometric assessment of a functional
oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil
86: 1516-1520. [Crossref]
15. Guedes
RLV, Angelis ECD, Chen AY, Kowalski LP, Vartanian JG (2013) Validation and
application of the M.D. Anderson Dysphagia Inventory in patients treated for
head and neck cancer in Brazil. Dysphagia 28: 24-32. [Crossref]
16. de Melo Alvarenga L, Ruiz MT,
Bertelli ECP, Ruback MJC, Maniglia JV et al. (2008) Epidemiologic
evaluation of head and neck patients in a university hospital of Northwestern
São Paulo State. Braz J
Otorhinolaryngol 74:
68-73. [Crossref]
17. Filho FSA, de Andrade Sobrinho J,
Rapoport A, Carvalho MB, Novo NF et al. (2003) Study of
demographic, occupational and co-carcinogenetic variables in squamous cell
carcinoma of base of the tongue in women. Rev Bras Otorrinolaringol 69:
472-478.
18. Quintão
B, Rocha C (2017) Epidemiological aspects of patients with head and neck
neoplasms undergoing radiotherapy in Juiz de Fora HU. Rev Juiz de Fora
43: 71-75.
19. Eytan
DF, Blackford AL, Eisele DW, Fakhry C (2018) Prevalence of Comorbidities among
Older Head and Neck Cancer Survivors in the United States. Otolaryngol Head
Neck Surg 160: 85-92. [Crossref]
20. Clark
HM, Solomon NP (2012) Age and sex differences in orofacial strength. Dysphagia
27: 2-9. [Crossref]
21. Vanderwegen
J, Guns C, Nuffelen GV, Elen R, Bodt MD (2013) The influence of age, sex, bulb
position, visual feedback, and the order of testing on maximum anterior and
posterior tongue strength and endurance in healthy belgian adults. Dysphagia
28: 159-166. [Crossref]
22. Sanders
I, Mu L (2013) A three-dimensional atlas of human tongue muscles. Anat Rec
(Hoboken) 296: 1102-1114. [Crossref]
23. Marchesan
IQ, Furkim AM, Santini CS (2018) Disfagias
orofaríngeas. São Paulo: Pró-Fono 3-18.
24. van der Geer SJ, van Rijn PV, Kamstra
JI, Roodenburg JLN, Dijkstra PU (2018) Criterion for
trismus in head and neck cancer patients: a verification study. Support Care
Cancer 27: 1129-1137. [Crossref]
25. van
der Geer SJ, Kamstra JI, Roodenburg JLN, van Leeuwen M, Reintsema H et al.
(2016) Predictors for trismus in patients receiving radiotherapy. Acta Oncol
55: 1318-1323. [Crossref]
26. Marrafon CS, Matos LL, Zenari MS,
Cernea CR, Nemr K (2018) Speech-language therapy program for mouth opening in
patients with oral and oropharyngeal cancer undergoing adjuvant radiotherapy: a
pilot study. Codas 30: e20160221. [Crossref]
27. den
Steen LV, Vanderveken O, Vanderwegen J, Gestel DV, Daisne JF et al. (2017)
Feasibility of tongue strength measurements during (chemo)radiotherapy in head
and neck cancer patients. Support Care Cancer 25: 3417-3423. [Crossref]
28. Kamal M, Barrow MP, Lewin JS, Estrella A, Gunn GB et al. (2019) Modeling symptom drivers of oral intake in long-term head and neck cancer survivors. Support Care Cancer 27: 1405-1415. [Crossref]
29. Andrade MS, Gonçalves AN, Guedes RLV, Barcelos CB, Slobodticov LDS et al. (2017) Correlation between swallowing-related quality of life and videofluoroscopy after head and neck cancer treatment Codas 29: e20150175. [Crossref]
