Effect of Cerebral Infarction on the Risk of Synchronous Brain Metastasis: Analysis of 307 Consecutive Patients of Newly Diagnosed Non-Small Cell Lung Cancer
A B S T R A C T
Background: Brain metastasis (BM) is a common complication of patients with non-small cell lung cancer (NSCLC) and associated with a poor prognosis. The study aimed to evaluate the effect of cerebral infarction (CI) on the risk of BM in NSCLC for preventive therapy strategy.
Methods: 307 patients with newly diagnosed NSCLC in Zhongda Hospital, Medical School of Southeast University from July 2013 to July 2018 were retrospectively analyzed. Depending on magnetic resonance imaging (MRI), the patients were divided into the BM group and the control group (without BM). Then, the prevalence of CI and baseline clinicopathological parameters were evaluated and compared between the two groups.
Results: Out of the 307 patients, 204 patients (66.4%) had CI, and 52 patients (16.9%) had BM. Especially, the prevalence of CI in the NSCLC patients with BM was 84.6%, which was significantly higher than that of 62.7% in the NSCLC patients without BM (p = 0.002). Following univariate logistic regression analysis and the multivariate model, the results demonstrated that CI was a significant independent risk factor for BM in NSCLC (odds rate [OR], 2.921; 95% confidence interval [CI], 1.242-6.873; p = 0.014). Moreover, CI contributed to a worse prognosis in NSCLC patients with BM. Finally, dynamical trace confirmed CI could promote BM in NSCLC patients.
Conclusions: CI could be associated with a metastatic tropism to the brain and then with an increased risk of BM in NSCLC patients. Therefore, the targeted intervention of the metastatic niche of CI could offer a promising approach for the prevention, prognostic evaluation, and therapy of BM in NSCLC patients for better clinical outcomes.
Keywords
Metastatic niche, non-small cell lung cancer, brain metastasis, cerebral infarction
Introduction
Lung cancer remains one of the most frequently diagnosed cancers as well as the leading cause of cancer-related mortality worldwide, in which non-small cell lung cancer (NSCLC) constitutes 85% cases [1, 2]. Unfortunately, in nearly 20% of NSCLC patients, brain metastasis (BM) is already present at diagnosis, with up to 50% of patients developing BM throughout the disease course [3, 4]. BM in NSCLC complicates the clinical picture and portends a poor prognosis with a median survival of 3-7 months [3, 4]. However, risk factors and underlying mechanisms of metastasis to the brain, the most common site of distant metastasis of lung cancer, remain a mystery and have not been well addressed. Thus, to best improve the overall survival (OS) and quality of life for NSCLC patients, it is highly significant to illustrate the clinical risk factors of target BM in NSCLC for prevention strategies and specific therapies.
The tumor metastases are formed by a complex interaction between cancer cells and microenvironment, which is the "seed-soil" hypothesis [5, 6]. The "seed-soil" hypothesis sets forth the concept that a conducive microenvironment, or metastatic niche, is necessary for disseminating cancer cells to engraft distant sites [7, 8]. The common functions of metastatic niche include anchorage, survival support, protection from external insults, licensing proliferation and outgrowth [9]. For a long time since, much attention has been focused on the molecular and genetic factors of cancer cells as “seed” endowed metastatic advantage. Meanwhile, the metastatic niche creating a fertile “soil” for cancer cells to lodge and grow has been largely neglected. Therefore, the microenvironment of organs as the risk factors to determine metastatic colonization are particularly important for exploration, which might be most amenable to therapeutic interventions.
A very interesting one appears in previous studies, which demonstrated that patients with lung cancer were prone to induced cerebral infarction (CI) when compared with non-cancer control [10, 11]. Moreover, CI occurrence could worsen patient’s prognosis in advanced or post-operative recurrent NSCLC [12]. These achievements inspire us from the perspective of “soil” to draw attention to the effect of CI as a metastatic niche on the risk of target-specific BM in NSCLC patients in the study, which may lead to detect therapeutic strategies and prevent metastasis at its earliest inception.
Patients and Methods
I Study Population
We conducted a retrospective study of all newly diagnosed NSCLC patients who were continuously admitted to Zhongda Hospital, Medical School of Southeast University from July 2013 to July 2018. The inclusion criteria were as follows: 1) all patients with histologically confirmed NSCLC, 2) did not receive any surgery, chemotherapy, radiotherapy, molecular targeted therapy, or immunotherapy in other hospitals before admission, 3) for the purpose of detecting distant organ metastasis, all patients accepted brain, chest, abdominal and pelvic imaging, such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission computed tomography/computed tomography (PET/CT), 4) complete medical records. The exclusion criteria were as follows: 1) NSCLC patients with synchronous distant metastases except for the brain, 2) patients with NSCLC accompanied by malignant tumors in other parts of the body, 3) patients with CI caused by other pathogenic factors. In total, 307 patients were enrolled in this study. The study was approved by the ethics committee of Zhongda Hospital, Southeast University.
II Data Collection
Data collected included age, gender, body mass index (BMI), smoking, hypertension, diabetes, atrial fibrillation, histological type, tumor (T) status, lymph node (N) status and primary tumor size and location. In addition, laboratory tests were also covered, including D-Dimer (D-D) and the tumor markers of carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125) and cytokeratin 19 fragment(CYFRA21-1). The survival time from the onset of BM was evaluated. The follow-up duration lasted until death or July 31, 2019. Overall survival (OS) from the diagnosis of BM was evaluated.
III Diagnosis of CI
The diagnostic criteria of CI refer to
IV Imaging Evaluation of BM in NSCLC
The patients had undergone a brain computed tomography (CT) scan. When brain lesion(s) could not be excluded, patients would undergo a magnetic resonance imaging (MRI). Depending on the imaging examination results of BM, NSCLC patients were divided into the BM group and the control group (without BM). Then, the prevalence of CI and baseline clinicopathological parameters were evaluated and compared between the two groups.
V Statistical Analysis
The prevalence of CI in the two groups was compared using the Chi square test; other patient characteristics were compared using either the Chi square test, the Fisher's exact test, or the Wilcoxon two-sample test. Univariate and multivariate analyses were performed using logistic regression to assess the risk factors for BM. OS was plotted using the Kaplan Meier method. Differences in OS were analyzed using the log-rank test. p < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS version 25.0 software.
Results
I Characteristics of NSCLC Patient
A total of 307 NSCLC patients (185 men and 122 women) with a median age of 64 years (range of 25–83 years) were confirmed for the analysis in our study. Among them, 52 patients (16.9%) had BM, and they were then categorized into the BM group. NSCLC patients without BM were regarded as the control group. In the 52 NSCLC patients with BM, 22 patients (42.3%) underwent a brain CT or MRI without any brain symptom, while other patients took the examination due to some symptoms, such as headache, hemiplegia and psychiatric disorder. The summary of patient characteristics of the BM group and the control group are shown in (Table 1).
Table 1: Baseline characteristics of NSCLC patients with/without BM.
|
|
|
Without BM (N=255) |
With BM (n=52) |
p |
|
Age (year) |
Median (range) |
62 (25-83) |
62.5 (43-78) |
0.92 |
|
BMI (kg/m²) |
Median (range) |
23.18 (16-34) |
22.85 (14-30) |
0.088 |
|
Sex |
Male (%) |
154 (60.4%) |
31 (59.6%) |
0.92 |
|
Smoking |
Yes (%) |
157 (61.6%) |
35 (67.3%) |
0.436 |
|
Hypertension |
Yes (%) |
105 (41.2%) |
25 (48.1%) |
0.359 |
|
Diabetes |
Yes (%) |
59 (23.1%) |
9 (17.3%) |
0.37 |
|
Yes (%) |
16 (6.3%) |
2 (3.8%) |
0.748 |
|
|
Primary tumor size (cm) |
Median (range) |
3 (0.4-14) |
3.6 (0.8-11) |
0.912 |
|
Primary tumor location (lobe) |
Upper (%) |
152 (59.6%) |
26 (50.0%) |
0.02 |
|
Lower (%) |
89 (34.9%) |
17 (32.7%) |
||
|
Middle (%) |
14 (5.5%) |
9 (17.3%) |
||
|
Histology (type) |
Adenocarcinoma (%) |
180 (70.6%) |
46 (88.5%) |
0.028 |
|
Squamous carcinoma (%) |
58 (22.7%) |
5 (9.6%) |
||
|
Other (%) |
17 (6.7%) |
1 (1.9%) |
||
|
CI |
Yes (%) |
160 (62.7%) |
44 (84.6%) |
0.002 |
|
T status |
T3-T4 (%) |
74 (29.0%) |
28 (53.8%) |
0.001 |
|
LN status |
N2-N3 (%) |
134 (52.5%) |
42 (80.8%) |
<0.001 |
|
D-D (ng/ml) |
Median (range) |
182 (0.11-14612) |
304.5 (52-7640) |
<0.001 |
|
CEA (ng/ml) |
Median (range) |
4.11 (0.1-746.7) |
9.675 (1.28-1068) |
<0.001 |
|
CA125 (U/ml) |
Median (range) |
19.44 (3.1-2846) |
49.15 (6.61-796.7) |
<0.001 |
|
CYFRA21-1 (ng/ml) |
Median (range) |
21.93 (0.3-971) |
28.56 (1.36-978) |
0.23 |
BM: brain metastasis; BMI: body mass index; CI: cerebral infarction; D-D: D-Dimer; CEA: carcinoembryonic antigen; CA125: carbohydrate antigen 125; CYFRA21-1: cytokeratin 19 fragment.
The result revealed a 66.45 % incidence of CI in patients with NSCLC, patients with acute cerebral infarction was 3.26%. Especially, the prevalence of CI in the NSCLC patients with BM was 84.6%, which was significantly higher than that of 62.7% in the NSCLC patients without BM (p = 0.002, χ 2 test). In addition, there were significant differences in primary tumor location (lobe), histology (type), T status, LN status, D-D, CEA and CA125 between BM group and control group. By contrast, there were no significant differences in age, BMI, sex, smoking, hypertension, diabetes, atrial fibrillation, primary tumor size and CYFRA21-1 between the two groups.
II Risk Factors for BM in NSCLC Patient
Following univariate logistic regression analysis, CI, primary tumor location (lobe), histology (type), D-D, CEA, and CA125 were chosen as risk factors for BM in NSCLC. As shown in (Table 2), the results illustrated that BM was significantly associated with CI (odds rate [OR], 3.266; 95% confidence interval [CI], 1.475-7.231; p = 0.004). Besides, adenocarcinoma (p = 0.011), middle region tumor location (p = 0.005), advanced T status(T3-T4) (p = 0.001), advanced LN status (N2-N3) (p< 0.001), increased CEA level (p = 0.002), increased CA125 level (p< 0.001) and higher D-D level (p = 0.027) were also linked with increased risks of BM in NSCLC.
Table 2: Univariate analysis of risk factors associated with BM in NSCLC patient.
|
|
|
Without BM (N=255) |
With BM (n=52) |
OR |
95%CI |
p |
|
CI |
Yes |
160 |
44 |
3.266 |
1.475-7.231 |
0.004 |
|
No |
95 |
8 |
||||
|
Primary tumor location (lobe) |
Middle |
14 |
9 |
3.603 |
1.468-8.845 |
0.005 |
|
Upper or Lower |
241 |
43 |
||||
|
Histology (type) |
180 |
46 |
3.194 |
1.309-7.797 |
0.011 |
|
|
Not Ad |
75 |
6 |
||||
|
T status |
T1-T2 |
181 |
24 |
2.854 |
1.553-5.245 |
0.001 |
|
T3-T4 |
74 |
28 |
||||
|
LN status |
N0-N1 |
121 |
10 |
3.793 |
1.824-7.887 |
<0.001 |
|
N2-N3 |
134 |
42 |
||||
|
D-D (ng/ml) |
>500 |
44 |
16 |
2.131 |
1.088-4.176 |
0.027 |
|
≤500 |
211 |
36 |
||||
|
CEA (ng/ml) |
>5 |
114 |
36 |
2.783 |
1.470-5.270 |
0.002 |
|
≤5 |
141 |
16 |
||||
|
CA125 (U/ml) |
>35 |
80 |
30 |
2.983 |
1.620-5.492 |
<0.001 |
|
≤35 |
175 |
22 |
BM: brain metastasis; CI: cerebral infarction; D-D: D-Dimer; CEA: carcinoembryonic antigen; CA125: carbohydrate antigen 125.
Next, we assessed the significance of CI with respect to BM by using the multivariate model according to the factors that are proven to be meaningful in the above univariate analysis. From (Table 3), we could find that CI (OR, 2.921; 95% CI, 1.242 - 6.873; p= 0.014) was a significant independent risk factor for BM in NSCLC. Besides, adenocarcinoma, middle region tumor location and advanced LN status (N2-N3) also remained as the risk factors for BM, whereas D-D, T status, tumor markers of CEA and CA125 was no longer significant.
Table 3: Multivariate analysis of risk factors associated with BM in NSCLC.
|
Variabe |
OR |
95%CI |
p |
|
CI (Yes vs No) |
2.921 |
1.242-6.873 |
0.014 |
|
T status (T1-T2 vs T3-T4) |
1.892 |
0.952-3.760 |
0.069 |
|
LN status (N0-N1 vs N2-N3) |
2.828 |
1.262-6.337 |
0.012 |
|
Location (lobe) (Middle vs Not middle) |
3.148 |
1.158-8.558 |
0.025 |
|
Histology (Ad vs Not Ad) |
4.032 |
1.542-10.542 |
0.004 |
|
D-D (ng/ml) (>500 vs ≤500) |
1.091 |
0.503-2.367 |
0.825 |
|
CEA (ng/ml) (>5 vs ≤5) |
1.370 |
0.657-2.858 |
0.401 |
|
CA125 (U/ml) (>35 vs ≤35) |
1.815 |
0.893-3.690 |
0.100 |
BM: brain metastasis; CI: cerebral infarction; D-D: D-Dimer; CEA: carcinoembryonic antigen; CA125: carbohydrate antigen 125.
III Effect of CI on the Survival of NSCLC Patient with BM
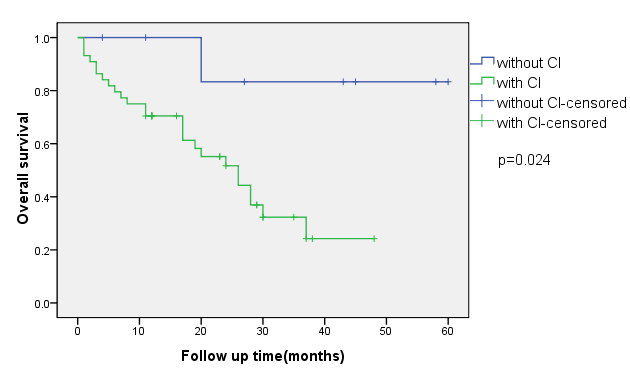
To assess the effect of CI on the survival of NSCLC patients with BM, OS was measured and compared according to the presence or absence of CI. As shown in (Figure 1), OS from diagnosis of BM was significantly shorter in NSCLC patients with CI than that in NSCLC patients with BM without CI (p = 0.024).
Figure 1: Overall survival from diagnosis of BM in NSCLS patient.
CI: cerebral infarction; BM: brain metastasis; NSCLC: non-small cell lung cancer.
IV Dynamic Tracing of CI as Metastatic Niche to Promote BM of NSCLC
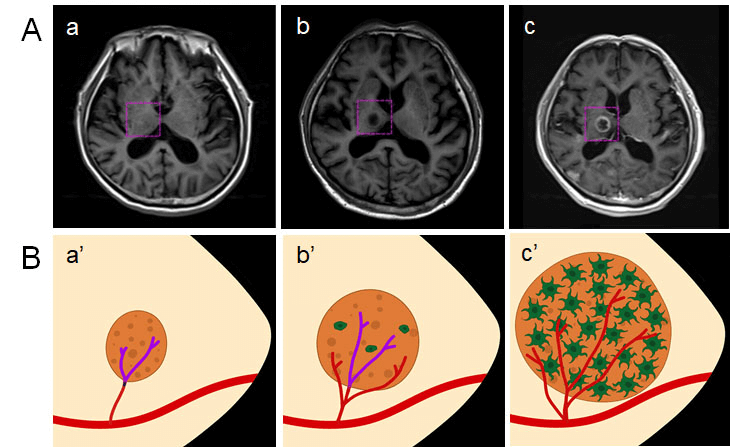
Figure 2 shows a typical case with NSCLC who developed BM due to CI. In this case, when diagnosed with lung adenocarcinoma, one 79-year-old male patient underwent radical resection of the upper right lung cancer on November 24, 2015. Regular examination and follow-up were conducted in the outpatient department after the surgery. More than one year, a new patchy ischemic lesion was revealed at the right basal ganglia, which showed iso- or hypo-intense signal on the T1weighted image (T1WI) of magnetic resonance (MR) on March 16, 2017 (Figure 2A a). As time went by, the lesion at the same site was significantly larger, which showed a low signal on T1WI (Figure 2A b) when follow-up on November 10, 2017. Combined with the medical history, BM could not be excluded for the patient. Unfortunately, on March 16, 2018, the lesion obviously progressed, which showed an abnormal nodular enhancement lesion (Figure 2A c). Thus, BM was confirmed in the NSCLC patient. The dynamic establishment of CI as a metastatic niche to promote BM of NSCLC is schemed in (Figure 2B). In brief, CI (Figure 2B a’) creates a fertile “soil” for cancer cell as “seed” to lodge (Figure 2B b’) and grow (Figure 2B c’).
Figure 2: Dynamically tracing of CI as metastatic niche to promote BM in NSCLC. A) a) MRI of CI; b) BM could not be excluded in MRI; c) confirmation of BM by MRI. B) the scheme of CI as metastatic niche to promote BM in NSCLC. a’) CI as a fertile “soil”; b’) cancer cell as “seed” to lodge; c’) metastatic colonization after proliferation and outgrowth. CI: cerebral infarction; BM: brain metastasis; NSCLC: non-small cell lung cancer; MRI: magnetic resonance imaging.
Discussion
Our study indicated a higher prevalence of BM in NSCLC patients with CI compared to that in those without CI, and CI was a dependent risk factor for BM in patients with NSCLC. Meanwhile, in NSCLC patients with BM, CI contributed to a worse prognosis in NSCLC patients with BM with shorter OS than that in those without CI. Cancer cells that disseminate from primary tumors are dependent on the microenvironment they encounter at secondary sites that determine their fate. Metastatic niche is the "soil" of the specific microenvironment established within the target organ to encourage the outgrowth of the incoming "seed" of cancer cells [7, 8]. It is very important to explore which microenvironment serves as metastatic niche for BM in NSCLC due to its significant mortality.
It is remarkable that the rate of cancer incidence is higher among ischemic stroke patients than the general population, and cancer itself may increase the risk of CI occurrence [14-22]. Once CI occurs in patients with cancer, neurological outcomes significantly worsen, and prognosis tends to be poor in addition to stroke-associated morbidity/mortality [23-25]. Among all cancer types, patients with lung cancer have the highest incidence of CI [10, 11]. Motoyasu et al. reported that the development of CI contributed to a worse prognosis in patients with advanced and recurrent NSCLC [2]. In our study, we found that the incidence of synchronous BM was 16.9% in NSCLC, which was in line with recent studies [3, 4]. In addition, the prevalence of CI was 66.4%, which was much higher than the previous clinical studies. The increased prevalence of CI in our study may be related to the reference of updated diagnostic criteria. In fact, according to the classical WHO definition, the incidence of acute brain infarction in our study was 3.25%, which was in accordance with earlier studies [10, 12].
Silent brain infarction, as the newly added subtype of CI, the prevalence varies with different disease states, the highest incidence could reach above 60% [26]. For our aim was to make an initial exploration of the relationship between ischemic brain damage and BM, the current diagnostic criteria are relatively appropriate. Our study also demonstrated a higher prevalence of BM in patients with CI at 84.6% compared to a prevalence of 62.7% in those without CI. All these findings shed us a light to explore a potential risk of CI to BM in patients with NSCLC. To date, there have been few studies that focused on the relationship between CI and BM. A case report performed by Surl et al. showed that a patient with adenosquamous carcinoma of cervix developed BM limited to an area of evolving infarction [27]. In another report, Yun-Peng et al. described a case that the patient complicated with CI and subsequent BM sarcoma after the initial cardiac tumor resection, which was confirmed by brain tissue pathology [28]. In our study, we conducted a retrospective study of all newly diagnosed 307 NSCLC patients, and our results demonstrated that CI was a dependent risk factor for BM in patients with NSCLC.
CI accounts for 80% of cerebrovascular diseases [29]. The microenvironment of CI as a metastatic niche thus might be a crucial pillar to explain the specificity that applies to the seeding and outgrowth of BM in NSCLC. CI causes the opening of the blood-brain barrier (BBB), active inflammation and edema formation [30-32]. Under the pathological condition of CI, the BBB can be disrupted, followed by the extravasation of blood components into the brain parenchyma. So, metastasizing NSCLC cancer cells could be arrested in the brain due to the BBB leakage. Furthermore, after initial cell death in CI, the clearance of debris in the lesion leaves a compartmentalized cavity that can accept a large volume transplant. Inflammation is an important driver of tumor development and metastasis [33]. The inflammatory response also plays an important role in the pathological process of CI, which is usually accompanied with the release of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF), interleukin 6 [IL-6], IL-1β, monocyte chemotactic protein 1, macrophage inflammatory protein 1α), microglial activation, and adhesion molecules [7]. The establishment of an inflammatory microenvironment in the CI brain, either prior to or at the same time as the arrival of circulating tumor cells, is helpful in the seeding, survival, and proliferation of tumor cells in the metastatic niche. Therefore, the roles of the microenvironment of CI are basically consistent with the common functions of different metastatic niches: anchorage, survival support, protection from external insults, licensing proliferation and outgrowth.
Metastases account for the majority of cancer deaths, and BM is the most lethal complication of NSCLC. Host factors determining metastatic colonization to secondary organs are particularly important for exploration. In our study, the association between CI and increased risk of BM in NSCLC patient was determined first; then the results of the effect of CI on survival in NSCLC patients with BM demonstrated that CI might contribute to a worse prognosis; finally, dynamical trace confirmed that CI could promote BM in NSCLC patient. Thus, in clinical practice, targeting the metastatic niche of CI may evolve into a promising avenue for therapy of BM in NSCLC.
Conclusion
CI could act as a metastatic niche to promote BM in patients with NSCLC. Therefore, the targeted intervention of CI could open up new strategies for the prevention, prognostic evaluation, and therapy of NSCLC patients with BM.
Acknowledgements
Not applicable.
Funding
None.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Author Contributions
DZ and HZ completed data collection, data analysis, manuscript drafting. WX contributed to the conception and design of the current study. HZ directed the arrangement of the study and supervised the whole writing of the manuscript and manuscript revision. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
Not applicable. No animal/human studies are presented in this manuscript.
Consent
No potentially identifiable human images or data is presented in this study.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 24, Apr 2020Accepted: Fri 08, May 2020
Published: Thu 14, May 2020
Copyright
© 2023 Haijun Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.05.07
Author Info
Dandan Zhou Haijun Zhang Hongming Zhang Wenwen Xu
Corresponding Author
Haijun ZhangDepartment of Oncology, Zhongda Hospital, Medical School of Southeast University, Nanjing, Jiangsu, P.R.China
Figures & Tables
Table 1: Baseline characteristics of NSCLC patients with/without BM.
|
|
|
Without BM (N=255) |
With BM (n=52) |
p |
|
Age (year) |
Median (range) |
62 (25-83) |
62.5 (43-78) |
0.92 |
|
BMI (kg/m²) |
Median (range) |
23.18 (16-34) |
22.85 (14-30) |
0.088 |
|
Sex |
Male (%) |
154 (60.4%) |
31 (59.6%) |
0.92 |
|
Smoking |
Yes (%) |
157 (61.6%) |
35 (67.3%) |
0.436 |
|
Hypertension |
Yes (%) |
105 (41.2%) |
25 (48.1%) |
0.359 |
|
Diabetes |
Yes (%) |
59 (23.1%) |
9 (17.3%) |
0.37 |
|
Yes (%) |
16 (6.3%) |
2 (3.8%) |
0.748 |
|
|
Primary tumor size (cm) |
Median (range) |
3 (0.4-14) |
3.6 (0.8-11) |
0.912 |
|
Primary tumor location (lobe) |
Upper (%) |
152 (59.6%) |
26 (50.0%) |
0.02 |
|
Lower (%) |
89 (34.9%) |
17 (32.7%) |
||
|
Middle (%) |
14 (5.5%) |
9 (17.3%) |
||
|
Histology (type) |
Adenocarcinoma (%) |
180 (70.6%) |
46 (88.5%) |
0.028 |
|
Squamous carcinoma (%) |
58 (22.7%) |
5 (9.6%) |
||
|
Other (%) |
17 (6.7%) |
1 (1.9%) |
||
|
CI |
Yes (%) |
160 (62.7%) |
44 (84.6%) |
0.002 |
|
T status |
T3-T4 (%) |
74 (29.0%) |
28 (53.8%) |
0.001 |
|
LN status |
N2-N3 (%) |
134 (52.5%) |
42 (80.8%) |
<0.001 |
|
D-D (ng/ml) |
Median (range) |
182 (0.11-14612) |
304.5 (52-7640) |
<0.001 |
|
CEA (ng/ml) |
Median (range) |
4.11 (0.1-746.7) |
9.675 (1.28-1068) |
<0.001 |
|
CA125 (U/ml) |
Median (range) |
19.44 (3.1-2846) |
49.15 (6.61-796.7) |
<0.001 |
|
CYFRA21-1 (ng/ml) |
Median (range) |
21.93 (0.3-971) |
28.56 (1.36-978) |
0.23 |
BM: brain metastasis; BMI: body mass index; CI: cerebral infarction; D-D: D-Dimer; CEA: carcinoembryonic antigen; CA125: carbohydrate antigen 125; CYFRA21-1: cytokeratin 19 fragment.
Table 2: Univariate analysis of risk factors associated with BM in NSCLC patient.
|
|
|
Without BM (N=255) |
With BM (n=52) |
OR |
95%CI |
p |
|
CI |
Yes |
160 |
44 |
3.266 |
1.475-7.231 |
0.004 |
|
No |
95 |
8 |
||||
|
Primary tumor location (lobe) |
Middle |
14 |
9 |
3.603 |
1.468-8.845 |
0.005 |
|
Upper or Lower |
241 |
43 |
||||
|
Histology (type) |
180 |
46 |
3.194 |
1.309-7.797 |
0.011 |
|
|
Not Ad |
75 |
6 |
||||
|
T status |
T1-T2 |
181 |
24 |
2.854 |
1.553-5.245 |
0.001 |
|
T3-T4 |
74 |
28 |
||||
|
LN status |
N0-N1 |
121 |
10 |
3.793 |
1.824-7.887 |
<0.001 |
|
N2-N3 |
134 |
42 |
||||
|
D-D (ng/ml) |
>500 |
44 |
16 |
2.131 |
1.088-4.176 |
0.027 |
|
≤500 |
211 |
36 |
||||
|
CEA (ng/ml) |
>5 |
114 |
36 |
2.783 |
1.470-5.270 |
0.002 |
|
≤5 |
141 |
16 |
||||
|
CA125 (U/ml) |
>35 |
80 |
30 |
2.983 |
1.620-5.492 |
<0.001 |
|
≤35 |
175 |
22 |
BM: brain metastasis; CI: cerebral infarction; D-D: D-Dimer; CEA: carcinoembryonic antigen; CA125: carbohydrate antigen 125.
Table 3: Multivariate analysis of risk factors associated with BM in NSCLC.
|
Variabe |
OR |
95%CI |
p |
|
CI (Yes vs No) |
2.921 |
1.242-6.873 |
0.014 |
|
T status (T1-T2 vs T3-T4) |
1.892 |
0.952-3.760 |
0.069 |
|
LN status (N0-N1 vs N2-N3) |
2.828 |
1.262-6.337 |
0.012 |
|
Location (lobe) (Middle vs Not middle) |
3.148 |
1.158-8.558 |
0.025 |
|
Histology (Ad vs Not Ad) |
4.032 |
1.542-10.542 |
0.004 |
|
D-D (ng/ml) (>500 vs ≤500) |
1.091 |
0.503-2.367 |
0.825 |
|
CEA (ng/ml) (>5 vs ≤5) |
1.370 |
0.657-2.858 |
0.401 |
|
CA125 (U/ml) (>35 vs ≤35) |
1.815 |
0.893-3.690 |
0.100 |
BM: brain metastasis; CI: cerebral infarction; D-D: D-Dimer; CEA: carcinoembryonic antigen; CA125: carbohydrate antigen 125.
CI: cerebral infarction; BM: brain metastasis; NSCLC: non-small cell lung cancer.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424. [Crossref]
- Patel N, Wu P, Zhang H (2017) Comparison of gefitinib as first- and second-line therapy for advanced lung adenocarcinoma patients with positive exon 21 or 19 del epidermal growth factor receptor mutation. Cancer Manag Res 9: 243-248. [Crossref]
- Hanibuchi M, Kim SJ, Fidler IJ, Nishioka Y (2014) The molecular biology of lung cancer brain metastasis: an overview of current comprehensions and future perspectives. J Med Invest 61: 241-253. [Crossref]
- Taillibert S, Le Rhun É (2015) [Epidemiology of brain metastases]. Cancer Radiother 19: 3-9. [Crossref]
- Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147: 275-292. [Crossref]
- Spano D, Zollo M (2012) Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis 29: 381-395. [Crossref]
- Liu Y, Cao X (2016) Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 30: 668-681. [Crossref]
- Ren G, Esposito M, Kang Y (2015) Bone metastasis and the metastatic niche. J Mol Med (Berl) 93: 1203-1212. [Crossref]
- Celià-Terrassa T, Kang Y (2018) Metastatic niche functions and therapeutic opportunities. Nat Cell Biol 20: 868-877. [Crossref]
- Chen PC, Muo CH, Lee YT, Yu YH, Sung FC (2011) Lung cancer and incidence of stroke: a population-based cohort study. Stroke 42: 3034-3039. [Crossref]
- Zheng R, Zeng H, Zuo T, Zhang S, Qiao Y et al. (2016) Lung cancer incidence and mortality in China, 2011. Thorac Cancer 7: 94-99. [Crossref]
- Kato M, Shukuya T, Mori K, Kanemaru R, Honma Y et al. (2016) Cerebral infarction in advanced non-small cell lung cancer: a case control study. BMC cancer 16: 203. [Crossref]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ et al. (2013) An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association / American Stroke Association. Stroke 44: 2064-2089. [Crossref]
- Gon Y, Okazaki S, Terasaki Y, Sasaki T, Yoshimine T et al. (2016) Characteristics of cryptogenic stroke in cancer patients. Ann Clin Transl Neurol 3: 280-287. [Crossref]
- Selvik HA, Thomassen L, Logallo N, Næss H (2014) Prior cancer in patients with ischemic stroke: the Bergen NORSTROKE study. J Stroke Cerebrovasc Dis 23: 919-925. [Crossref]
- Quintas S, Rogado J, Gullón P, Pacheco-Barcia V, Dotor García-Soto J et al. (2018) Predictors of unknown cancer in patients with ischemic stroke. J Neurooncol 137: 551-557. [Crossref]
- Karlińska AG, Gromadzka G, Karliński MA, Członkowska A (2015) The activity of malignancy may determine stroke pattern in cancer patients. J Stroke Cerebrovasc Dis 24: 778-783. [Crossref]
- Qureshi AI, Malik AA, Saeed O, Adil MM, Rodriguez GJ et al. (2015) Incident cancer in a cohort of 3,247 cancer diagnosis free ischemic stroke patients. Cerebrovasc Dis 39: 262-268. [Crossref]
- Lee EJ, Nah HW, Kwon JY, Kang DW, Kwon SU et al. (2014) Ischemic stroke in patients with cancer: is it different from usual strokes? Int J Stroke 9: 406-412. [Crossref]
- Guo YJ, Chang MH, Chen PL, Lee YS, Chang YC et al. (2014) Predictive value of plasma (D)-dimer levels for cancer-related stroke: a 3-year retrospective study. J Stroke Cerebrovasc Dis 23: e249-e254. [Crossref]
- Grazioli S, Paciaroni M, Agnelli G, Acciarresi M, Alberti A et al. (2018) Cancer-associated ischemic stroke: A retrospective multicentre cohort study. Thromb Res 165: 33-37. [Crossref]
- Wang JY, Zhang GJ, Zhuo SX, Wang K, Hu XP et al. (2018) D-dimer >2.785 μg/ml and multiple infarcts ≥3 vascular territories are two characteristics of identifying cancer-associated ischemic stroke patients. Neurol Res 40: 948-954. [Crossref]
- Chung JW, Cho YH, Ahn MJ, Lee MJ, Kim GM et al. (2018) Association of Cancer Cell Type and Extracellular Vesicles With Coagulopathy in Patients With Lung Cancer and Stroke. Stroke 49: 1282-1285. [Crossref]
- Emre U, Gunes T, Pinar I, Kokturk F, Liman E et al. (2018) Evaluation of ischemic stroke patients with systemic cancer. Ideggyogy Sz 71: 178-183. [Crossref]
- Kneihsl M, Enzinger C, Wünsch G, Khalil M, Culea V et al. (2016) Poor short-term outcome in patients with ischaemic stroke and active cancer. J Neurol 263: 150-156. [Crossref]
- Fanning JP, Wong AA, Fraser JF (2014) The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med 12: 119. [Crossref]
- Nielsen SL, Posner JB (1983) Brain metastasis localized to an area of infarction. J Neurooncol 1: 191-195. [Crossref]
- Sun YP, Wang X, Gao YS, Zhao S, Bai Y (2017) Primary cardiac sarcoma complicated with cerebral infarction and brain metastasis: A case report and literature review. Cancer Biomark 21: 247-250. [Crossref]
- Zhang AW, Han XS, Xu XT, Fang YN, Chen HB et al. (2019) Acute phase serum cathepsin S level and cathepsin S/cystatin C ratio are the associated factors with cerebral infarction and their diagnostic value for cerebral infarction. Kaohsiung J Med Sci 35: 95-101. [Crossref]
- Bhasin A, Srivastava MVP, Mohanty S, Vivekanandhan S, Sharma S et al. (2016) Paracrine Mechanisms of Intravenous Bone Marrow-Derived Mononuclear Stem Cells in Chronic Ischemic Stroke. Cerebrovasc Dis Extra 6: 107-119. [Crossref]
- Mohan Rajwani K, Crocker M, Moynihan B (2017) Decompressive craniectomy for the treatment of malignant middle cerebral artery infarction. Br J Neurosurg 31: 401-409. [Crossref]
- Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH et al. (2015) Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits After Stroke. Stem Cells Transl Med 4: 841-851. [Crossref]
- Sirniö P, Väyrynen JP, Klintrup K, Mäkelä J, Karhu T et al. (2019) Alterations in serum amino-acid profile in the progression of colorectal cancer: associations with systemic inflammation, tumour stage and patient survival. Br J Cancer 120: 238-246. [Crossref]
