Dysregulation of DNA Methylation During Development as a Potential Mechanism Contributing to Obsessive Compulsive Disorders and Autism
A B S T R A C T
Autism Spectrum Disorder (ASD) is a pervasive developmental disorder, that is rising at a concerning rate. However, underlying mechanisms are still to be discovered. Obsessions and compulsions are the most debilitating aspect of these disorders (OCD), and they are the treatment priority for patients. SAPAP3 knock out mice present a reliable mouse model for repetitive compulsive behaviour and are mechanistically closely related to the ASD mouse model Shank3 on a molecular level and AMPA receptor net effect. The phenotype of SAPAP3 knock out mice is obsessive grooming that leads to self-inflicted lesions by 4 months of age. Recent studies have accumulated evidence, that epigenetic mechanisms are important effectors in psychiatric conditions such as ASD and OCD. Methylation is the most studied mechanism, that recently leads to drug developments for more precise cancer treatments. We injected SAPAP3 mice with an epigenetic demethylation drug RG108 during pregnancy and delayed the onset of the phenotype in the offspring by 4 months. This result gives us clues about the possible mechanisms involved in OCD and ASD. Additionally, it shows that the modulation of methylation mechanisms during development might be explored as a preventative treatment in the cases of the high inherited risk of certain mental health conditions.
Keywords
Epigenetic mechanisms, methylation, autism, compulsions, OCD, development,novel epigenetic cancer drug, mental health,a transgenic mouse model
Introduction
Autism is described in the ICD-10 and DSM-5 as a delay or abnormal development with onset before 3 years leading to impairments in social communication, and repetitive and stereotyped patterns in behavior, interests, and activities. Many researchers support the hypothesis that the mechanisms underlying autism are polygenic and epistatic, and that environmental factors interact with genetic factors that lead to increase risk [1, 2]. The aspect of obsessions and compulsions are the most debilitating facet of ASD, and they are the treatment priority from the patient’s perspective [3]. Epigenetic mechanisms [4, 5] regulate normal development and cell differentiation in order to generate and maintain different specialized tissue-specific phenotypes based on the same genome [6]. Mental health disorders developing during childhood are hypothesized to be especially affected by epigenetic mechanisms [7].
Another important factor affecting autism spectrum disorders (ASD) and obsessive-compulsive disorders (OCD), seems to be an immune response. Brain imaging and neuropathological techniques have shown developmental macroscopic and microscopic aberrations proposing neuroinflammation in the frontal cortex and cerebellar regions, consisting of cytokine production and activation of microglia and astrocytes [8, 9]. Additionally, allergies and autoimmune diseases were significantly higher in children with autism than among controls [10]. These dysregulated immune responses in ASD and OCD patients were shown to affect synaptic autophagy and appropriate synaptic pruning mechanisms, which play an important role in regular development [11, 12].
Zinc has been shown to play an important role in immune response and ASD [13, 14]. Zinc seems to be crucial for the development and function of cells mediating innate immunity, neutrophils, and NK cells, macrophages, phagocytosis, intracellular killing, cytokine production, and growth and function of T and B cells, which were all shown to be impaired by zinc deficiency. It was observed that interferon-γ (IFN-γ production is decreased as a result of zinc deficiency and may affect cell-mediated immunity adversely. Furthermore, zinc seems to function as an anti-oxidant and to be involved in membrane stabilization suggesting a role in the prevention of free radical-induced damaging especially during inflammatory processes [15]. In another study, the authors suggested a strong interaction between social behavior and IFN-γ-driven responses. They demonstrated that inhibitory neuron activity is directly affected by IFN-γ, which lead to an increase in GABAergic (γ-aminobutyric-acid) currents in projection neurons. This demonstrates a molecular link between meningeal immunity and neural circuits involved in social behavior in which IFN-γ plays a crucial role [16].
In young hippocampal neurons, zinc was recently shown to increase the timing of AMPAR-mediated synaptic currents, in which Shank2 and Shank3 played a crucial role by promoting the removal of GluA1 while enhancing recruitment of GluA2. This regulation shows a zinc-dependent mechanism in young neurons and their dependence on Shank2 and Shank3. This could represent an underling mechanism in ASD and OCD, that is modified by genetic mutations and environmental factors during early development, which lead to the observed impairment of synaptic maturation and circuit formation.
It has been demonstrated that DNA methylation controls key aspects of immune responses regulating peripheral immune system effector cell development, in which especially DNMT1 and DNMT3b seem to play a major role during development [17]. DNMT3b seems to be highly expressed in the murine neural tube between embryonic day (E)7.5 and E9.5, indicating that DNMT3b might have a very specific role in the early stages of neurogenesis [18]. DNMT1 was shown to be involved in regulating gene expression of GAD67 protein in the GABAergic neurons of the cortex [19]. DNMT1 was shown to be regulated by the Sp1 and Sp3, a family of transcription factors that are united by a combination of three conserved Cys2His2 zinc fingers that form the sequence-specific DNA-binding domain [20].
All these results seem to indicate that DNA methylation and zinc play an important role in regulating immune response, neural development and circuit activity in brain regions involved in ASD and OCD. We wanted to know if we would be able to ameliorate the stereotypic and repetitive behavior involved in ASD and OCD by the administration of a comparatively low toxic demethylation drug RG108 during development in a disorder mouse model. For this study, we used the transgenic mouse model that shows the most severe phenotypic behavior for repetitive compulsive behavior, which is the SAPAP3 knock out (KO) mouse. SAPAP3, Shanks, and PSD-95 are master scaffolding proteins that cross-link neurotransmitter receptors, signaling molecules and cytoskeletal components [21]. SAPAP3 KO mice lack one of SAP90/PSD-95-associated proteins (SAPAPs, also known as guanylate cyclase, associated proteins), a family of scaffold proteins in postsynaptic density [22]. The net effect of deleting SAPAP3 appears to be the reduction of the sensitivity to AMPAR-mediated glutamatergic signaling, which is the same net effect shown for zinc mediated AMPA receptor modulations (mentioned above).
Methods
I Animals
We used 3-8 months old male and female Sapap3 KO mice. All mice were housed individually in home-cages with 12 h light/dark cycle with food and water ad libitum. The research complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and approved by Animal Welfare Committee, NY, USA.
II Behavior
Total time spent grooming, number and duration of grooming bouts, and body region groomed (head, core or tail) was quantified by observers blind to experimental conditions. Open field behavior and performance in an elevated plus maze were be tested after the stimulation protocol as general measures of locomotion and anxiety-like behavior and analyzed with EthioVision XT software.
III Drug
RG108 (Sigma) was dissolved in 10% DMSO and sterile saline and injected subcutaneously in a 0.8 mg/kg dosage.
IV Statistical Analysis
All data were presented as the mean ± standard error. All statistics were performed using GraphPad Prism 5.0b. Comparison between two groups was evaluated by unpaired Student’s t-test. Differences in different treatment groups were carried out using one-way or two-way ANOVA with Tukey post hoc comparison tests, Mann-Whitney test. Statistical significance was defined as p < 0.05.
Results and Discussion
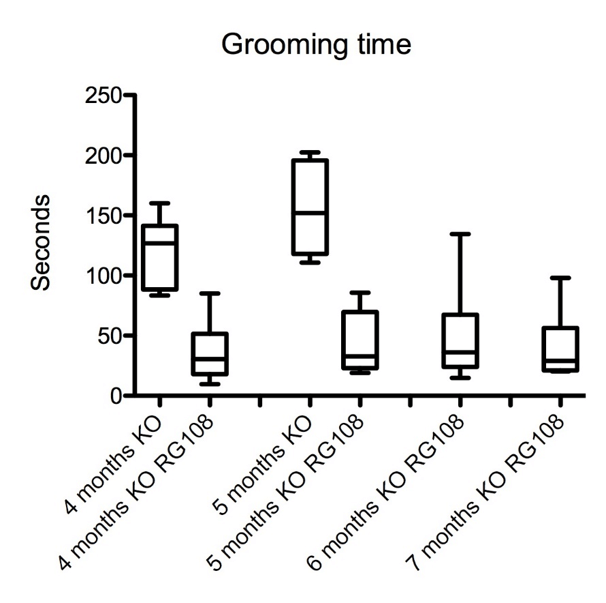
Figure 1: Prolonged amelioration of repetitive grooming behavior in offspring of RG108 treated pregnant (day 10) SAPAP3 KO mice (n=6 in each group, p < 0.05).
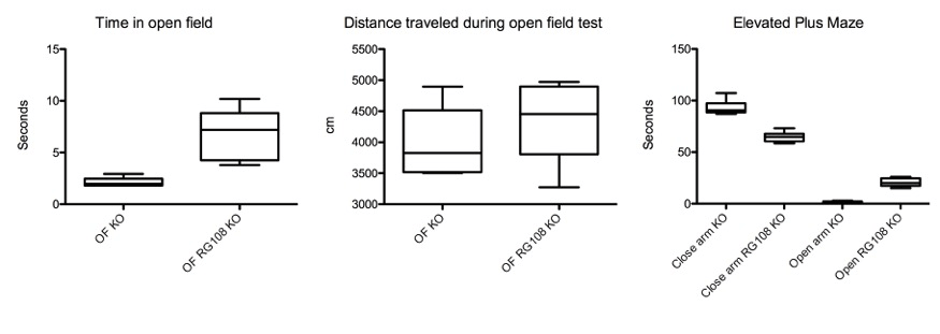
We injected one dose of RG108, a non-nucleoside DNA methyltransferase (DNMT) inhibitor, in pregnant SAPAP3-deficient mice which prevented repetitive grooming behavior in the offspring significantly and delayed its phenotypic behavior by 4 months (p < 0.05) (Figure 1). We also recorded an improvement of fear-related behavior tested in the open field and elevated plus-maze tests (p < 0.05), (Figure 2). The effect of the drug was remarkably long, compared to injections of RG108 in adult SAPAP3-deficient mice, which ameliorated the phenotypic behavior for only 3 days (Todorov et al., 2019).
Figure 2: Reduced fear related behavior in offspring of RG108 treated pregnant (day 10) SAPAP3 KO mice (n=6 in each group, p < 0.05).
Based on our results, we hypothesize that by injection of RG108 we suppress relatively excessive (in the conditions of reduced sensitivity to AMPAR-mediated glutamatergic signaling) DNMT activity in this mouse model. We theorize that especially DNMT1 and DNMT3b would play an important role in the amelioration of the phenotypic behavior based on the fact that DNMT3b appears to be active in a fairly short window during embryonic development (early phase of neurogenesis) and much less active in the adult brain (see above) and DNMT1 is highly active in embryonic neural precursor cells and that activity is distinct from DNMT1 activity in adult post-mitotic neurons. Additionally, the involvement of DNMT1 in inhibition of the cortex is another reason why we think that it’s mechanisms are involved in the aberrant behavior in SAPAP3 KO mice since an over-activity in the cortex has been reported in this mouse model and ASD [23, 24].
Sp1 and/or Sp3 zinc finger proteins activate DNMT1 transcription. Therefore, zinc deficiency during pregnancy may lead to DNMT1 inhibition and have a similar effect to DNMT1 inhibition in SAPAP3 mice, which we would propose should be tested in these mice. Additionally, synaptic pruning is affected by DNMT inhibition in early development and may have long-term effects via (among other things) effects on development in ASD and OCD patients [25]. In the light of direct involvement of IFN-γ regulating of social anxiety-related behavior and GABAergic synaptic transmission in the cortex, which in turn is modulated through DNMT1 and zinc, one should also consider recording immune response markers in ASD and OCD mice models. There is evidence that these underlying immune mechanisms lead to deficient/abnormal synaptic pruning (and microglia/immune) in ASD and OCD, which is affected in early development may have long-term effects [11, 12]. Synaptic pruning is also (directly) affected by the activity of the complement system, especially C3. In fact, C3 is decreased in autism [26]. Also, IFN-γ increases expression of the complement components C3 and C4 may indirectly increase synaptic pruning. Conversely, low IFN-γ at crucial points during development might cause a decrease in synaptic pruning [27]. This may be one of the mechanisms by which IFN-γ signaling may be involved in disorders such as ASD and OCD.
Even though targets of these mechanisms remain unclear, these findings provide strong motivation to evaluate therapeutic interventions that may target the mentioned mechanisms to achieve a rebalancing of synaptic activity. When molecular targets are clarified, one will be in the position of designing more specific repertoire of drugs aiming nuclear signaling and gene regulation for the treatment of mental health disorders.
Author Contributions
German Todorov and Catarina Cunha conceived of the presented idea. Catarina Cunha developed the experiment design, performed experiments and oversaw the project. German Todorov analyzed and verified the data and analytical methods. Karthikeyan Mayilvahanan and David Ashurov assisted Catarina Cunha with performing experiments and analyzing blindly behavior data. All authors discussed the results and contributed to the final manuscript.
Competing Interests
None.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 24, Jan 2020Accepted: Wed 19, Feb 2020
Published: Wed 26, Feb 2020
Copyright
© 2023 Catarina Cunha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.01.04
Author Info
Catarina Cunha David Ashurov German Todorov Karthikeyan Mayilvahanan
Corresponding Author
Catarina CunhaEmotional Brain Institute, Nathan Kline Institute, Orangeburg, New York, USA
Figures & Tables
References
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B et al. (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68: 1095-1102. [Crossref]
- Gardener H, Spiegelman D, Buka SL (2011) Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 128: 344-355. [Crossref]
- Matson JL, Nebel Schwalm MS (2007) Comorbid psychopathology with autism spectrum disorder in children: an overview. Res Dev Disabil 28: 341-352. [Crossref]
- Avery OT, Macleod CM, McCarty M (1944) Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type III. J Exp Med 79: 137-158. [Crossref]
- Hotchkiss RD (1948) The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem 175: 315-332. [Crossref]
- Holliday R, Pugh JE (1975) DNA modification mechanisms and gene activity during development. Science 187: 226-232. [Crossref]
- Tordjman S, Somogyi E, Coulon N, Kermarrec S, Cohen D et al. (2014) Gene × Environment interactions in autism spectrum disorders: role of epigenetic mechanisms. Front Psychiatry 5: 53. [Crossref]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57: 67-81. [Crossref]
- Attwells S, Setiawan E, Wilson AA, Rusjan PM, Mizrahi R et al. (2017) Inflammation in the Neurocircuitry of Obsessive-Compulsive Disorder. JAMA Psychiatry 74: 833-840. [Crossref]
- Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B et al. (2015) Immune mediated conditions in autism spectrum disorders. Brain Behav Immun 46: 232-236. [Crossref]
- Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY et al. (2017) Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry 22: 1576-1584. [Crossref]
- Frick L, Pittenger C (2016) Microglial Dysregulation in OCD, Tourette Syndrome, and PANDAS. J Immunol Res 2016: 8606057. [Crossref]
- Ha HTT, Leal Ortiz S, Lalwani K, Kiyonaka S, Hamachi I et al. (2018) Shank and Zinc Mediate an AMPA Receptor Subunit Switch in Developing Neurons. Front Mol Neurosci 11: 405. [Crossref]
- Prasad AS (2008) Zinc in human health: effect of zinc on immune cells. Mol Med 14: 353-357. [Crossref]
- Shankar AH, Prasad AS (1998) Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 68: 447S-463S. [Crossref]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W et al. (2016) Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535: 425-429. [Crossref]
- Teitell M, Richardson B (2003) DNA methylation in the immune system. Clin Immunol 109: 2-5. [Crossref]
- Okano M, Bell DW, Haber DA, Li E (1999) DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99: 247-257. [Crossref]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R et al. (2004) DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A 101: 348-353. [Crossref]
- Kishikawa S, Murata T, Kimura H, Shiota K, Yokoyama KK (2002) Regulation of transcription of the Dnmt1 gene by Sp1 and Sp3 zinc finger proteins. Eur J Biochem 269: 2961-2970. [Crossref]
- Chua JJ, Kindler S, Boyken J, Jahn R (2010) The architecture of an excitatory synapse. J Cell Sci 123: 819-823. [Crossref]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J et al. (2007) Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 448: 894-900. [Crossref]
- Manning EE, Dombrovski AY, Torregrossa MM, Ahmari SE (2019) Impaired instrumental reversal learning is associated with increased medial prefrontal cortex activity in Sapap3 knockout mouse model of compulsive behavior. Neuropsychopharmacology 44: 1494-1504. [Crossref]
- Ha S, Sohn IJ, Kim N, Sim HJ, Cheon KA (2015) Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan. Exp Neurobiol 24: 273-284. [Crossref]
- Kavalali ET, Nelson ED, Monteggia LM (2011) Role of MeCP2, DNA methylation, and HDACs in regulating synapse function. J Neurodev Disord 3: 250-256. [Crossref]
- Fagan K, Crider A, Ahmed AO, Pillai A (2017) Complement C3 Expression Is Decreased in Autism Spectrum Disorder Subjects and Contributes to Behavioral Deficits in Rodents. Mol Neuropsychiatry 3: 19-27. [Crossref]
- Mitchell TJ, Naughton M, Norsworthy P, Davies KA, Walport MJ et al. (1996) IFN-gamma up-regulates expression of the complement components C3 and C4 by stabilization of mRNA. J Immunol 156: 4429-4434. [Crossref]
