Dual Chest Pathology Presenting with Acute Respiratory Failure: A Case Report and Review of Literature
A B S T R A C T
Congenital diaphragmatic hernia (CDH) is an uncommon neonatal pediatric surgical problem. About 5-25% of cases had delayed presentation, making diagnostic challenge and requiring a high index of suspicion. Combination of diaphragmatic hernia and severe pneumonia with rapid deterioration of the case up to respiratory failure is a rare occasion. Herein, we present a case of 2-years-old female referred to our hospital intubated, ventilated due to type 2 respiratory failure resulting from dual chest pathology (severe pneumonia and left sided congenital diaphragmatic hernia). She was admitted at the pediatric intensive care unit (PICU), stabilized then operated for repair of her diaphragmatic hernia. Post-operatively, she remained intubated, ventilated for 7 days then weaned gradually from the ventilator. The cardiorespiratory status has much improved and she started oral feeding by the 8th post-operative day and discharged home in a stable condition.
Keywords
Congenital diaphragmatic hernia, delayed presentation, pneumonia, hernia repair, septic shock, respiratory failure
Introduction
Congenital diaphragmatic hernia (CDH) refers to a congenital defect in the posterolateral diaphragm at the “Foramen of Bochdalek.” It is a relatively common cause of neonatal respiratory distress with an overall incidence of 1:3000 (1 in 2000-5000) live births, about 33% of cases are stillborn (it predominates in females if stillborn fetuses are counted) [1, 2]. Embryologically, it results from failure of development of different parts of the diaphragm which occurs during 8th gestational week resulting in patency of the pleuroperitoneal canal [2]. Classically, CDH manifest early after birth. But, sometimes, it has a delayed diagnosis in older children, adolescents or even adults. The presenting symptoms can be vague and equivocal in most instances.
Case Details
A 2-year-old female patient referred to our hospital intubated, ventilated due to type 2 respiratory failure (hypercapnia pCO2: 82 mmHg & hypoxia pO2: 28 mmHg) resulting from dual pathology (severe pneumonia and left sided congenital diaphragmatic hernia) with no history of trauma. The patient was the result of consanguineous marriage with past history of 3 abortions. Her mother had uneventful antenatal follow up course. She was delivered by Caesarian section at near term (36 weeks gestation) with birth weight of 1200 gm and incubated at neonatal intensive care unit (NICU) for 5 days because of low birth weight (LBW) and jaundice. Again, she was admitted in the incubator for 10 days because of high fever with chest infection. Within the previous 2 years, she did not complaint of any pathology. She weighs 11 kg with delayed motor developmental milestones (still only walks with stretcher support). The parents declared that she was well and good tell 3 days back with fever, nasal discharge, and productive cough for which her parents sought medical advice, then she became cyanotic with pO2 saturation dropped to 72% for which she had been intubated & ventilated.
On examination, she had high fever (39°C) with cold extremities, nasal congestion & discharge, shortness of breathing, grunting, bronchial labored breathing with diminished air entry on left side & bilateral crepitations, and productive cough followed by nausea and vomiting and she was lethargic before intubation then fallen comatose. Vital signs included tachypnea (30 breaths/min), sinus tachycardia (166 beat/min) and hypotension (70/44 mmHg). Orogastric tube was inserted for bowel deflation. The baby was investigated by basic laboratory investigations that revealed leukocytosis (WBCs 19.300/ ml3), with absolute neutrophilia monophilia, basophilia, anemia, thrombocytopenia (93.000/ ml3), C-reactive protein (CRP) 0.29 mg/dl, severe respiratory acidosis (pH 6.88, HCO3 10 mmol/L, Base excess -14.5 mmol/L), elevated urea level, normonatremia (142 mmol/L), normokalemia (4.1 mmol/L), hyperchloremia (132 mmol/L), critical hypocalcemia (0.62 mmol/L), hypomagnesemia, stress hyperglycemia, elevated alanine aminotransferase (ALT 214 U/L), and hypoalbuminemia (34 gm/L).
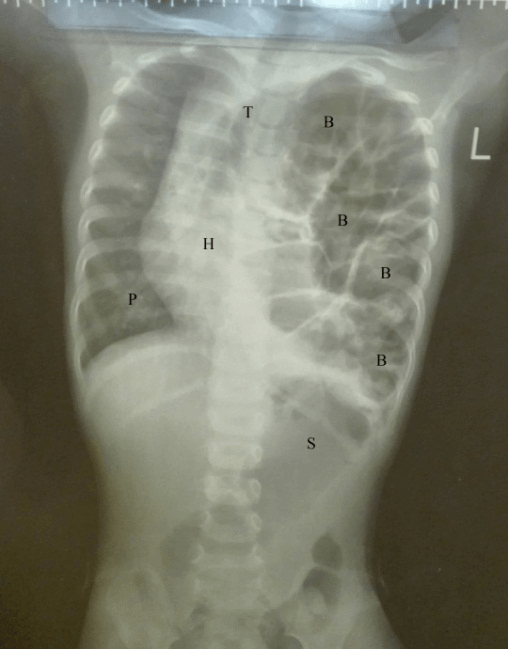
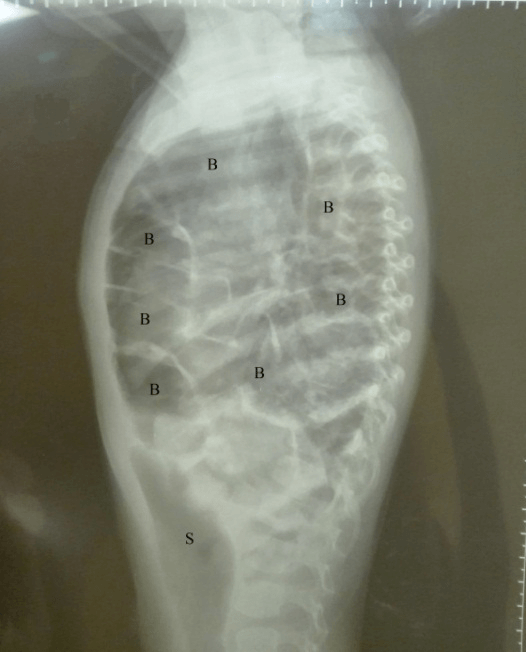
Figure 1: Chest radiography Posteroanterior view (to the left) and Lateral view (to the right) revealed large left sided congenital diaphragmatic hernia with intrathoracic bowel loops reaching the left apical region and marked mediastinal shift and compressing the right lung (S: Stomach; B: Bowel; T: Trachea; H: Heart; P: Pneumonic Patches).
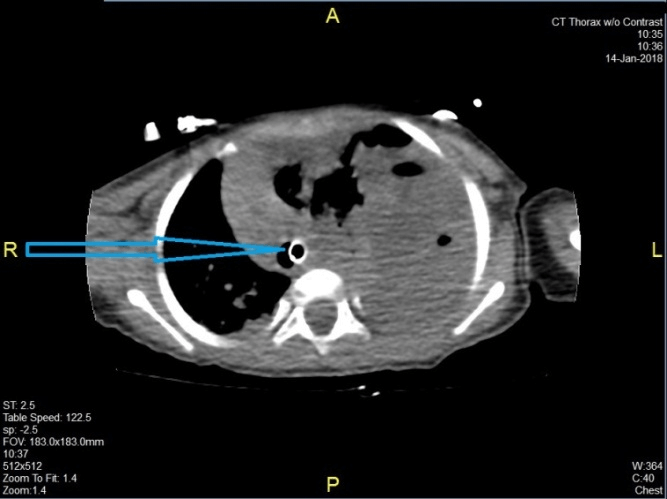
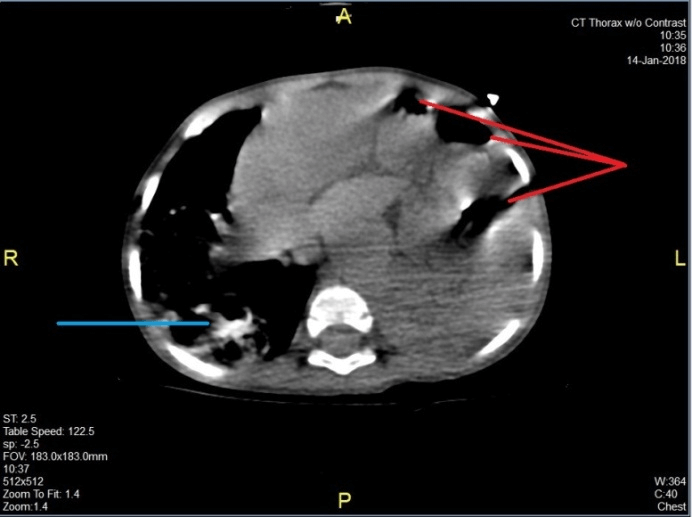
Plain chest radiography (Figure 1) revealed bowel loops in the left hemithorax, intra-abdominal stomach, marked mediastinal shift to the right side, compressed right lung, severe bilateral pneumonia. CT chest without contrast revealed the endotracheal tube with its tip at a satisfactory position (Figure 2). There is a large left diaphragmatic hernia as evident by bowel loops seen within the left hemithorax (Figure 3) causing significant mass effect and collapse of the left lung as well as marked mediastinal shift to the right side, absent left diaphragmatic outline, intra-abdominal stomach. There are multiple bilateral patchy airspace opacities with large right lower lobe airspace consolidation with air bronchogram seen (Figure 4). At the emergency department (ER), due to collapsed peripheral veins, 2 central venous lines were inserted in left internal jugular and right femoral veins as life-saving followed by infusion of 3 boluses of normal saline and sedatives (fentanyl & midazolam), dextrose in normal saline, Epinephrine as inotropic agent with incrementing doses (from 0.05 to 0.1 to 0.5 microgram/kg/minutes) cefotaxime, vancomycin, piperacillin/tazobactam, calcium chloride slowly intravenously, paracetamol as antipyretic, packed RBCs, fresh frozen plasma, and glucocorticoids to reverse the septic shock and severe stress. She was admitted at the pediatric intensive care unit (PICU), isolated for assessment of coronavirus infection that revealed negative.
Figure 2: CT chest without contrast revealed the endotracheal tube tip at satisfactory level (blue arrow).
After stabilization of the case and correction of acid-base/ fluid & electrolytes panel derangements and optimization of the general condition, she was operated for repair of her diaphragmatic hernia that was accomplished by minilaparotomy on the left upper abdomen (due to fragile status of the patient & surgeon preference) followed by reduction of the bowel loops and closing the diaphragmatic defect primarily without need for any prosthetic patch. Post-operatively, she remained intubated, ventilated for 7 days then weaned gradually from the ventilator. The cardiorespiratory status has much improved and she started oral feeding by the 8th post-operative day and discharged home after tolerating full oral intake and normal defecation. Mask ventilation was avoided to prevent further bowel distension. High frequency oscillatory ventilation (HFOV) was the best option for respiratory support.
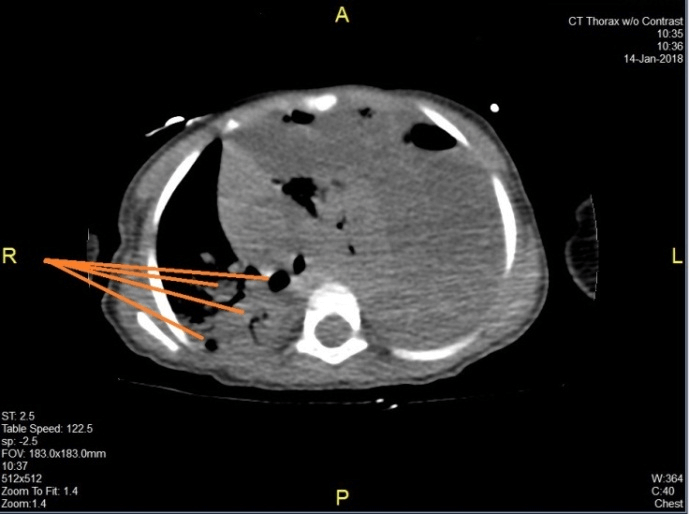
Figure 3: CT chest without contrast revealed numerous bowel loops filling the left hemithorax (red lines) and right lower lobe pneumonia (blue line).
Figure 4: CT chest without contrast revealed right lower lobe pneumonia consolidation with multiple airspaces (orange lines).
Discussion
Congenital diaphragmatic hernia (CDH) is one of the common congenital anomalies with estimated incidence 1:3500 live births (between 1in 2000 to 5000 births) [2]. The exact etiology of CDH is not completely known. It is presumably a multifactorial pathology with exposed genetically susceptible fetus to pharmacologic & environmental teratogens especially the recognized association between CDH and vitamin A deficiency [2, 3]. This case was dealt with seriously because of suspected Corona virus infection from fear of the China epidemic episode of 2019-nCoV or endemic MERS-CoV in Saudi Arabia. The resulting severe pneumonia may be explained by occurring during the current epidemic novel Corona virus (2019-nCoV) or other viruses causing severe acute respiratory syndrome (MERS-CoV) or by gram negative bacteria from perforated bowel inside the chest. The first possibility was excluded by nasopharyngeal lavage for Corona virus assay that revealed negative. The second explanation is not an accepted possibility in the setting of the distended bowel in the chest as shown in the radiologic images (bowel loops are distended with no pleural effusion and, no pneumoperitoneum). Pneumonia in the case has rapidly deteriorated as the patient has markedly limited respiratory reserve.
Lately presenting CDH is defined as being discovered beyond the first month of life. It may be incidentally discovered during radiologic evaluation of unrelated cause, can mimic tension pneumothorax, or during assessment for recurrent chest infections or postprandial discomfort or presented acutely as emergency by bowel/ gastric strangulation or perforation. Many articles in the literature describe cases of lately presenting CDH with several scenarios ranging from asymptomatic cases to cases with respiratory distress to the extremis with acute catastrophic gastrointestinal conditions [4-7].Our case has been diagnosed by chest radiography and chest CT scan as in similar articles [8, 9]. Fortunately, no major associated congenital anomalies were present in our patient, predicting the good outcome. The better outcome of delayed presentation of CDH is the usual event as it is ameliorated by absence of pulmonary hypertension and lung hypoplasia. The peculiar issue in the current case is the strange clinical scenario of rapidly deteriorating respiratory distress up to type 2 respiratory failure associated with septic shock with pending multi-organ failure due to combined pathology (the congenital diaphragmatic hernia and the acquired severe pneumonia).
A similar case of CDH in 2 years old male child presented lately masquerading as tension pneumothorax has been reported by Choi et al., 2019. The CDH was diagnosed only by CT scan chest [10]. Similar articles described the same presentation [11-13]. Also, Blood et al. describes a case of Morgagni hernia presented with intestinal malrotation. Also, they cited a study conducted at 2019 at a high-volume center in the Netherlands between 2000 and 2015 consisting of retrospective analysis of 197 patients with CDH finding malrotation in 76 patients (39%) with higher incidence of bowel herniation in the malrotation group [2]. Pradeep et al. described a case of lately presented CDH with iatrogenic gastric perforation by chest tube insertion on assumption of left sided pyothorax [13]. Similar cases have been reported with chest tube insertion leading to iatrogenic perforation in patients with undiagnosed diaphragmatic hernia [14, 15].
Conclusion
To our knowledge, no previous case of dual chest pathology (one congenital lesion with delayed presentation and the other acquired) resulting in respiratory failure has been reported. Combination of congenital diaphragmatic hernia and severe pneumonia with rapid deterioration of the case up to respiratory failure is a rare occasion that makes the physician raises his alertness threshold for doing prompt evaluation of the case before issuance of respiratory failure.
Consent
Written informed consent was obtained from the parents to publish the images & details. No written details or photos in this article could lead to identification of the relevant case.
Funding
None.
Conflicts of Interest
None.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Acknowledgements
We thank the parents for their acceptance to publish the case images and findings.
Article Info
Article Type
Case Report and Review of LiteraturePublication history
Received: Tue 04, Feb 2020Accepted: Thu 20, Feb 2020
Published: Wed 26, Feb 2020
Copyright
© 2023 Muhammad Elsayed Abdelhafez Mahmoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2020.01.09
Author Info
Ahmed Nabil Abdelhadi Khaled Zamel Aldaraan Mohamed Hany Hassab Muhammad Elsayed Abdelhafez Mahmoud
Corresponding Author
Muhammad Elsayed Abdelhafez MahmoudPediatric Surgery Department, Prince Mohammed bin Abdulaziz Hospital (PMAH), Riyadh, Kingdom of Saudi Arabia
Figures & Tables
References
- Roehr CC, Proquitte H, Jung A, Ackert U, Bamberg C et al. (2009) Impaired somatic growth and delayed lung development in infants with congenital diaphragmatic hernia--evidence from a 10-year, single center prospective follow-up study. J Pediatr Surg 44: 1309-1314. [Crossref]
- Blood AG, Whaley ZL, Kadenhe Chiweshe AV, Spigland NA (2020) Case report: 19M old boy with Morgagni Hernia associated with intestinal malrotation. J Pediatr Surg Case Rep 52: 101341.
- Green JJ, Babiuk RP, Thebaud B (2003) Etiology of congenital diaphragmatic hernia: the retinoid hypothesis. Pediatr Res 53: 726-730. [Crossref]
- Robinson PD, Fitzgerald DA (2007) Congenital diaphragmatic hernia. Pediatr Respir Rev 8: 323-334. [Crossref]
- Elhalaby EA, Abo Sikeen MH (2002) Delayed presentation of congenital diaphragmatic hernia. Pediatr Surg Int 18: 480-485. [Crossref]
- Bagłaj M (2004) Late-presenting congenital diaphragmatic hernia in children: a clinical spectrum. Pediatr Surg Int 20: 658-669. [Crossref]
- Vega MT, Maldonado RH, Vega GT, Vega AT, Liévano EA et al. (2013) Late-onset congenital diaphragmatic hernia: A case report. Int J Surg Case Rep 4: 952-954. [Crossref]
- Oh KS, Newman B, Bender TM, Bowne A (1988) Radiologic evaluation of the diaphragm. Radiol Clin North Am 26: 355-364. [Crossref]
- Yoonyoung YC, Aoife BR, Roy MK (2019) Congenital diaphragmatic hernia in a 2-year-old resembling a tension pneumothorax. J Pediatr Surg Case Rep 47: 101228.
- Ng J, Rex D, Sudhakaran N, Okoye B, Mukhtar Z (2013) Tension gastrothorax in children: introducing a management algorithm. J Pediatr Surg 48: 1613-1617. [Crossref]
- Naess PA, Wiborg J, Kjellevold K, Gaarder C (2015) Tension gastrothorax: acute life-threatening manifestation of late onset congenital diaphragmatic hernia (CDH) in children. Scand J Trauma Resusc Emerg Med 23: 49. [Crossref]
- Balks MF, Gosemann JH, Sorge I, Lacher M, Hirsch FW (2018) Congenital Diaphragmatic Hernia Presenting with Tension Pneumothorax in a 3-Year-Old Boy. European J Pediatr Surg Rep 6: e63-e65. [Crossref]
- Kajal P, Bhutani N, Goyal M, Kamboj P (2017) Iatrogenic gastric perforation in a misdiagnosed case of late presenting congenital diaphragmatic hernia: Report of an avoidable complication. Int J Surg Case Rep 41: 154-157. [Crossref]
- Wadhwa R, Ahmad Z, Kumar M (2014) Delayed traumatic diaphragmatic hernia mimicking hydropneumothorax. Indian J Anaesth 58: 186-189. [Crossref]
- Greer JJ (2013) Current concepts on the pathogenesis and etiology of congenital diaphragmatic hernia. Respir Physiol Neurobiol 189: 232-240. [Crossref]
