Diagnosis and Therapeutic Care of Infantile Myofibroma Of the Jaws: A Case Report and Literature Systematic Review
Diagnosis and Therapeutic Care of Infantile Myofibroma Of the Jaws: A Case Report and Literature Systematic Review
A B S T R A C T
Myofibroma is a rare and benign mesenchymal tumor defined by a proliferation of myofibroblastic tissue, most often affecting children. We report the clinical, radiological and histological features of a case of myofibroma of the mandible in a 3-year-old-girl, followed by a systematic review to define the diagnostic and therapeutic criteria.
The literature systematic review shows that the age of the patients was between a few weeks of life and the end of adolescence. our case includes most of the parameters encountered in the literature. 86% of the tumors affected the mandible versus 14% for the maxilla. Dental damages are possible. The clinical and radiological aspects are not typical. Only the histological examination confirms the diagnosis. The first-line treatment always includes surgical excision of the tumor. Recurrences are rare. This pathology requires the intervention of a multidisciplinary team and a regular follow-up.
Keywords
Child, jaw neoplasm, myofibroma
Introduction
Myofibroma is a rare and benign mesenchymal tumor defined by a proliferation of myofibroblastic tissue. They may be isolated (myofibroma) or disseminated (myofibromatosis). It most commonly occurs on children during the first decade [1, 2]. The most frequent locations are head and neck region. Bone involvement of the mandible and maxilla is relatively rare [3]. The preservation of deciduous or permanent teeth germs involved by tumor is a challenge for surgical management. The authors report a case of myofibroma of the mandible in a 3-year-old-girl. A systematic review on the myofibroma of the jaw bone focuses on its clinical and radiological features, followed by the treatment options.
Case-report
A 3-year-old girl was referred in the department of Paediatric Dentistry of the Hospital of Strasbourg for a facial asymmetry observed by her parents. The swelling, developed over for a few months, concerned the right side of the mandible. Concerning the familial history, the authors noted a Gardner’s syndrome of the father and grandfather. This type of familial adenomatous polyposis, linked to the APC gene mutation, is at the origin of multiple colorectal polyps. Osteomas may affect the jaw. Although genetic analysis in search of the APC gene mutation was strongly advised, parents did not want to perform the test on their kid. The older brother had been tested and carries the mutation, but he did not have any symptoms yet.
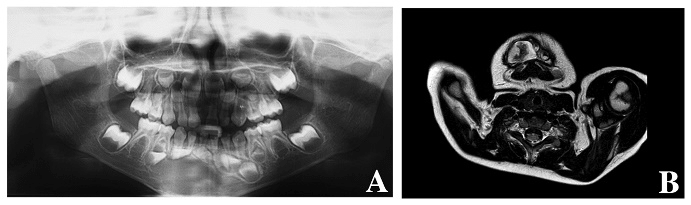
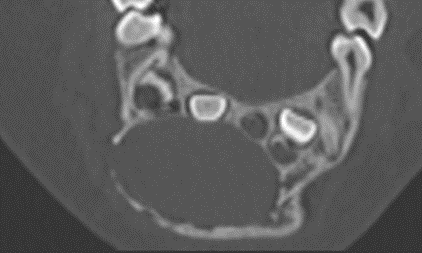
The clinical examination revealed a firm extensive mass, partly filling the vestibule. The oral mucosa was normal and free of ulceration. The patient had no pain, no lymphadenopathy and no difficulties in feeding. Primary teeth were intact, non-mobile and not displaced. The panoramic radiography revealed an ill-defined radiolucent lesion extending from the first left central incisor to the first right molar (Figure 1A). The germs of the permanent teeth were displaced. The teeth showed no radicular resorption. The CT scan showed a bone expansion with a cortical thinning and multiple perforations (Figure 2). The MRI revealed an heterogenous tumor whose size is 20,82 x 28,34 mm (Figure 1B).
Figure 1 A: Panoramic radiography showing an extensive radiolucent lesion of the mandible. B: Heterogenous aspect of the tumor on MRI (T2).
Figure 2 : CT scan showing multiple cortical perforations
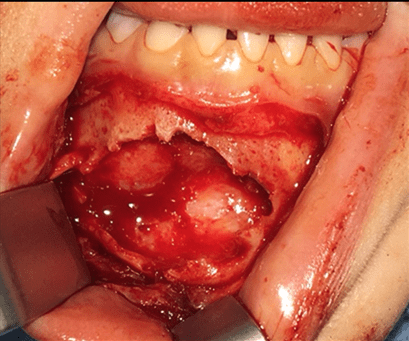
Figure 3: Visual appearance during surgical procedure
Figure 4: Hematoxylin-eosin staining
One of the difficulties was the potential for teeth and other odontogenic structures to be involved by tumor. Under general anesthesia, the vestibular approach revealed about ten cortical perforations. The tumor was whitish and grayish with a firm plurinodular aspect (Figure 3). The surgeon performed a complete resection of the tumor, respecting a safety margin of 5mm at the soft tissue level. The preservation of bone continuity and the integrity of permanent teeth was a challenge. However, the residual bone around the germs guided the surgeon to preserve them. The sampling was sent for anatomopathological analysis. The tumor consisted of a proliferation of spindled cells arranged in a fascicular pattern (Figure 4A). The myofibroblasts with small nuclei showed no atypia. A hemangyiopericytoma like pattern gave the lesion a ramified vascular aspect (Figure 4B). The clinical and radiologic follow-up allowed to control the bone healing, the evolution of the permanent teeth and the absence of recurrence. The patient had been on follow up for 18 months without any tumor recurrence.
Review
The objective of this review is to analyze the cases reported in the literature, to define the clinical and radiological features of myofibroma and myofibromatosis affecting the jaw bones.
Methods
Two independent researchers selected the articles, first from the abstracts and then from the full text. Only cases involving the mandible or the maxilla in children were selected. There were no restrictions regarding sex, general health, date of therapeutic management and health professional. MEDLINE, COCHRANE, GOOGLE SCHOLAR and OPENGREY databases were used with the following key words: (myofibromatosis AND mandible) OR (myofibroma AND mandible) OR (myofibromatosis AND jaw) OR (myofibroma AND jaw) OR (myofibromatosis AND maxillary) OR (myofibroma AND maxillary). All types of articles were retained, including case reports. The duplicate records were excluded. The following data were extracted from the articles: author, year, number of patients, age, localization, medical history, circumstances of discovery, clinical data, imaging exams, radiographic characteristics, histological analysis, treatment, recurrence/follow-up. Descriptive statistics were computed.
Results
The research strategy retained 61 articles. After screening the titles and abstracts, 41 manuscripts were selected for full text reading. The excluded items were due to the age of the patients, the localization of the tumor or diagnosis (one case of myofibroblastoma). After full reading, 4 articles were rejected because of other localization or other diagnosis. Finally, 35 articles published between 1984 and 2017 and one article submitted for publication were selected for the review [1-35]. The flow chart diagram of the systematic review is depicted in (Figure 5)
Figure 5: Flow-chart of the search strategy
In total, 90 cases of myofibromatosis or myofibroma of the jaw were listed in the literature, with only 3 articles reporting myofibromatosis with involvement of the mandible or maxilla [9, 17, 25]. Multicentric forms with mandibular or maxillary involvement are therefore quite rare. The age of the patients at the time of surgery was between a few weeks of life and the end of adolescence [9, 10, 17, 27]. 11% were less than 1 year old and 34% less than 10 years old. 54% were males and 46% females. Looking at medical history, trauma was reported for 4 children but most of the time the history did not bring a contributory information [5, 16, 30, 32]. 56 patients visited the pratician following the discovery of a mass, a swelling, a facial asymmetry, a hypoesthesia, or a trismus [2, 4-7, 11, 13, 18, 20, 21, 23-27, 29-34]. The youngest case was referred by his parents for decreased oral intake and bleeding from the hard palate [17]. Seven cases were discovered during routine examination [1, 16, 28]. The majority of the tumors affected the mandible (86% versus 14% for the maxilla). At the mandible, the posterior lateral sectors were the most affected (93%). The right side seemed twice more involved. Concerning clinical examination, the oral mucosa was considered as normal state in most cases. The literature reported only 2 cases of ulcerations [1, 17]. Palpation revealed a firm mass in all cases. The lesions were almost always painless and nerve implication rare (only one case of hypoesthesia) [26].
Regarding the teeth, only two patients presented tooth mobility but no loss of vitality was noted [1, 13]. 12 patients had dental displacements, affecting most off the time the germs. Concerning imaging exams, most practitioners used conventional radiography and CT scan. Panoramic radiography is used to have an overview. MRI was not realized systematically (only 6 patients). In case of suspicion of multicentric myofibromatosis, other examinations could be performed like skull X-ray, chest X-ray, abdominal ultrasound, Body-CT-scan. Radiographic exams revealed in more than half of cases bone expansion and cortical thinning. 19 cases reported cortical perforations. 16 lesions were well-defined and 5 described as ill-defined radiolucency. Approximately half of the lesions were unilocular and the rest had a multilocular appearance. Displacements of the germs or the teeth located near the tumor were often described but the dental resorptions are evoked only in 4 cases. These resorptions could concern one or more teeth. The first-line treatment always included surgical excision under general anesthesia. In the case of myofibromatosis with a location in the maxilla in a 2-year-old child, chemotherapy using vinblastin and methotrexate was performed before complete surgical resection [17]. Once the intra- or extra-oral surgical approach was performed, the tumor was always described as firm and white, yellow or grey. Many of the authors described it as non-encapsulated and non-bleeding solid tumor. In most cases the tumors were easily removed from their bone site. Most of surgical resections were performed in order to get margins free of tumor. The determination of these margins did not bring out a consensus, ranging from bone curettage to margins of one centimeter [20]. 3 cases required interruptive resection of the mandible [21, 31, 32]. 12 surgical procedures required removal of teeth or germs and in one case the teeth were preserved at first and then finally extracted after a recurrence [36].
Table 1: Immunohistochemical data for myofibroma
|
|
Actin |
Vimentin |
Calponin |
HHF-35 |
Desmin |
P-63 |
CD-34 |
CD-31 |
Protein S-100 |
H-caldesmon |
Keratin |
Neuron specific enolase |
|
Abramowicz, 2012 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Allon, 2007 |
+ |
+ |
|
|
|
|
|
|
|
|
|
|
|
Chattaraj, 2017 |
+ |
+ |
+ |
|
- |
- |
|
|
|
- |
|
|
|
Chtourou, 2007 |
+ |
+ |
|
|
- |
|
- |
|
- |
|
|
|
|
Damera, 2013 |
+ |
+ |
|
+ |
|
|
|
|
|
|
|
|
|
Ech Charif, 2008 |
+ |
+ |
|
|
|
|
|
|
|
|
|
|
|
Haspel, 2012 |
+ |
|
|
|
- |
|
|
- |
- |
- |
- |
|
|
Jones, 1994 |
+ |
+ |
|
|
- |
|
|
|
|
|
|
|
|
Kaur, 2016 |
+ |
+ |
|
|
|
|
|
|
- |
|
|
|
|
Lingen, 1995 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
Lopes, 2015 |
+ |
+ |
|
|
- |
|
- |
|
- |
|
- |
|
|
Mahajan, 2017 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Matthews, 1990 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Montgomery, 2000 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Nirvikalpa, 2011 |
+ |
+ |
|
|
- |
|
|
|
- |
|
- |
|
|
Odell, 2004 |
|
+ |
|
|
|
|
|
|
|
|
|
|
|
Oudijk, 2012 |
+ |
|
|
|
- |
|
|
|
|
|
|
|
|
Rai, 2014 |
+ |
|
|
|
- |
|
+ |
|
- |
|
|
|
|
Rokos, 2011 |
+ |
|
|
|
- |
|
- |
|
- |
- |
|
|
|
Sheper, 2005 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Shibuya, 2008 |
+ |
+ |
|
|
- |
|
- |
|
- |
|
|
|
|
Souza, 2009 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
Sugatani, 1995 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
- |
|
Tabrizi, 2013 |
+ |
|
|
|
- |
|
|
|
|
|
|
|
|
Urs, 2014 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
Vigneswaran, 1992 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
White, 2015 |
+ |
|
|
|
|
|
|
|
|
|
|
|
Histological examination confirmed the diagnosis of myofibroma. Hematoxylin eosin or Masson’s trichrome staining highlighted spindled cell proliferation of myofibroblast and fibroblast arranged in fascicles alternating with hemangiopericytoma-like pattern. Few or no mitosis were observed. The histological features showed no difference between solitary myofibromas and multicentric forms. Myofibroma was always immunoreactive for actin and vimentin and typically negative for desmin and protein S-100 (Table 1). Recurrences were rare (only 2) and most of the time attributable to incomplete excision or to multicentric forms [7, 36]. The follow-up ranged from 6 months to 17 years (mean 4 years). One of the cases of maxillary myofibroma recurred twice despite surgical management. The medical team chose a chemotherapy approach for the second recurrence using vincristine, actinomycin and cyclophosphamide. It allowed a regression of the tumor
Discussion
27 selected articles reported only one case, 5 articles reported between two and four cases and only two large case series were identified (12 and 34 cases). Thus, the authors included all the articles in their review strategy, even the case-reports. The etiology of myofibroma is unknown and the diagnosis is usually realized after the appearance of swelling, as in the case presented previously, or through routine radiographic examination. As described in most cases, the patient reported in this case-report had a healthy gum without ulceration. This lesion presented the features of a myofibroma, which are most commonly found in literature : firm and painless mass, with no symptomatic nervous involvment, radiolucent with bone expansion. This mandibular localization is the most frequently encountered. However, the reported localizations are more often posterior whereas in this case the tumor is rather anterior. Although dental lesions are reported in some cases of the literature, our patient had no abnormalities in temporary teeth, no displacement or mobility, but the germs of the permanent teeth were displaced.
The symptomatology is not specific, and the clinical examination alone is not sufficient to establish the diagnosis. Complementary CT scan or MRI imaging examinations allow the patrician to perform differential diagnosis and to approach the surgical phase in optimal conditions. The radiological aspect is not constant. Lesions are not well-defined, with or without internal calcifications. Only the histological analysis of the anatomical piece leads to the diagnosis of myofibroma. For the case reported previously, only the histological analysis allowed to establish the diagnosis, the appearance of the tumor in situ was not typical. The use of chemotherapy remains uncommon but seems to give satisfactory results in multicentric forms or for extensive tumors. It seems to result in the regression of the tumor before resection or even in complete disappearance. There is no consensus about the choice of therapeutic molecules. This medical treatment could avoid extensive interruptive resection of the mandible, particularly crippling during growth in children.
Conclusion
Myofibroma of the jaw is a rare tumor in children and literature mainly gathers case-reports. Its therapeutic management requires the intervention of a multidisciplinary team including surgeons, oncologists, radiologists and anatomopathologists. Indeed, the diagnosis must be confirmed by the histological examination of the anatomical piece. Most often surgical resection is preferred, and the recurrence rate of the tumor is low. Chemotherapy treatments are mentioned to treat the most aggressive forms. A conservative approach and regular follow-up seems to be a good consensus for the management of myofibroma in children.
Acknowledgments
The authors report no conflicts of interest related to this study. The authors thank Dr. Luc Marcellin for histology.
Article Info
Article Type
Case ReportPublication history
Received: Wed 24, Apr 2019Accepted: Thu 18, Jul 2019
Published: Fri 30, Aug 2019
Copyright
© 2023 Marion Strub. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2019.03.02
Author Info
François Clauss Caroline Dissaux Eléonore Blein Marie-Cécile Maniere Marion Strub
Corresponding Author
Marion StrubHôpitaux Universitaires de Strasbourg, Department of Pediatric Dentristry, 1 place de l'Hôpital, 67000 Strasbourg, France
Figures & Tables
Table 1: Immunohistochemical data for myofibroma
|
|
Actin |
Vimentin |
Calponin |
HHF-35 |
Desmin |
P-63 |
CD-34 |
CD-31 |
Protein S-100 |
H-caldesmon |
Keratin |
Neuron specific enolase |
|
Abramowicz, 2012 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Allon, 2007 |
+ |
+ |
|
|
|
|
|
|
|
|
|
|
|
Chattaraj, 2017 |
+ |
+ |
+ |
|
- |
- |
|
|
|
- |
|
|
|
Chtourou, 2007 |
+ |
+ |
|
|
- |
|
- |
|
- |
|
|
|
|
Damera, 2013 |
+ |
+ |
|
+ |
|
|
|
|
|
|
|
|
|
Ech Charif, 2008 |
+ |
+ |
|
|
|
|
|
|
|
|
|
|
|
Haspel, 2012 |
+ |
|
|
|
- |
|
|
- |
- |
- |
- |
|
|
Jones, 1994 |
+ |
+ |
|
|
- |
|
|
|
|
|
|
|
|
Kaur, 2016 |
+ |
+ |
|
|
|
|
|
|
- |
|
|
|
|
Lingen, 1995 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
Lopes, 2015 |
+ |
+ |
|
|
- |
|
- |
|
- |
|
- |
|
|
Mahajan, 2017 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Matthews, 1990 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Montgomery, 2000 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Nirvikalpa, 2011 |
+ |
+ |
|
|
- |
|
|
|
- |
|
- |
|
|
Odell, 2004 |
|
+ |
|
|
|
|
|
|
|
|
|
|
|
Oudijk, 2012 |
+ |
|
|
|
- |
|
|
|
|
|
|
|
|
Rai, 2014 |
+ |
|
|
|
- |
|
+ |
|
- |
|
|
|
|
Rokos, 2011 |
+ |
|
|
|
- |
|
- |
|
- |
- |
|
|
|
Sheper, 2005 |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
Shibuya, 2008 |
+ |
+ |
|
|
- |
|
- |
|
- |
|
|
|
|
Souza, 2009 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
Sugatani, 1995 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
- |
|
Tabrizi, 2013 |
+ |
|
|
|
- |
|
|
|
|
|
|
|
|
Urs, 2014 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
Vigneswaran, 1992 |
+ |
+ |
|
|
- |
|
|
|
- |
|
|
|
|
White, 2015 |
+ |
|
|
|
|
|
|
|
|
|
|
|


References
- Abramowicz S, Simon LE, Kozakewich HP, Perez-Atayde AR, Kaban LB et al. (2012) Myofibromas of the jaws in children. J Oral Maxillofac Surg 70: 18804-1884. [Crossref]
- Allon I, Vered M, Buchner A, Dayan D (2007) Central (intraosseous) myofibroma of the mandible: clinical, radiologic, and histopathologic features of a rare lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103: 45-53. [Crossref]
- Chang JYF, Kessler HP (2008) Masson trichrome stain helps differentiate myofibroma from smooth muscle lesions in the head and neck region. J Formos Med Assoc 107: 767-773. [Crossref]
- Chattaraj M, Gayen S, Chatterjee RP, Shah N, Kundu S (2017) Solitary Myofibroma of the Mandible in a Six-Year Old-Child: Diagnosis of a Rare Lesion. J Clin Diagn Res 11: 13-15. [Crossref]
- Chtourou I, Krichen Makni S, Dhouib M, Khabir A, Fakhfakh I et al. (2007) Pediatric mandibular myofibromatosis. Rev Stomatol Chir Maxillofac 108: 461-464. [Crossref]
- Damera NCR, Vallabhaneni KC, Tripuraneni SC, Madala S, Diddi RR (2013) Non malignant maxillary lesions: our experience. Indian J Otolaryngol Head Neck Surg 65: 74-79. [Crossref]
- Ebert CS Jr, Zdanski C, Ardeshirpour F, Patel M, Hart CF et al. (2008) Recurrent infantile myofibromatosis: a report of conservative management and discussion of treatment strategies. Ear Nose Throat J 87: E4. [Crossref]
- Ech-Charif S, Benhammou A, Maher M, Séfiani S (2008) Solitary myofibroma of the mandible: a case report. Rev Laryngol Otol Rhinol (Bord) 129: 337-340. [Crossref]
- Foss RD, Ellis GL (2000) Myofibromas and myofibromatosis of the oral region: A clinicopathologic analysis of 79 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89: 57-65. [Crossref]
- Haspel AC, Coviello VF, Stevens M, Robinson PG (2012) Myofibroma of the mandible in an infant: case report, review of the literature, and discussion. J Oral Maxillofac Surg 70: 1599-1604. [Crossref]
- Jones AC, Freedman PD, Kerpel SM (1994) Oral myofibromas: A report of 13 cases and review of the literature. J Oral Maxillofac Surg 52: 870-875. [Crossref]
- Kadlub N, Kreindel T, Belle Mbou V, Coudert A, Ansari E et al. (2014) Specificity of paediatric jawbone lesions: tumours and pseudotumours. J Craniomaxillofac Surg 42: 125-131. [Crossref]
- Kaur P, Chowalta R, Lata J (2016) Central myofibroma of the maxilla. Contemp Clin Dent 7: 71-74. [Crossref]
- Kim JS, Kim SE, Kim JD (2006) Myofibroma of the mandible: A case report. Korean J Oral Maxillofac Radiol 36: 211-215.
- Lingen MW, Mostofi RS, Solt DB (1995) Myofibromas of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 80: 297-302. [Crossref]
- Lopes RN, Alves F de A, Rocha AC, Suassuna TM, Kowalski LP et al. (2015) Head and neck solitary infantile myofibroma: Clinicopathological and immunohistochemical features of a case series. Acta Histochem 117: 431-436. [Crossref]
- Mahajan P, Hicks J, Chintagumpala M, Venkatramani R (2017) Myofibroma in Infancy and Childhood. J Pediatr Hematol Oncol 39: e136-e139. [Crossref]
- Matthews MS, Tabor MW, Thompson SH, Gross PD (1990) Infantile myofibromatosis of the mandible. J Oral Maxillofac Surg 48: 884-889. [Crossref]
- Montgomery E, Speight PM, Fisher C (2000) Myofibromas presenting in the oral cavity: A series of 9 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89: 343-348. [Crossref]
- Nirvikalpa N, Narayanan V (2011) Intraosseous infantile myofibroma of the mandible. Ann Maxillofac Surg 1: 87-90. [Crossref]
- Odell EW, Aldred M, Carlos R, Curran A, Heikinheimo K et al. (2004) Clinico-pathological conference 2002. Ann Acad Med Singap 33: 53-58. [Crossref]
- Oudijk L, den Bakker MA, Hop WCJ, Cohen M, Charles AK et al. (2012) Solitary, multifocal and generalized myofibromas: clinicopathological and immunohistochemical features of 114 cases. Histopathology 60: E1-E11. [Crossref]
- Rai B, Ludusan E, McGovern B, Sharif F (2014) Mandibular swelling in a 5-year-old child--mandibular myofibroma. BMJ Case Rep 2014. [Crossref]
- Rokos J, Carlos R, Romañach MJ (2011) Clinical Pathologic Conference Case 6: Infantile Myofibroma. Head Neck Pathol 5: 292-295. [Crossref]
- Scheper MA, DiFabio VE, Sauk JJ, Nikitakis NG (2005) Myofibromatosis: A case report with a unique clinical presentation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99: 325-330. [Crossref]
- Shibuya Y, Takeuchi J, Sakaguchi H, Yokoo S, Umeda M et al. (2008) Myofibroma of the mandible. Kobe J Med Sci 54: E169-E173. [Crossref]
- Slootweg PJ, Müller H (1984) Localized infantile myofibromatosis. Report of a case originating in the mandible. J Maxillofac Surg 12: 86-89. [Crossref]
- Souza DP, Loureiro CC, Rejas RA, Sousa SO, Raitz R (2009) Intraosseous myofibroma simulating an odontogenic lesion. J Oral Sci 51: 307-311. [Crossref]
- Sugatani T, Inui M, Tagawa T, Seki Y, Mori A et al. (1995) Myofibroma of the mandible. Clinicopathologic study and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 80: 303-309. [Crossref]
- Tabrizi R, Bahramnejhad E, Kazemi H, Asadzadeh M, Ranjbaran H (2013) Rapidly growing lesions involving the maxilla in infants: a two-case presentation and deferential diagnosis. J Craniofac Surg 24: 434-438. [Crossref]
- Troulis MJ, Williams WB, Kaban LB (2004) Staged protocol for resection, skeletal reconstruction, and oral rehabilitation of children with jaw tumors. J Oral Maxillofac Surg 62: 335-343. [Crossref]
- Urs AB, Mohanty S, Arora S, Augustine J, Kumar P et al. (2014) Pediatric solitary intraosseous infantile myofibroma of the mandible. J Dent Child 81: 42-46. [Crossref]
- Venkatesh V, Kumar BP, Kumar KA, Mohan AP (2015) Myofibroma-a rare entity with unique clinical presentation. J Maxillofac Oral Surg 14: 64-68. [Crossref]
- Vigneswaran N, Boyd DL, Waldron CA. Solitary infantile myofibromatosis of the mandible. Report of three cases. Oral Surg Oral Med Oral Pathol 73: 84-88. [Crossref]
- White D, Franklin LA (2015) Clinical pathologic conference case 3: a 15-year-old male with a radiolucent jaw lesion. Oral Surg Oral Med Oral Pathol Oral Radiol 119: e295-e297. [Crossref]
