Journals
Detailed clinical data of The Cancer Genome Atlas muscle-invasive bladder cancer database with focus on gender and ethnic stratification
A B S T R A C T
Introduction: Bladder cancer is one of the most common neoplasms, especially in men. About 25% of the patients present with the more aggressive type of bladder cancer, the muscle-invasive type, which carries a significant risk of death. The Cancer Genome Atlas (TCGA) had recently published extensive genomic data of 412 muscle-invasive bladder cancer (MIBC) patients, with modest clinical information. The aim of the current study was to analyze several aspects of the clinical data of TCGA bladder cancer database.
Methods: Using NCI Genomic Data Commons portal and cBioPortal, we extracted data on 412 patients with MIBC from the TCGA database. We then analyzed and statistically compared these data using different statistical methods. Aside from clinical data, we also extracted data on disease-free and overall survival.
Results: The database included 104 female patients and 297 male patients. The vast majority were Caucasians (79.0%), followed by Asians (11%) and African-Americans (5.5%). The female group was older than the male group (70.0 vs 67.3 years, P=0.02). Incidental prostate cancer was seen in 82 male patients (27.6%). Kaplan-Meier curves for disease-free survival and overall survival showed no difference between male and female patients, and no difference between the three ethnics groups.
Conclusions: TCGA provides an invaluable source for genomic alterations of MIBC. In this study, we have shown that it also provides adequate clinical information that can be compared with genomic data and future studies. No statistically significant differences were shown in disease-free and overall survival between male and female patients in the TCGA MIBC database.
K E Y W O R D S
Muscle-invasive bladder cancer, overall survival, TCGA
I N T R O D U C T I O N
Bladder cancer is one of the most common neoplasms in humans, and it is predicted that 85,991 Americans will have a new diagnosis of bladder cancer in 2020 and 141,469 new cases of bladder cancer will be diagnosed in the European Union in the same year [1]. Muscle-invasive bladder cancer (MIBC) represents only about 25% of patients diagnosed with bladder cancer; however, it carries a significant risk of death [2].
Although men are more likely to develop bladder cancer than women, women tend to present with more advanced disease [2]. However, whether this translates into worse survival rates is still debatable. While a meta-analysis of nearly 28,000 patients showed a worse survival in women compared to men [3], several other studies showed no difference in recurrence-free or survival rates [4, 5]. Moreover, survival and recurrence data on non-Caucasians patients are scarce.
TCGA has recently published the genomic data of 412 MIBC patients in their database. They reported the clinical data of the patients, though briefly. The aim of this study was to extensively report the clinical, survival and recurrence data of TCGA MIBC database, focusing on gender and ethnic background differences.
Materials and methods
Using the NCI Genomic Data Commons portal [6] and cBioPortal [7, 8], clinical and survival data of 412 patients with MIBC from the TCGA database were extracted. We excluded 11 patients who had M1 disease. Patients were then divided into groups based on gender (male vs female) and ethnics (Caucasian vs African American vs Asian).
Baseline characteristics were compared using t-test or Fisher’s exact test, as appropriate. Kaplan-Meier curves were generated for recurrence-free survival and overall-survival based on gender and ethnic groups. Kaplan-Meier curves were statistically compared using log-rank test. Two-tailed Pvalue of < 0.05 was considered statistically significant. All statistical analyses were done using SPSS v22 (IBM corp., Armonk, NY).
Tables
Table 1. Baseline, clinical and pathological data of female and male patients
|
Parameter |
Males (n=297) |
Females (n=104) |
P value |
|
Age years Median Range |
67.3±10.6 68 34-90 |
70.0±10.5 72.5 43-90 |
0.02 |
|
Ethnicity no.(%) Caucasian African American Asian NR |
244 (80.3) 13 (4.3) 36 (11.8) 11 (3.6) |
83 (76.8) 10 (9.3) 8 (7.4) 7 (6.5) |
0.07 |
|
AJCC Stage no.(%) I II III IV |
2 (0.7) 98 (32.9) 102 (34.3) 95 (31.9) |
0 (0) 33 (31.7) 40 (38.5) 31 (29.8) |
0.7 |
|
Pathological T no.(%) pT1 pT2 pT3 pT4 NR |
3 (1.0) 91 (30.6) 137 (46.2) 43 (14.5) 23 (7.7) |
0 (0) 29 (27.8) 53 (51.0) 14 (13.5) 8 (7.7) |
0.6 |
|
Pathological N no.(%) pN0 pN1 pN2 pN3 NR |
174 (57.2) 38 (12.5) 55 (18.1) 6 (2.0) 31 (10.2) |
65 (60.2) 9 (8.3) 21 (19.4) 2 (1.9) 11 (10.2) |
0.7 |
|
History of NMIBC* no.(%) |
52 (17.5) |
10 (9.6) |
0.05 |
|
Incidental Prostate cancer no.(%) |
82 (27.6) 7 (8.5) 0 |
- |
- |
AJCC - American Joint Committee on Cancer; NR – not reported; NMIBC – non-muscle invasive bladder cancer; *data is missing on ~25% of patients
Table 2. Subgroup analysis based on ethnicity
|
Parameter |
Caucasians (n=317) |
African Americans (n=22) |
Asians (n=44) |
P value |
|
Gender, no.(%) |
236 (74.4) 81 (25.6) |
13 (59.0) 9 (41.0) |
36 (81.8) 8 (18.2) |
0.1 |
|
Age years Median Range |
68.9±10.0 70.0 44-90 |
67.1±11.8 66.0 43-90 |
62.7±12.7 62.5 34-85 |
0.003* |
|
AJCC Stage no.(%) I II III IV |
1 (0.3) 87 (27.4) 116 (36.6) 113 (35.6) |
0 (0) 8 (36.4) 8 (36.4) 6 (27.2) |
1 (2.3) 30 (68.2) 11 (25.0) 2 (4.5) |
0.0001 |
|
Pathological T no.(%) pT1 pT2 pT3 pT4 NR |
2 (0.6) 74 (23.4) 163 (51.4) 50 (15.8) 28 (8.8) |
0 (0) 8 (36.4) 7 (31.8) 5 (22.7) 2 (9.1) |
1 (2.3) 30 (68.2) 12 (27.2) 0 (0) 1 (2.3) |
0.0001 |
|
History of NMIBC* no.(%) |
57 (17.9) |
1 (4.5) |
1 (2.3) |
0.009 |
|
Incidental PCa no.(%) |
76 (23.9) |
3 (13.6) |
0 (0) |
0.001 |
R e s u l t s
TCGA database included 104 female patients (25.9%) and 297 male patients (74.1%). The vast majority of the cohort included Caucasian patients (79.0%), followed by Asian ethnicity (11%) and lastly by African-Americans (5.5%). Ethnicity was not reported for 18 patients (4.5%). As shown in table 1, female patients were older than male patients with mean age of 70.010 years compared to 67.310 years (P=0.02).
AJCC staging showed an almost equal distribution between stages II, III and IV. Pathological T staging showed pT2 in only 30.0% of men and 27.8% of women. All other patients (except 3 males) had pT3 or higher. Most of the patients had uninvolved pelvic lymph nodes; however, almost third of the men and 28% of the women had pathologically confirmed positive lymph nodes (data was not available on 10% of the patients).
In table 2, we summarized the differences between the 3 ethnic groups. The male-to-female ratio was different between the groups, but not statistically significant. However, compared to Caucasians, Asians were, in average, 6 years younger (P=0.003). Asians patients presented with more favorable AJCC stages, with only 4.5% diagnosed at AJCC stage IV, compared to 27.2% of African Americans and 35.6% of Caucasians (P=0.0001). African Americans and Asians were far less likely to have a history of NMIBC compared to Caucasians (P=0.009). Lastly, out of 44 Asians men, none was diagnosed with concomitant prostate cancer, compared to 13.6% of African Americans and 23.9% of Caucasians (P=0.001). The overall rate of incidental prostate cancer for the studied male patients was 27,6%. Of the 82 males in this group none had stage pT4, 7 were staged as pT3 and all the others were staged at pT2c or lower. Gleason scores were not reported.
Data on the history of non-muscle invasive bladder cancer (NMIBC) was only available for 74.3% of the patients; analyzing these data showed that only 62 patients (20.8%) of the entire cohort had a known history of NMIBC. Women had much lower incidence of NMIBC before diagnosing MIBC (9.6% vs 17.5%, P=0.05).
Neo-adjuvant chemotherapy was administered to 10 patients only. Following radical cystectomy, 77 patients were treated with adjuvant Chemotherapy and 10 patients were treated with adjuvant Radiotherapy.
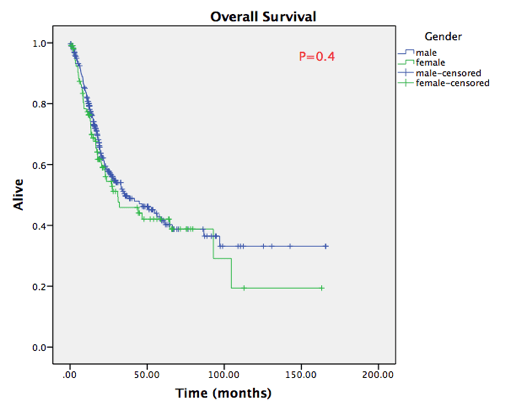
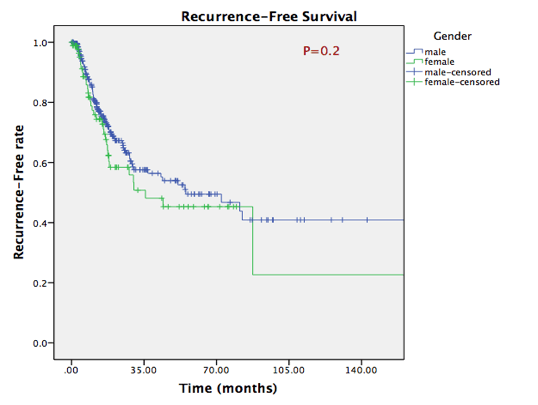
During the follow-up, recurrence was observed in 40 female patients (38%) and 97 male patients (33.6%), with no statistically significant difference (P=0.4). Overall, 125 males and 49 females died during follow-up (cancer and non-cancer deaths). Median survival estimate was 32 months in Caucasians, 22 months in African Americans and 55 months in Asians. However, no statistically significant differences were seen in recurrence-free survival or overall survival rates between female and male patients, as shown in figure 1A. No statistically significant differences in survival rates were shown in any ethnic sub-group as well (figure 1B).
D i s c u s s i o n
Several large studies published in late 1990s reported that women with MIBC have worse survival compared to men with MIBC [9-13]. Mungan et al showed that women with bladder cancer (urothelial or non-urothelial) were diagnosed with higher stages than men [14]. This could, at least partially, explain the survival disadvantage for women. Other studies from the same time period did not show any difference in gender-specific survival among bladder cancer patients [15-17].
Most of these articles have some limitations; some included NMIBC patients in their analysis, and others are relatively old and do not represent the current standard of care. In a review on the effect of gender on bladder cancer, Shariat et al [18] summarized three recent publications on this topic, showing survival advantage for men over women [19-21]. Two studies have used the Surveillance, Epidemiology and End Results (SEER) data base [20, 21], and one of them failed to show survival advantage for men over women in stages I-III of their disease [20]. Explanations provided for this disparity, included higher stage at presentations, longer time to diagnosis and inequalities in healthcare [22]. The current study which is based on TCGA data base, as we eluded before, included only MIBC patients who underwent radical cystectomy according to contemporary standard of care.
Although there were several ‘advantages’ for Asians over the other two subgroups, such as younger age at diagnosis, lower T and AJCC stages and less history of NMIBC, as shown in table 2, our study showed a largely similar recurrence-free and overall survivals in the three ethnics. This could be a result of small percentage of Asians in this cohort. In fact, a large study from Japan showed similar stage distribution to Caucasians in TCGA cohort and similar mean age at diagnosis to Caucasians in our study [23].
Its noteworthy that patients in TCGA database have higher risk disease than most reference cystectomy series, and therefore have lower disease-free survival rates [24]. This partly could be explained by the fact that many of the patients included in this data base were from high volume academic centers and their disease characteristics reflect a referral pattern of more difficult cases. However, the outcomes were non-inferior when adjusted for the risk of disease, as Seiler et al showed [24].
In this cohort of patients, incidental prostate cancer was diagnosed in 27.6% of male patients. Such proportion is relatively low compared to old studies (around 40%) and may be the results of the widespread use of PSA testing in the USA that have led to diagnosis of more prostate cancer before cystectomy This percentage, however is highly consistent with contemporary studies, as was shown in a review by Damiano et al [25]. It should be mentioned that none of these patients was Asians; this is in contrast to other studies reporting a similar incidence of incidental prostate cancer in Asians and Caucasians [26].
Though included only MIBC, TCGA still has some inherent limitations, the most important of which is the fact the database included non-consecutive patients from several centers, and excluded patients who received neo-adjuvant chemotherapy. This could lead to an obvious selection bias. Moreover, although the clinical data is extensive, some patients do lack some data. Lastly, patients in this cohort had more aggressive disease than most reported series in the literature. Regarding the non-inferiority in women survival compared to men, a large, contemporary meta-analysis should probably be done to answer this question.
A c k n o w l e d g e m e n t s
The results published here are based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/
Conflicts of interest
None
Article Info
Article Type
Research ArticlePublication history
Received: Sat 03, Mar 2018Accepted: Sun 11, Mar 2018
Published: Mon 26, Mar 2018
Copyright
© 2023 Zaher Bahouth. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2018.10.003
Author Info
Ofer Nativ Sarel Halachmi Yasmine Ghantous Zaher Bahouth
Corresponding Author
Zaher BahouthDepartment of Urology, Bnai-Zion Medical Center, Haifa, Israel
Figures & Tables
Table 1. Baseline, clinical and pathological data of female and male patients
|
Parameter |
Males (n=297) |
Females (n=104) |
P value |
|
Age years Median Range |
67.3±10.6 68 34-90 |
70.0±10.5 72.5 43-90 |
0.02 |
|
Ethnicity no.(%) Caucasian African American Asian NR |
244 (80.3) 13 (4.3) 36 (11.8) 11 (3.6) |
83 (76.8) 10 (9.3) 8 (7.4) 7 (6.5) |
0.07 |
|
AJCC Stage no.(%) I II III IV |
2 (0.7) 98 (32.9) 102 (34.3) 95 (31.9) |
0 (0) 33 (31.7) 40 (38.5) 31 (29.8) |
0.7 |
|
Pathological T no.(%) pT1 pT2 pT3 pT4 NR |
3 (1.0) 91 (30.6) 137 (46.2) 43 (14.5) 23 (7.7) |
0 (0) 29 (27.8) 53 (51.0) 14 (13.5) 8 (7.7) |
0.6 |
|
Pathological N no.(%) pN0 pN1 pN2 pN3 NR |
174 (57.2) 38 (12.5) 55 (18.1) 6 (2.0) 31 (10.2) |
65 (60.2) 9 (8.3) 21 (19.4) 2 (1.9) 11 (10.2) |
0.7 |
|
History of NMIBC* no.(%) |
52 (17.5) |
10 (9.6) |
0.05 |
|
Incidental Prostate cancer no.(%) |
82 (27.6) 7 (8.5) 0 |
- |
- |
AJCC - American Joint Committee on Cancer; NR – not reported; NMIBC – non-muscle invasive bladder cancer; *data is missing on ~25% of patients
Table 2. Subgroup analysis based on ethnicity
|
Parameter |
Caucasians (n=317) |
African Americans (n=22) |
Asians (n=44) |
P value |
|
Gender, no.(%) |
236 (74.4) 81 (25.6) |
13 (59.0) 9 (41.0) |
36 (81.8) 8 (18.2) |
0.1 |
|
Age years Median Range |
68.9±10.0 70.0 44-90 |
67.1±11.8 66.0 43-90 |
62.7±12.7 62.5 34-85 |
0.003* |
|
AJCC Stage no.(%) I II III IV |
1 (0.3) 87 (27.4) 116 (36.6) 113 (35.6) |
0 (0) 8 (36.4) 8 (36.4) 6 (27.2) |
1 (2.3) 30 (68.2) 11 (25.0) 2 (4.5) |
0.0001 |
|
Pathological T no.(%) pT1 pT2 pT3 pT4 NR |
2 (0.6) 74 (23.4) 163 (51.4) 50 (15.8) 28 (8.8) |
0 (0) 8 (36.4) 7 (31.8) 5 (22.7) 2 (9.1) |
1 (2.3) 30 (68.2) 12 (27.2) 0 (0) 1 (2.3) |
0.0001 |
|
History of NMIBC* no.(%) |
57 (17.9) |
1 (4.5) |
1 (2.3) |
0.009 |
|
Incidental PCa no.(%) |
76 (23.9) |
3 (13.6) |
0 (0) |
0.001 |


References
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed December 20, 2017.
2. Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, et al. (2014) EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2013 Guidelines. Eur Urol 65: 778-792. [Crossref]
3. Liu S, Yang T, Na R, Hu M, Zhang L, et al. (2015) The impact of female gender on bladder cancer-specific death risk after radical cystectomy: a meta-analysis of 27,912 patients. Int Urol Nephrol 47: 951-958. [Crossref]
4. Patafio FM, Robert Siemens D, Wei X, Booth CM (2015) Is there a gender effect in bladder cancer? A population-based study of practice and outcomes. Can Urol Assoc J 9: 269-274. [Crossref]
5. Pichler R, Fritz J, Heidegger I, Oberaigner W, Horninger W, et al. (2017) Gender-related Outcome in Bladder Cancer Patients undergoing Radical Cystectomy. J Cancer 8: 3567-3574. [Crossref]
6. Grossman RL, Heath AP, Ferretti V, Varmus HE, Lowy DR, et al. (2016) Toward a Shared Vision for Cancer Genomic Data. N Engl J Med. 375: 1109-1112. [Crossref]
7. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, et al. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6: 1. [Crossref]
8. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, et al. (2012) The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov 2: 401-404. [Crossref]
9. Micheli A, Mariotto A, Giorgi Rossi A, Gatta G, Muti P (1998) The prognostic role of gender in survival of adult cancer patients. Eur J Cancer 34: 2271-2278. [Crossref]
10. Gatta G, Buiatti E, Conti E, et al. (1997) Variations in the survival of adult cancer patients in Italy. Tumori 83: 497-504.
11. Kiemeney LALM, Coebergh JWW, Koper NP, et al. (1994) Bladder cancer incidence and survival in the south-eastern part of the Netherlands, 1975–1989. Eur J Cancer 30: 1134-1137.
12. Horstmann M, Witthuhn R, Falk M, Stenzl A (2008) Gender-specific differences in bladder cancer: A retrospective analysis. Gend Med 5: 385-394. [Crossref]
13. Mungan NA, Aben KK, Schoenberg MP, Visser O, Coebergh JW, et al. (2000) Gender differences in stage-adjusted bladder cancer survival. Urology 55: 876-880. [Crossref]
14. Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA (2000) Gender differences in stage distribution of bladder cancer. Urology 55: 368-371. [Crossref]
15. Koch M, McPhee MS, Gaedke H, Williams Y (1988) Five-year follow-up of patients with cancer of the bladder--the Northern Alberta experience. Clin Investig Med Med Clin Exp 11: 253-258.
16. Levi F, Mezzanotte G, Van TC, La CV (1989) Cancer survival from the incident cases of the Registry of Vaud, Switzerland. Tumori 75: 83-89. [Crossref]
17. Timberg G, Rahu M, Gornoi K, Aareleid T, Baburin A (1997) Bladder Cancer in Estonia, 1968-1992: Incidence, Mortality, Prevalence and Survival. Scand J Urol Nephrol 31:337-342. [Crossref]
18. Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, et al. (2010) The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int 105: 300-308. [Crossref]
19. Umbreit EC, Crispen PL, Farmer SA, Blute ML, Frank I (2009) Postoperative surveillance model following cystectomy for clinically localized urothelial carcinoma of the bladder. J Urol 4: 629.
20. Datta GD, Neville B, Datta NS, Earle C (2006) Gender disparities in bladder cancer survival: An assessment of socio-demographic factors. Cancer Epidemiol Prev Biomark 15: 38.
21. Jeldres C, Isbarn H, Capitanio U, et al. (2009) Gender is an important predictor of cancer-specific survival in patient with transitional cell carcinoma after radical cystectomy. J Urol 4: 635.
22. Taub DA, Hollenbeck BK, Cooper KL, Dunn RL, Miller DC, et al. (2006) Racial disparities in resource utilization for cystectomy. Urology 67: 288-293. [Crossref]
23. Go Anan, Shingo Hatakeyama, Naoki Fujita, Hiromichi Iwamura, Toshikazu Tanaka, et al. (2017) Trends in neoadjuvant chemotherapy use and oncological outcomes for muscle-invasive bladder cancer in Japan: a multicenter study. Oncotarget 8: 86130-86142. [Crossref]
24. Seiler R, Black P, Thalmann G, Stenzl A, Todenhöfer T (2017) Clinical and outcome characteristics of the cancer genome atlas (TCGA) bladder cancer cohort: Is it representative? Eur Urol Suppl 16: 1558.
25. Damiano R, Di Lorenzo G, Cantiello F, De Sio M, Perdonà S, et al. (2007) Clinicopathologic Features of Prostate Adenocarcinoma Incidentally Discovered at the Time of Radical Cystectomy: An Evidence-Based Analysis. Eur Urol. 52: 648-657. [Crossref]
26. Yang X, Monn MF, Liu L, Liu Y, Su J, et al. (2015) Incidental prostate cancer in Asian men: high prevalence of incidental prostatic adenocarcinoma in Chinese patients undergoing radical cystoprostatectomy for treatment of bladder cancer and selection of candidates for prostate-sparing cystectomy. The Prostate 75: 845-854. [Crossref]
