Designing and Validating a New Method the TUNEL Microwave (TUNEL-MW) for Rapid Quantification of Apoptosis in Islets Following Isolation and Post-Thaw
Designing and Validating a New Method the TUNEL Microwave (TUNEL-MW) for Rapid Quantification of Apoptosis in Islets Following Isolation and Post-Thaw
A B S T R A C T
Background: Among the current quality control assays used in islet transplantation, there is an urgent need for more appropriate assays that measure cell damage via apoptosis that are accurate and rapid. Although the Terminal Uridine Nucleotide End Labeling (TUNEL) is a popular marker for apoptosis, the protocol takes 4 hours to complete. In this regard, microwave assisted histoprocessing, which shortens the time taken for processing, holds promise. Keeping this in mind, a new TUNEL Microwave (TUNEL-MW) method, for rapid quantification of apoptosis, was designed, developed and validated.
Method: Two lots of post-thaw isolated human islets cultured for 24 hours, 3 days, 5 days and 7 days i.e. 8 samples, were used for the study. Dewaxed and rehydrated tissues were processed for routine histology, stained with haematoxylin and eosin (H&E) and the conventional TUNEL was carried out as per manufacturer’s instructions. For the TUNEL-MW, kit instructions were modified and microwave-assisted histoprocessing was done. The assessment of apoptotic index (AI%) by light microscopy (LM) was carried out by a pathologist who was completely blinded to the study.
Results: The new TUNEL-Microwave (TUNEL-MW) developed by us reduced processing time from 4 hours to 30 minutes (saving 3½ hours). Results were validated by univariate linear regression (r2>0.990), coefficient of variation (<5% between all three methods) and the Bland Altman plot comparing AI% determined by the new TUNEL-MW with the conventional TUNEL and with LM (gold standard).
Conclusion: TUNEL Microwave appears to be an ideal method. It is simple and takes just 30 minutes to perform and can therefore be used along with existing quality control measures to rule out or measure apoptosis prior to islet release for islet transplantation.
Keywords
Islet transplantation, cryopreservation, post-thaw culture, islet isolation, TUNEL
Introduction
The Terminal Uridine Nucleotide End Labeling (TUNEL) assay is a popular method for the detection of apoptosis [1-18]. Apoptosis first described by Kerr et al. is the endpoint of an energy-dependent cascade of molecular events initiated by numerous stimuli [19-24]. It is a vital process and is a natural form of programmed cell death designed to eliminate unwanted cells. It occurs during development, during situations where tissue homeostasis must be restored, as a defense mechanism and following cell injury [19-24]. The relevance of this phenomenon to islet transplantation is that both cold and warm ischaemia of the post-mortem pancreas and islets on cryopreservation and indeed the very process of islet isolation itself are known to make islets vulnerable to apoptosis [25-28]. Ischaemia and hypoxia seen in post-mortem pancreata, isolated islets and in post-thaw isolated islets activate intracellular signals and cause the leakage of cytochrome c into the cytosol which results in a cascade of events that trigger apoptosis within the islets.
Similar molecular changes occur during the disruption of islet cell-matrix interactions with acinar tissue during islet isolation [29-39]. The loss of interstitial matrix and the basement membrane following poorly controlled digestion compromises islet cell viability and function, regulating both cell differentiation and survival leading to induction of apoptosis [29-39]. Given these conditions, the inference is that all islets for transplant, whether freshly isolated or post-thaw, are potentially vulnerable to apoptosis. Therefore, it is prudent that standard quality control measures prior to islet release for transplantation incorporate assays that measure apoptosis, thus ensuring the administration of a safe, pure, potent and viable product, namely the islets. Among the many assays specifically directed at measuring cell damage via apoptosis, current quality control assays used in islet transplantation are restricted to membrane integrity assays [40, 41]. However, loss of membrane integrity is more a measure of necrosis while the hallmark of apoptosis is loss of nuclear integrity [19-24, 40-54]. Another drawback of membrane integrity assay is that apoptotic bodies are known to exclude dyes and therefore underestimation of the percentage of damaged cells is a strong possibility [19-24, 40-54].
One of the reasons for the continued preference of membrane integrity as a measure of cell damage is that, while there are several more appropriate assays that measure cell damage via apoptosis and necrosis, they do not meet the criteria for their selection as quality control assays used in islet transplantation. Specifically, while a quality control assay must be sensitive, appropriate, repeatable and valid, it must also be rapid and simple to perform [23-36]. The other obstacle when evaluating cell damage in isolated or native islets is that not all assays are appropriate for cell clusters [40, 41]. Among the markers of apoptosis, cell morphology still serves as the gold standard for the identification of apoptosis and its distinction from necrosis [11, 22]. The light microscopic morphology of apoptosis includes shrinkage of cells, condensation of nuclear chromatin peripherally under the nuclear membrane and formation of apoptotic bodies by fragmentation of the cells and the nuclei [11, 22].
The TUNEL assay used to detect apoptosis is based on the concept that following the leakage of cytochrome c into the cytosol, caspases are activated which on hydrolysis cause cleavage of the cytoskeletal and nuclear matrix proteins. The cytoskeletal proteins are converted into shrunken cells (apoptotic bodies) and the nuclear DNA is broken down into 180 to 200 base pairs by Ca++ and Mg++ dependent endonucleases [1-18]. The double-stranded, low molecular weight DNA fragments as well as single strand breaks (‘nicks’) in high molecular weight DNA can be identified by labeling free 3’-OH termini with modified nucleotides in an enzymatic reaction [1-18]. The existing TUNEL assay that measures apoptosis in terms of loss of nuclear integrity is a simple assay to perform. The drawback is that it takes approximately 4 hours to complete. It was hypothesized that if incubation times can be reduced via the use of the microwave then the possibility of the incorporation of the TUNEL as a quality control parameter checking for apoptosis, in isolated or native islets will enhance the scope of existing quality control tests.
Why the Microwave?
The microwave oven consists of metal walls and a magnetron which emits microwaves at a frequency of 2.45 GHz. This frequency is ideal for both scientific heating and cooking purposes. Whenever rapid diagnosis is required, microwaves are utilized as they provide quick fixation, rapid processing and support the performance of immunohistochemical stains. The work of several pioneers like; Mayers, Kok and Boon and Anthony Leong showed that the microwave helped reduce the processing and paraffin sectioning time for cell blocks and fresh tissues to as low as 32 minutes [55-60]. “Microwave transparent materials” such as glass and plastic, allow total transmission of these waves to biological tissue. Biological materials allow penetration and absorption of microwaves to varying degrees following which depolarization of molecules like water, cell membrane proteins etc. occur.
This biophysical change causes breakage of protein bonds, alteration of membrane permeability and the unmasking of hidden surface receptors. These physiological alterations form the basis for rapid fixation, histological processing, antigen retrieval, staining and labelling. However, the penetrative capacity for the medium, the type and size of the tissue, the size, shape and type of container used, the procedure being carried out and the positioning of the load, determine the duration and output required for processing. By controlling the input of the current using a range of power settings, the level of emitted waves can be controlled and rapid methods for processing standardised [55-60].
Methodology
Two lots of post-thaw isolated human islets (Case-I and Case-II) cultured for 24 hours, 3 days, 5 days and 7 days i.e. 8 samples of post-thaw islets were used for the study. The kit used for the quantification of apoptosis was the In-Situ Cell Death Detection Kit, (Fluorescein, Cat. No. 1 684 795, Roche Diagnostics Corporation, Roche Applied Science, Indianapolis, USA). The post-thaw, culture, islet fixation, agar and paraffin embedding of islets and the TUNEL protocols used have been described in detail by Priya et al. 2013 [61]. Pancreatic tumor tissue following therapy was used as the control as it has a naturally high apoptotic index. For the negative control the TUNEL reaction mixture contained only label (fluorescein) and for the positive control, the reaction mixture contained both label and enzyme.
As the combined staining of the samples with fluorescein (label-TUNEL) and the nuclei using haematoxylin (H&E) stain reduced the intensity of fluorescence the same section could not be used to evaluate apoptosis by light microscopy and the TUNEL. This drawback was circumvented by staining the “reference section” with H&E stain and the sections on either side of the reference section were used for the two TUNEL procedures namely, the conventional TUNEL and the newly developed TUNEL-Microwave (TUNEL-MW). Thus, the distance between a TUNEL section and the reference section was 5μ m and that between the two TUNEL sections was 10μ m. Thus, the possibility of variations in results due to sampling variations was avoided. The Olympus Compound Microscope was used for light microscopic (LM) assessment of apoptosis in post-thaw cultured islets and the Confocal Microscope LSM 510, Zeiss was used for the TUNEL and TUNEL-MW. For both the TUNELs and LM; sections were sampled in 20 random fields at a total magnification of x400. Thus, the possibility of bias due to geographical variation in apoptosis in the section examined was avoided.
I New TUNEL Microwave (TUNEL-MW)
i Microwave Oven Used
The oven should have a 1 to 10 range preferably. The power output of the microwave oven should be low (between 800 to 900W); the reason being, that domestic microwave ovens have a cycle time, each lasting one minute and consisting of three phases. The “preheat time” of the magnetron followed by “full emission” of 800W or 900W for a short time and then a short duration during which the magnetron is completely “switched off”. The various ranges are obtained by altering the time of the switching ‘on’ and ‘off’ of the magnetron and not by controlling the amount of emission. Thus, our calculation of 350W output required for tissue processing which is approximately at the range of power 3 or power 4 ensures that the tissue is exposed to 800W for a shorter time within each cycle time [55-60]. Thus, a microwave oven with very high output like 1250W could harm the tissue and cause false positives.
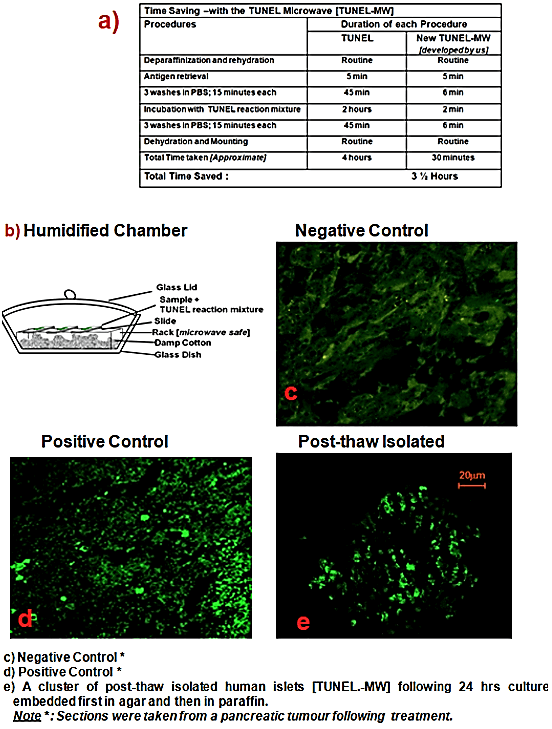
Dewaxed and rehydrated tissue was permeabilized and antigen retrieval was carried out by microwave irradiation using 200ml 0.1 M Citrate buffer, pH 6.0 at 350 W for 5 min (like the manufacturer’s protocol). Following this, three rinses in cold PBS were carried out in the microwave at Power 4 (350W) for 2 minutes each. The TUNEL mixture was applied and incubation was carried out at Power 4 for 2 minutes in a humidified chamber as shown in (Figure 1a, processing time for TUNEL procedure) and (Figure 1b, humidified chamber). The reaction was stopped by three rinses in cold PBS at Power 4 (350W) for 2 minutes each and the slides were then mounted using anti-fade. Samples were directly viewed using the confocal microscope LSM 510 (Figure 1e), using the same excitation wavelength as mentioned in the manufacturer’s protocol.
Figure 1: New TUNEL Microwave (TUNEL-MW) method.
ii Precautions Taken to Prevent False Positives
i. Artificial nicks and uncoiling of the DNA occurs at temperatures above 90°C. To circumvent this, the temperature was maintained below 90°C using both short cycles and rapid cooling (chilled buffers and ice).
ii. A positive and negative control of pancreatic tumor tissue following therapy was used with each run.
iii. As islets are composed of a cluster of cells, the resulting combined fluorescence interferes with the accuracy of the counting. By using the confocal microscope LSM 510 “point focusing” was made possible.
II Statistical Analysis
Validation was by univariate linear regression, coefficient of variation and the Bland Altman plot [62-64].
Results
Figures 1 a-e presents the images of results of the newly developed TUNEL-MW method taken via the confocal microscope LSM 510. The negative control (Figure 1c) was strongly negative for the TUNEL while the positive control (Figure 1d) was strongly positive for the same, indicative of the specificity of the TUNEL. The table (Figure 1a) illustrates the ability of the new method to cut down processing time from approximately 4 hours to 30 minutes.
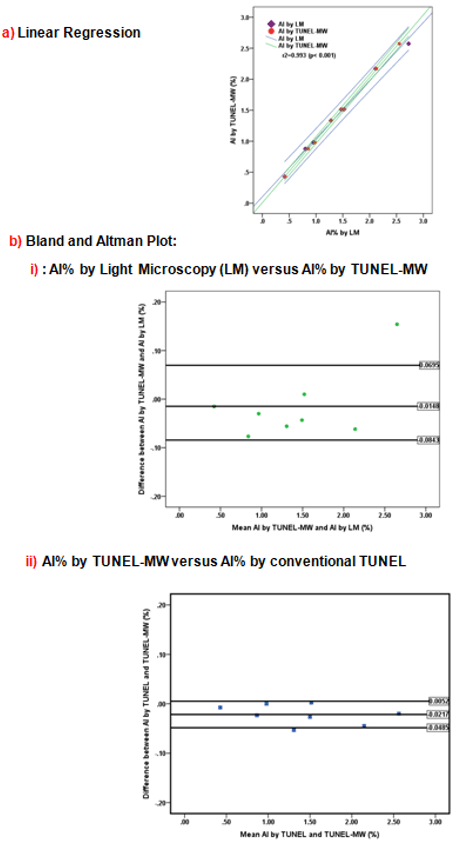
Validation of the AI% obtained by the New TUNEL-MW against the AI% obtained by the conventional TUNEL and via LM (gold standard). Figure 2a presents the univariate linear regression as a scatter plot of the results of the AI% by LM (gold standard) versus AI% by TUNEL-MW, r2=0.993 (p< 0.001) and AI% determined by conventional TUNEL versus AI% by TUNEL-MW r2=0.999 (p<0.001) (result not shown). The coefficient of variation was <5% for all three methods. To confirm that AI% by the new TUNEL-MW did not exceed ±2SD, which is the statistically allowed limit, the Bland & Altman plot (217-219) was used (Figures 2bi & 2bii ). The mean difference for the AI% by LM (gold standard) versus the AI% by new TUNEL-MW (Figure 2bi) was -0.0148 and the 1.96SD ranged from +0.0695 to -0.0843. The mean difference for the AI% by the classic TUNEL versus the AI% by new TUNEL-MW (Figure 2bii) was -0.0217 and the 1.96SD ranged from +0.0052 to -0.0485. To determine the measure of consistency or agreement of values within cases for all three methods, the intra class coefficient of correlation was determined. The intra class “coefficient of correlation” between the AI determined by LM and the TUNEL-MW was r=0.997 and that between the conventional TUNEL and the TUNEL-MW was r=0.999.
Figure 2: Validation of the New TUNEL Microwave (MW).
Discussion
The battery of quality control assays in current use in islet transplantation include functionality assays (insulin vs. glucose response), sterility assays (mycoplasma and endotoxin levels), islet quantification (via islet equivalents) and membrane integrity assays (fluorescent dyes exclusion assays) [40, 41]. The protocols followed in islet isolation and cryopreservation increase the vulnerability of islets towards apoptosis, the lack of definite quality control measures to evaluate apoptosis prior to islet release for transplantation presents a serious drawback [40, 41]. One of the reasons for the current lack of suitable measures of apoptosis is the lack of assays that are reliable, appropriate and quick. Early markers of apoptosis like Bcl-2, caspases etc. might yield high false positives due to a transient decline (as in Bcl-2) or elevation (as in caspases) during islet isolation and thaw [1-18]. Their levels might be restored to normal physiological limits when islets are transplanted into suitably primed recipients. Therefore, markers directed at the hallmark of apoptosis namely of nuclear integrity might be more appropriate in indicating the percentage of cells that are irreversibly damaged and whose loss will affect the volume of the functional islet mass post-transplant [1-18].
The conventional TUNEL, an established assay to quantify apoptosis was therefore selected for this study [1-18]. Its advantage is that the simplicity of the protocol and the assessment of results allow it to be carried out by a trained technician. Its drawback is that it takes approximately 4 hours to perform. In order to cut the processing time for the TUNEL, we designed a new method which incorporated the use of the microwave which we named as TUNEL-Microwave (TUNEL-MW) to serve as a candidate quality control measure to be used in routine islet transplantation. Figure 1a summarizes the processing time for the new TUNEL-MW assay developed; the new TUNEL-MW method cuts down processing time by approximately 3½ hours. By successfully decreasing incubation time for the TUNEL by the new TUNEL-MW together with confirmation of the reliability of the TUNEL as a measure for apoptosis, we propose that the TUNEL-MW could be used as a quality control measure in routine islet transplantation.
The gold standard for the evaluation and quantification of apoptosis is histomorphology via electron microscopy [11, 22]. The drawback with electron microscopy is that it is time consuming, expensive and the area of tissue that can be sampled/examined is extremely small. Further, when the apoptotic index of a cell population is relatively low it is impractical to use electron microscopy as a method of measuring apoptosis [65]. Light microscopy however affords a wider area of tissue sampling, is inexpensive and when strict criteria is adhered to by skilled pathologists, is a very reliable technique [11, 22]. To validate the new TUNEL-MW, the apoptotic index measured by this method was compared to that measured by the classic TUNEL and the reference method chosen for this study namely light microscopy (H&E stain). The three methods were found to be highly correlated (r >0.90, p<0.001) and the measure of consistency or agreement measured by the intra class coefficient of correlation for all three methods was r>0.990 (p<0.001).
The following are the advantages of the New TUNEL Microwave Method:
i. With the use of a humidified chamber this method prevents drying of the sample and the fluorescein label used during the microwaving process.
ii. It allows for diagnosis of apoptosis in both native islets, and fresh and post-thaw isolated islets.
iii. It significantly reduced incubation times, thus reducing the timing of the entire procedure considerably.
iv. Does not require highly specialized equipment; a simple domestic microwave may be used, thus keeping the costs down.
v. As no special experience is essential for the adoption of the microwave TUNEL method, and the TUNEL kit being a simple one, it provides a new convenient assay for the rapid detection of apoptosis.
One of the limitations is that the procedure requires standardization based on the microwave oven’s power output, type and thickness of tissue sample and size of the load used. The TUNEL method is also sensitive to the duration of fixation that a tissue is exposed to. In conclusion, the new TUNEL-MW to detect and measure apoptosis has reliability and reproducibility that matches both the apoptotic index as measured by light microscopy (LM) and the classic TUNEL. Simple, and requiring 30 minutes to perform, it can be used to complement existing quality control measures to rule out or measure apoptosis prior to release of islets for transplantation.
Acknowledgements
The post-thaw human isolated islets used in this study were kindly provided by Dr Lakey JRT and Dr Shapiro AMJ of the Clinical Islet Transplant Program, Edmonton, Canada. We thank CMC Vellore Fellowship, Lady Tata Memorial Fellowship, MDRF Travel and Training Fellowship and University of Alberta, Edmonton, Canada for their support. We thank Dr Thiagarajan V (Department of Biotechnology) and Dr AB Peter, Dr B Murali Manohar, Dr S, Vairamuthu (Department of Pathology) Madras Veterinary College for their guidance and support. We thank Dr Meera Govindarajan, Dr S Ganthimathy and Dr Manjula Datta for their guidance and support. We thank Kimberli Sawarin for her help with the wax embedding of agar embedded post-thaw cultured isolated islets. With regards to imaging of the TUNEL, we thank Dr VD Ramanathan, Deputy Director, Department of Pathology, Tuberculosis Research Centre [TRC] both for letting us used the confocal microscope and the hands-on training he provided.
Author Contributions
Dr Priya Miranda, Dr Lakey JRT, and Dr Mohan V conceived the study. Dr Lakey JRT, and Dr Mohan V, supervised the study and revised the manuscript. Dr Priya Miranda conceived, coordinated, performed the study, performed the statistical analysis and wrote the first draft of the article. Dr Meera Govindarajan. supervised the microwave assisted histoprocessing technique. Cell turnover was assessed by Dr Ganthimathy S and she also revised the manuscript. Dr Anjana RM gave valuable input and revised the manuscript. Dr Thiagarajan V and Dr S Gunasekaran revised the manuscript.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 06, Jul 2020Accepted: Mon 20, Jul 2020
Published: Fri 31, Jul 2020
Copyright
© 2023 Jonathan Lakey. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2020.02.05
Author Info
Priya M Miranda Viswanathan Mohan Sekhar Ganthimathy Meera Govindarajan Ranjit M Anjana S Gunasekaran Venkatachalam Thiagarajan Michael Alexander Jonathan Lakey
Corresponding Author
Jonathan LakeyUniversity of California, Irvine, California, USA
Figures & Tables
References
- Ansari B, Coates PJ, Greenstein BD, Hall PA (1993) In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol 170: 1-8. [Crossref]
- Eastman A, Barry MA (1992) The origins of DNA breaks: a consequence of DNA damage, DNA repair, or apoptosis? Cancer Invest 10: 229-240. [Crossref]
- Grasl Kraupp B, Ruttkay Nedecky B, Koudelka H, Bukowska K, Bursch W et al. (1995) In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21: 1465-1468. [Crossref]
- Strater J, Gunthert AR, Bruderlein S, Moller P (1995) Microwave irradiation of paraffin-embedded tissue sensitizes the TUNEL method for in situ detection of apoptotic cells. Histochem Cell Biol 103: 157-160. [Crossref]
- Tamura T, Said S, Lu W, Neufeld D (2000) Specificity of TUNEL method depends on duration of fixation. Biotech Histochem 75: 197-200. [Crossref]
- Tateyama H, Tada T, Hattori H, Murase T, Li WX et al. (1998) Effects of prefixation and fixation times on apoptosis detection by in situ end-labeling of fragmented DNA. Arch Pathol Lab Med 122: 252-255. [Crossref]
- Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP et al. (1994) Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 71: 219-225. [Crossref]
- Didenko VV, Ngo H, Baskin DS (2003) Early necrotic DNA degradation: presence of blunt-ended DNA breaks, 3' and 5' overhangs in apoptosis, but only 5' overhangs in early necrosis. Am J Pathol 162: 1571-1578. [Crossref]
- Negoescu A, Guillermet C, Lorimier P, Brambilla E, Labat Moleur F et al. (1998) Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed Pharmacother 52: 252-258. [Crossref]
- Negoescu A, Lorimier P, Labat Moleur F, Drouet C, Robert C et al. (1996) In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem 44: 959-968. [Crossref]
- Vagunda V, Kalabis J, Vagundova M (2000) Correlation between apoptotic figure counting and the TUNEL technique. Anal Quant Cytol Histol 22: 307-310. [Crossref]
- Herrmann M, Lorenz HM, Voll R, Grunke M, Woith W et al. (1994) A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 22: 5506-5507. [Crossref]
- Walker PR, Kokileva L, LeBlanc J, Sikorska M (1993) Detection of the initial stages of DNA fragmentation in apoptosis. Biotechniques 15: 1032-1040. [Crossref]
- Olive PL, Wlodek D, Banath JP (1991) DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Res 51: 4671-4676. [Crossref]
- Chapman RS, Chresta CM, Herberg AA, Beere HM, Heer S et al. (1995) Further characterisation of the in-situ terminal deoxynucleotidyl transferase (TdT) assay for the flow cytometric analysis of apoptosis in drug resistant and drug sensitive leukaemic cells. Cytometry 20: 245-256. [Crossref]
- Gong J, Traganos F, Darzynkiewicz Z (1994) A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem 218: 314-319. [Crossref]
- Wijsman JH, Jonker RR, Keijzer R, van de Velde CJ, Cornelisse CJ et al. (1993) A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem 41: 7-12. [Crossref]
- Gavrieli Y, Sherman Y, Ben Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493-501. [Crossref]
- Kerr JFR, Searle J, Harmon BV, Bishoop CJ (1987) Apoptosis In: Perspectives on Mammalian Cell Death. Potten CS, (ed). Oxford University Press, Oxford 93-128.
- Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239-257. [Crossref]
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495-516. [Crossref]
- Cotran RS, Kumar V, Collins T (1999) Cellular Pathology 1: Cell Injury and Cell Death. In: Robbin’s Pathologic Basis of Disease. Sixth Edition. Cotran RS, Kumar V, Collins T, (eds). WB Saunders Company 260-327.
- Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20: 175-193. [Crossref]
- Rastogi RP, Richa, Sinha RP (2009) Apoptosis: molecular mechanisms and pathogenicity. EXCLI J 8: 155-181.
- Benhamou PY, Watt PC, Mullen Y, Ingles S, Watanabe Y et al. (1994) Human islet isolation in 104 consecutive cases. Factors affecting isolation success. Transplantation 57: 1804-1810. [Crossref]
- Schulak JA, Kisthard J (1984) The effect of warm ischemia and cold-storage preservation on rat pancreas transplantation. J Surg Res 36: 134-139. [Crossref]
- Abouna GM, Sutherland DE, Florack G, Najarian JS (1987) Function of transplanted human pancreatic allografts after preservation in cold storage for 6 to 26 hours. Transplantation 43: 630-636. [Crossref]
- Nishihara M, Sumimoto R, Asahara T, Fukuda Y, Southard JH et al. (1996) Effect of donor fasting on survival of pancreas and heart grafts after warm ischemia. Hiroshima J Med Sci 45: 93-97. [Crossref]
- Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L (2000) Cell loss in isolated human islets occurs by apoptosis. Pancreas 20: 270-276. [Crossref]
- Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ et al. (1999) Pancreatic islet cell survival following islet isolation: the role of cellular interactions in the pancreas. J Endocrinol 161: 357-364. [Crossref]
- Paraskevas S, Duguid WP, Maysinger D, Feldman L, Agapitos D et al. (1997) Apoptosis occurs in freshly isolated human islets under standard culture conditions. Transplant Proc 29: 750-752. [Crossref]
- Hui H, Dotta F, Di Mario U, Perfetti R (2004) Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J Cell Physiol 200: 177-200. [Crossref]
- Cattan P, Berney T, Schena S, Molano RD, Pileggi A et al. (2001) Early assessment of apoptosis in isolated islets of Langerhans. Transplantation 71: 857-862. [Crossref]
- Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D et al. (1999) Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery 126: 209-304. [Crossref]
- Wang RN, Rosenberg L (1999) Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinology 163: 181-190. [Crossref]
- Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124: 619-629. [Crossref]
- Ricordi C, Alejandro R, Rilo HH, Carroll PB, Tzakis AG et al. (1995) Long-term in vivo function of human mantled islets obtained by incomplete pancreatic dissociation and purification. Transplant Proc 27: 3382. [Crossref]
- Paraskevas S, Duguid WP (1996) Apoptosis and Islet Cell Survival. In:. Cellular Inter-relationships in the Pancreas-Implications for Islet Transplantation. Rosenberg L, Duguid WP, Williams PL, Warwick, Dyson M and Bannister LH, (eds). RG Landes Company 99-123.
- Hill DJ (1996) Extracellular Matrix in Islet growth and Function. In: Cellular Inter-relationships in the Pancreas-Implications for Islet Transplantation. Rosenberg L, Duguid WP (eds). RG Landes Company 87-97.
- Ricordi C (1991) Quantitative and qualitative standards for islet isolation assessment in humans and large mammals. Pancreas 6: 242-244. [Crossref]
- Scharp DW (1992) Islet quality control testing and the islet isolation laboratory. In: Pancreatic Islet Cell Transplantation; 1892-1992: One Century of Transplantation for Diabetes. Ricordi C, Austin, TX, (eds). R.G. Landes Company 82-88.
- Boffa DJ, Waka J, Thomas D, Suh S, Curran K et al. (2005) Measurement of apoptosis of intact human islets by confocal optical sectioning and stereologic analysis of YO-PRO-1-stained islets. Transplantation 79: 842-845. [Crossref]
- Edidin M (1970) A rapid, quantitative fluorescence assay for cell damage by cytotoxic antibodies. J Immunol 104: 1303-1306. [Crossref]
- Yang H, Acker J, Chen A, McGann L (1998) In situ assessment of cell viability. Cell Transplant 7: 443-451. [Crossref]
- Miyamoto M, Morimoto Y, Nozawa Y, Balamurugan AN, Xu B et al. (2000) Establishment of fluorescein diacetate and ethidium bromide (FDAEB) assay for quality assessment of isolated islets. Cell Transplant 9: 681-686. [Crossref]
- London NJ, Contractor H, Lake SP, Aucott GC, Bell PR et al. (1990) A fluorometric viability assay for single human and rat islets. Horm Metab Res Suppl 25: 82-87. [Crossref]
- London NJ, Contractor H, Lake SP, Aucott GC, Bell PR et al. (1989) A microfluorometric viability assay for isolated human and rat islets of Langerhans. Diabetes Res 12: 141-149. [Crossref]
- Gray DW, Morris PJ (1987) The use of fluorescein diacetate and ethidium bromide as a viability stain for isolated islets of Langerhans. Stain Technol 62: 373-381. [Crossref]
- Chen T, Acker JP, Eroglu A, Cheley S, Bayley H et al. (2001) Beneficial effect of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology 43: 168-181. [Crossref]
- Reers M, Smiley ST, Mottola Hartshorn C, Chen A, Lin M et al. (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260: 406-417. [Crossref]
- Gorczyca W, Melamed MR, Darzynkiewicz Z (1998) Analysis of apoptosis by flow cytometry. Methods Mol Biol 217-238. [Crossref]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139: 271-279. [Crossref]
- Schmid I, Uittenbogaart CH, Keld B, Giorgi JV (1994) A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods 170: 145-157. [Crossref]
- Vermes I, Haanen C, Steffens Nakken H, Reutlingsperger C (1995) A novel assay for apoptosis, Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immun Methods 184: 39-51. [Crossref]
- Kok LP, Mathilde B (1992) Microwave Exposure and DNA in situ Hybridization. In: Microwave cookbook for Microscopists. 3rd Edition, Kok LP, Mathilde B (eds). Coulomb Press Leyden 366-378.
- Lucassen PJ, Labat Moleur F, Negoescu A , Campagne MVL (2000) Microwave-enhanced in situ end-labeling of apoptotic cells in tissue sections: Pitfalls and Possibilities. In: Antigen Retrieval Techniques: Immunohistochemistry and Molecular Morphology. Shi RS, Jiang G and Taylor CR (eds). Eaton Publishing 71-91.
- Singla K, Sandhu SV, Pal RAGK, Bansal H, Bhullar RK et al. (2017) Comparative evaluation of different histoprocessing methods. Int J Health Sci (Qassim) 11: 28-34. [Crossref]
- Katoh K (2016) Microwave-Assisted Tissue Preparation for Rapid Fixation, Decalcification, Antigen Retrieval, Cryosectioning, and Immunostaining. Int J Cell Biol 2016: 7076910. [Crossref]
- Shruthi BS, Vinodhkumar P, Kashyap B, Reddy PS (2013) Use of microwave in diagnostic pathology. J Cancer Res Ther 9: 351-355. [Crossref]
- Amrutha N, Patil S, Rao RS (2014) Microwaves: a revolution in histoprocessing. J Contemp Dent Pract 15: 149-152. [Crossref]
- Miranda PM, Mohan V, Ganthimathy S, Anjana RM, Gunasekaran S et al. (2013) Human islet mass, morphology, and survival after cryopreservation using the Edmonton protocol. Islets 5: 188-195. [Crossref]
- Bland JM, Altman DG (1986) Statistical method for assessing agreement between two methods of clinical measurement. Lancet 1: 307-310. [Crossref]
- Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8: 135-160. [Crossref]
- Dewitte K, Fierens C, Stöckl D, Thienpont LM (2002) Application of the Bland-Altman plot for interpretation of method-comparison studies: a critical investigation of its practice. Clin Chem 48: 799-801. [Crossref]
- Bateman AC, Turner SM, Thomas KSA, McCrudden PR, Fine DR et al. (2002) Apoptosis and proliferation of acinar and islet cells in chronic pancreatitis: evidence for differential cell loss mediating preservation of islet function. Gut 50: 542-548. [Crossref]
