Could Reported Sex Differences in Hypertonic Saline-induced Muscle Pain be a dose Issue?
A B S T R A C T
Higher levels of experimental muscle pain induced by injection of the same volume of noxious substances have been reported by women compared to men. This could hypothetically be related to the difference in muscle volume between men and women. The aim of this study was to investigate if the sex differences reported by intramuscular injection of hypertonic saline would disappear if a larger dose is given to men than women under similar conditions.
Methods: Fifty-six healthy volunteers (25 men and 31 women) received hypertonic saline injection into the masseter muscle, 0.5 mL for men and 0.3 mL for women, to evoke pain. Pain intensity was assessed with 0-100 mm visual analogue scale (VAS) every 15 seconds until pain subsided or maximum 300s. VAS was also used to assess perceived unpleasantness and anxiety. Pain drawings were used to assess maximal pain distribution, and the McGill pain questionnaire to assess pain quality.
Results: There was no sex difference in maximum pain intensity, unpleasantness, anxiety or pain drawing area, but the evoked pain had larger total pain area (p=0.005), and longer duration (p<0.001) in the men than women. The sexes also used some different pain descriptors.
Conclusions: This study shows that the previously reported higher pain levels in women were abolished when a lower dose of hypertonic saline was injected into the masseter muscle of the women than men. This might indicate that the sex differences reported to hypertonic-induced muscle pain may be a dose issue. Further studies are required to validate these results
Keywords
experimental pain, hypertonic saline solution, intramuscular injections, masseter, sex difference, temporomandibular joint disorder
Introduction
Pain is an enormous global health problem [1]. Based on the International Association for the study of Pain (IASP), pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage” [2]. Chronic pain is defined as pain of longer duration than 3 months and hence, lacks the acute warning function of physiological nociception [3]. The prevalence of chronic pain has been recognized as 20% worldwide and it is often associated with sleep disorders, mood disturbances and low quality of life [1, 4-5]. It has been estimated that 20% of patients with chronic pain suffer from depressive symptoms resulting in low work function (e.g. sick leave) and high health care utilization [6, 7]. Temporomandibular disorders (TMDs) is a term embracing conditions in the temporomandibular joint (TMJ) and masticatory muscles. It is the most prevalent chronic pain condition in the orofacial region and affects 5-10% of the population worldwide [8, 9]. In similarity to many other musculoskeletal pain conditions and headache the prevalence of TMD is higher in women than men with a female to male ratio of approximately 2:1 [9-12].
To study mechanisms behind chronic pain, experimental pain models are commonly used. This approach provides the possibility to evaluate pain quantitatively and the mechanisms involved in pain initiation and transmission as well as the perception of pain. Experimental research can be applied both in the laboratory and the clinic through activation of the nociceptive system with specific stimuli for pain induction and measurement of the evoked pain under normal and/or pathophysiological situations [13]. Pain stimuli may be electrical, thermal, chemical, and mechanical of which, chemical stimuli provide a more dynamic model to study sex differences in experimental pain [12-14]. Intramuscular injection of hypertonic saline is one of the most frequent methods to provoke human myalgia [15]. Hypertonic saline injection elicits pain with short, deep and diffuse character that mimics chronic muscle pain and is regarded a valid experimental model for chronic muscle pain albeit it has an acute character. A few previous researches have reported elevated levels of glutamate and serotonin in patients with chronic masseter myalgia and recently these biomarkers were also reported elevated after hypertonic saline injection, which further lend support for the validity of the model [16-20]. Furthermore, the model is regarded as safe and no serious side effects have been reported [21]. Although penetration of the skin and muscle may cause bleeding and ensuing edema, this rarely occur and can be avoided by proper injection technique.
Many previous human experimental pain studies using chemical modalities to induce pain in the orofacial region as well as in other part of the body have reported higher pain intensity, longer pain duration, and larger pain area in women compared to men [12, 22-30]. However, in all those studies the same volume (dose) of the compound was used for both sexes.
The reasons behind sex-differences in clinical and experimental pain are not fully understood but a combination of biological, psychological, and socio-cultural factors has been suggested to be involved [31]. Biological factors include e.g. sex hormones, genotype, sex-related cortical differences during the processing of pain-related stimuli and in the endogenous opioid system [12]. For instance, estradiol and progesterone exert pro-nociceptive effects on pain, while testosterone seems to have anti-nociceptive effect and be protective in nature [32]. Apart from these factors for sex difference in the perceived experienced pain, one additional reason for higher levels of pain in women could potentially be related to their generally smaller muscle mass [33, 34]. Studies have shown that the risk of diffusion increases with higher dose, concentration, and volume of the injected compound [35]. Therefore, the aim of this study was to investigate the hypothesis that the higher pain intensity, larger area and longer pain duration reported after intramuscular injection of hypertonic saline in previous studies would disappear if a larger dose is given to men than women under similar conditions.
Methods
I Participants
Fifty-six healthy participants, 25 men and 31 women, with a mean (SD) age of 25.3 (4.5) years among men and 27.1 (6.9) years among women participated in the study. The participants were recruited through advertisement at the Department of Dental Medicine at the Karolinska Institutet, Huddinge, Sweden, where the study was conducted during 2016-2017. The study protocol followed Good Clinical Practice and was conducted in accordance with the Helsinki Declaration. The Regional Ethical Review Board in Stockholm approved the study (reg nr 2015/1659-31/2). All participants received verbal and written information about the study. Informed consent was obtained from all individual participants included in the study prior to participation.
Inclusion criteria were age between 18 to 40 years and good general health. Exclusion criteria were 1) any pain-related TMD diagnosis or other orofacial pains, 2) diagnosed systemic muscular or joint diseases (e.g. fibromyalgia and rheumatoid arthritis), 3) whiplash-associated disorder, 4) neuropathic pain or neurological disorders, 5) frequent or chronic headache, 6) pregnancy or lactation, and 7) usage of analgesics 24h before experiment.
II Experimental Protocol
The participants were first screened for orofacial pain with the Symptom Questionnaire included in the Diagnostic criteria for TMD (DC/TMD), and a medical history was taken to ensure that they were healthy [36]. A clinical examination according to DC/TMD was then performed to ensure that also their jaw system was healthy. The participants were first asked to complete the Swedish version of the Axis II Questionnaire included in the Diagnostic criteria for TMD (DC/TMD) which includes questions of demographic characteristics (sex, age, place of birth, marital status, living condition, education/job status), general health, presence of pain in temples, jaws or ears, headache, presence of clicking, luxation, history of trauma, graded chronic pain scale (GCPS), oral behavior checklists (OBC-6), jaw functional limitation scale (JFLS), depression and anxiety (PHQ-4), and perceived stress scale (PSS-4) [36].
The participants sat in a conventional dental chair in a relaxed position during the experiment. They were asked to bite on the posterior teeth to identify the most prominent point of the masseter, which was used for injection. The skin of the cheek was disinfected with a tissue soaked in alcohol prior to the injection. Muscle pain was evoked by a single intramuscular injection of sterile hypertonic saline (58.5 mg/mL) with a 2-mL syringe with a 19-mm needle (diameter 0.4 mm) inserted into the masseter muscle to a depth of approximately 15 mm without any surface anesthesia. The experimental side was the subject’s dominant side i.e. the left side for left-handed participants and the right side for right-handed.The hypertonic saline solution was prepared by mixing one part of concentrated solution (Addens-Natriumklorid B. Braun 234 mg /mL, Braun Medical AB, Danderyd, Sweden) with three parts of Sterile water (Fresenius Kabi AB, Uppsala, Sweden). Preparation of the solution and syringe was made shortly before injection. The men received an injection of 0.5 mL and the women 0.3 mL. The same injection rate was strived for (5 s/0.1 mL), i.e. for the women the solution was injected during approximately 15 s and for men during approximately 25 s. The injections were given by three researchers that were trained in the technique. The participants were told that they would receive a saline injection and that they should assess any pain evoked, but they were not aware of the different volume used for the other sex. As the individual masseter mass of the participants was unknown, we chose a dose for the men that we expected would be able to alter results.
III Assessment of pain
Pain was assessed with a 0–100 mm visual analogue scale (VAS) marked with the end-points "no pain" and "the worst pain experienced" before and after injection. Immediately after the injections the participants were instructed to mark their pain level on the scale and then every 15th second until pain had subsided with a maximum duration of 300 s. Furthermore, after termination of the experiment participants were asked to recall and mark the maximum level of their perceived unpleasantness and anxiety evoked by the injections on a 0-100 mm VAS. The scales were anchored at each end with "Not at all unpleasant" and "The most unpleasant experience imaginable" and "No anxiety" and "The most intense anxiety imaginable", respectively. The procedure was done in accordance with previously studies [37, 38]. Thereafter the participants were asked to mark the quality of their experienced pain from the Swedish version of the McGill Questionnaire (MPQ) [39]. The Swedish version of MPQ has 73 pain descriptors categorizing into 20 subclasses of 4 major subscales; sensory (subclasses 1–10), affective (subclasses 11–15), evaluative (subclass 16), and miscellaneous (subclasses 17–20). The total number of words chosen by the participants was calculated, and the sum of the ranks was calculated as the pain rating index PRI(R) [40]. PRI is interpreted both in terms of quantity and quality of the pain and higher score represents worse pain. Finally, the participants were asked to draw their maximum distribution of perceived pain evoked by the injection on a lateral view of the face. The pain drawing area was digitalized, calculated in Photoshop CS6 (Adobe Systems Software Inc., San José, CA, USA) and presented in arbitrary units [41].
IV Menstrual cycle
The female participant’s menstrual cycle was assessed by asking the women about which day in the cycle she was counted from the first day of the last menses. The menstrual cycle was divided into five phases: menstrual (days 1-5), follicular (days 6-11), preovulatory (days 12-16), luteal (days 17-23), and premenstrual (days 24-28) [42].
V Statistical analyses
The statistical analyses and graphs were done by SigmaPlot version 14, SysStat Software Inc., San José, CA, USA. The normality of the data was tested with the Shapiro-Wilks test. Most variables were not normally distributed thus non-parametric statistics were used. Descriptive data are presented as number of participants (n) and median with interquartile range (IQR), except for age that is presented with mean (SD). From the individual data of pain assessments after injection the following data was retrieved: the maximum pain intensity (occurring at any time point), the pain duration (max 300s), the pain intensity at 300s (last assessment), the number of participants with pain at 300s, and the area under the curve (AUC), i.e. the cumulated pain intensities multiplied by the pain duration.
Mann-Whitney U–test was applied to compare variables between men and women. Chi-square test was used to find significant differences in frequencies between sexes. Spearman’s test with Bonferroni correction was used for correlation analyses between pain variables, unpleasantness and anxiety. ANOVA on ranks (Kruskal-Wallis test) was used to test the difference in maximum pain intensity between menstrual cycle phases in the women. A P-value less than 0.05 were considered statistically significant (p<0.01 for correlation analyses).
Results
Participants in both groups were healthy without any TMD pain disorders or systemic disease, but one woman reported a history of jaw locking. There were no statistically significant differences in any variables between men and women. The dominant side used for injection was the right side in 84% of the men and in 87% of the women.
Figure 1: Median (IQR) pain intensity assessed every 15 s up to 300 s after injection of 58.5 mg/mL hypertonic saline into the masseter muscle of 25 men and 31 women. Men received an injection with 0.5 ml hypertonic saline and women the injection with 0.3 ml. Among men, pain increased gradually until it reached a peak after 60-90 seconds and then decreased slowly. Most women reported the maximum level of pain immediately after injection (at 0 seconds) and pain then decreased more rapidly than in the men and remained fairly constant after 120 seconds.
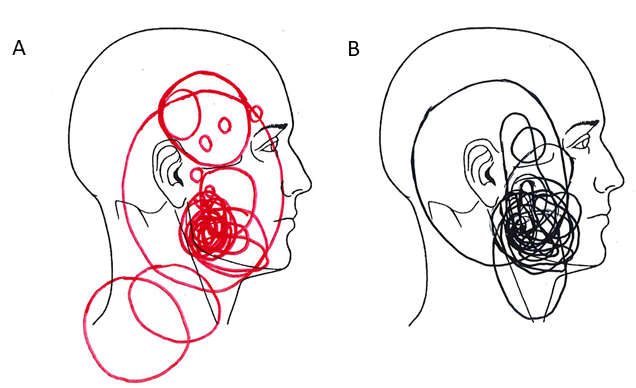
Figure 2: Drawings of perceived pain area and spread after injection of hypertonic saline into the masseter muscle (0.5 mL in men and 0.3 mL in women) of healthy participants. A) Women (n = 31), B) Men (n = 25).
I Pain-related responses
The injection of hypertonic saline solution evoked pain with a different pattern among men and women for 300 seconds (Figure 1). As is illustrated by the graph, the pain intensity among men increased gradually until it reached a peak after 60-90 seconds and then decreased slowly. However, most women reported the maximum level of pain immediately after injection and pain then decreased more rapidly than in the men and remained fairly constant after120 seconds.
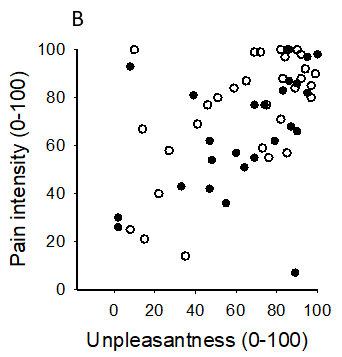
Table 1 shows pain related variables evoked by the injection. There were no statistical differences between men and women in the maximum pain intensity, unpleasantness, anxiety or pain-drawing areas evoked by the injection. AUC was significantly greater and the pain duration longer in the men compared to women. The majority of men (76%) still reported pain after 300 seconds, while only 29% of women reported pain after 300 seconds. The pain intensity at 300 seconds was also higher in the men. The hypertonic saline-evoked pain was felt not only at the site of injection but also showed referral to other ipsilateral regions of the face and head (teeth in upper and lower jaws, temporal muscle, TMJ, ear, neck and eye) in 14 men (56%) and 17 women (55%), as assessed by the pain drawings (Figure 2). There was no difference between men and women in terms of number of participants with pain referral. The men used in total 65 words and the women 57 words from the MPQ to describe hypertonic saline pain. The most frequently chosen words (n of participants) by the men were: intense (13), tender (11), sharp (9) aching (9) and tiring (7). In the women the most frequently chosen words were pressing (14), pulsing (13), hurting (11), aching (11), sharp (11), and tender (11). There was a significant difference between men and women in the frequency of participants who had chosen pulsing (p<0.001) and hurting (p=0.035). The median (IQR) PRI(R) derived from the MPQ was 17 (10) in the men and 20 (25) in the women (p=0.779). In both sexes alike, maximum pain intensity, unpleasantness and anxiety evoked by the injection, inter-correlated (corrected p’s<0.001-0.007) (Figure. 3). There were no significant correlations between these variables and pain drawing area or MPQ (Table 1).
Figure 3: Scatterplots of associations between A) pain intensity and anxiety, B) pain intensity and unpleasantness, and C) unpleasantness and anxiety evoked by hypertonic saline injection in 25 healthy men and 31 healthy women. See also Table 2 for correlation coefficients.
Table 1: Pain variables assessed after injection of hypertonic saline solution (58.5 mg/mL) into the masseter muscle of healthy participants. The men received an injection of 0.5 ml and the women 0.3 ml. Variables are presented as median (interquartile range).
|
Pain measures |
Men (n=25) |
Women (n=31) |
P-value |
|
Max pain intensity (VAS 0-100) |
68.0 (34.0) |
84.0 (31.5) |
0.171 |
|
Pain duration (s) |
300 (7.5) |
255 (180) |
<0.001 |
|
AUC (au) |
251100 (266400) |
133800 (184200) |
0.005 |
|
Pain after 300s (n) |
19 |
9 |
<0.001 |
|
Pain intensity at 300 s |
14.0 (54.0) |
0 (1.0) |
<0.001 |
|
Pain unpleasantness (VAS 0-100) |
69.0 (39.5) |
75.0 (46.0) |
0.786 |
|
Anxiety (VAS 0-100) |
26.0 (64.5) |
23.0 (53.0) |
0.649 |
|
Pain drawing area (Pixel) |
20355 (36341) |
18327 (16320) |
0.856 |
N=number of subjects, AUC = area under the curve, VAS = visual analogue scale
Table 2: Spearman correlation coefficients (rs) for associations between pain intensity (0-100), unpleasantness (0-100), anxiety (0-100), pain drawing area (pixel), and pain rating index (0-73) evoked by hypertonic saline injection into the masseter muscle of 25 healthy men and 31 healthy women.
|
|
|
Anxiety |
Unpleasantness |
Pain drawing area |
Pain Rating Index |
|
Pain intensity |
|
|
|
|
|
|
|
Men |
0.621 |
0.665 |
0.192 |
0.218 |
|
|
Women |
0.519 |
0.474 |
0.088 |
0.090 |
|
Anxiety |
|
|
|
|
|
|
|
Men |
|
0.641 |
0.285 |
0.274 |
|
|
Women |
|
0.426 |
0.345 |
0.306 |
|
Unpleasantness |
|
|
|
|
|
|
|
Men |
|
|
0.202 |
0.335 |
|
|
Women |
|
|
0.261 |
0.186 |
|
Pain drawing area |
|
|
|
|
|
|
|
Men |
|
|
|
0.284 |
|
|
Women |
|
|
|
0.279 |
Bold Figures denote significant differences after Bonferroni correction for multiple comparisons (p<0.01).
II Menstrual cycle and pain experience
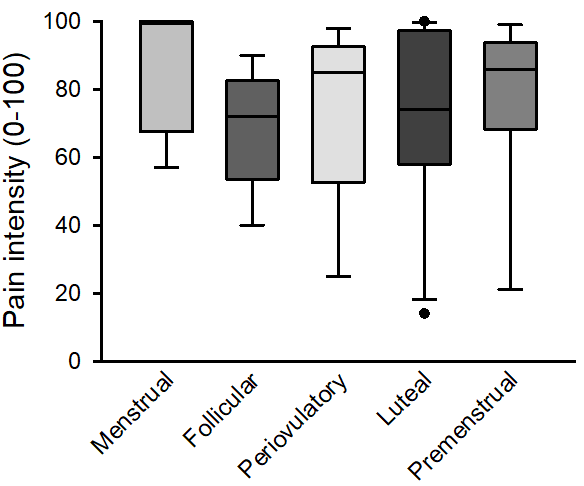
Four of the women were in the menstrual phase, 6 were in the follicular phase, 4 were in the preovulatory phase, 12 were in the luteal phase, and 5 women were in the premenstrual phase on the menstrual cycle. There was no difference in maximum pain intensity between women in different phases of the menstrual cycle (Figure 4).
Figure 4: The maximum pain intensity in 31 women after injection of 0.3 ml of hypertonic saline into the masseter muscle divided into menstrual cycle phase. There was no difference in maximum pain intensity between women in different phases (p=0.342).
Discussion
This study investigated sex differences to experimentally-induced muscle pain by hypertonic saline injection into the masseter muscle. The main findings were that there were no significant differences in pain intensity, unpleasantness, anxiety or pain drawing areas between sexes when a greater volume of hypertonic saline was injected into the masseter of the men. There was also a different pattern of hypertonic saline-evoked masseter muscle pain among men and women, where the women experienced the maximum pain intensity much sooner after injection and a shorter duration of hypertonic-saline evoked pain than the men. Overall, the results from this study support the hypotheses that sex differences previously reported to experimentally-induced muscle pain by chemical compounds may be a volume effect. To our knowledge, this study is the first in which women received a lower volume of hypertonic saline than men in an attempt to adjust the dose to their generally smaller muscle mass. Most previous studies that have reported sex-differences in experimentally-induced muscle pain by different chemical modalities have used the same dose among both sexes [20, 23, 25, 26]. One study showed that the sex-related differences in pain intensities by glutamate injection into the masseter muscle remained even when the dose was reduced with 50% for both sexes [43]. Thus, our results indicate that if a higher dose is injected in men than women the sex differences in pain intensity may disappear. Nevertheless, one cannot exclude that there may be sex differences if other methods or techniques are used to evoke experimental pain. For example, multiple injections instead of a single injection, use of other compounds such as glutamate or capsaicin and in different concentration, or if pain is induced in other tissues.
We also found some interesting effects for other pain variables. Women reported the maximum level of pain directly immediately after injection, whereas for the men the maximum pain level occurred after about one minute. Then, pain decreased more rapidly among women compared to men. Furthermore, only 29% of the women compared to 76% of the men still reported pain after 300s and the pain intensity at 300 seconds was also higher in the men. Also, there was no sex difference in pain distribution from pain drawings after hypertonic saline injections, in contrast to an earlier study [26]. The most plausible explanation to these findings is that these variables also are influenced by the dose, in line with the findings of a previous study showing that peak pain rating, overall amount of pain (AUC) and duration of pain are influenced by the concentration of the injected compound [23]. However, no significant interaction between gender and compound concentration was reported in that study [23]. The different pain profile in women and men is also noteworthy and could perhaps be explained by a sex difference in nocebo response. A recent systematic review reported that women responded with larger nocebo effects induced by conditioning procedures compared to men due to a higher increase in anxiety and stress level among them [44]. On the other hand, there was no sex difference in anxiety levels evoked by the injection in the present study. This also contrasts another study showed that men reported more anxiety than women after application of topical capsaicin on their face [25]. The difference across studies may be due to different compounds used to evoke pain and anxiety as well as different tissues and sites. In addition, capsaicin evokes a more burning type of pain compared to hypertonic saline that usually is regarded as ‘aching’, ‘taut’, ‘tight’, ‘spreading’, and ‘radiating’ [45]. However, in line with the study by Frot and co-workers (2004), pain intensity, unpleasantness and anxiety were positively inter-correlated in our study emphasizing the link between pain and emotions. Participants reported pain spread beyond the masseter, i.e. to the ipsilateral teeth, temporal muscle, TMJ, ear, neck and eye. This is related to the characteristics of the referred pain in the trigeminal area, which does not often cross the midline [46].
Another finding in this study was that even though there was no sex difference in PRI, there were some words that were more often used in one sex, for example “pulsing” and “hurting”, while other words were among the most frequently chosen in both sexes, such as “sharp”, “tender”, and “aching”. Many of these were also among the most frequently chosen words in a previous study regarding hypertonic saline injection into the masseter muscle [22, 47]. In the present study, women experienced the same level of maximum pain intensity in different phases of the menstrual cycle (Figure 4) which contrasts previous studies showing varying pain intensities across the menstrual cycle in both TMD-related and experimental pain [12, 48]. However, previous studies are not consistent. In one study the pain threshold for mechanical, ischemic and thermal modalities was higher during the follicular phase (low to moderate level of estradiol and progesterone) compared to premenstrual phase of the menstrual cycle (decreasing level of estradiol and progesterone) [42]. In another study, the electrical pain threshold as well as pain evoked by injection of capsaicin was lower in the luteal phase compared to the follicular phase [49].
The different results may be due to the fact that; menstrual cycle length is not stable during the reproductive life. For example, the length of the follicular phase may decrease with chronological age. Moreover, the concentration of different hormones varies in the same phase; early in the follicular phase the concentration of estrogen and progesterone is low, however in the mid follicular phase, estrogen is high independently from progesterone. Another difference may a type-II error due to low number of women in the different phases.
Strengths and limitation of the study
In this study, experimental pain was evoked among healthy men and women with similar background characteristics by injection of different volumes of hypertonic saline. Therefore, it provides a new perception regarding experienced pain intensity among men and women. The sample size was big enough to result in reliable estimates. Also, the number of missing data in the study sample was very low.An obvious study limitation is that the ratio in hypertonic saline volume between men and women was chosen based on an estimate of difference in average masseter muscle volume between men and women. Computerized tomography or magnet resonance imaging could be used to evaluate masseter muscle mass to determine the precise dose of hypertonic injection among men and women, but this was not possible in the present study. In a recent systematic review, it was reported that muscle thickness, length and cross-sectional area tend to be smaller in women than men. It was further reported that the mean volume of the masseter muscle among women is approximately 50% smaller compared to men [34]. Thus, the doses used seem to have been a reasonably good estimate, although the inter-individual variation in muscle volume was unknown. Also, the body mass index (BMI) could have given an estimate of muscle volume, but unfortunately BMI was not calculated in the present study. Nevertheless, the BMI cannot distinguish between muscle and fat why BMI would be a very crude measure of muscle mass. It is unknown if other factors such as masseter microcirculation, show sex differences. If one of the sexes would have a higher blood flow, the clearance of the solution would be faster which also could affect the results.
In this study we chose not to include a control group. This can be regarded as another study limitation. However, the aim of this study was to get an idea of if it is possible to alter sex differences in pain responses to injections of algesic substances using different doses for men and women, not to investigate if hypertonic saline evokes more pain than a control solution. It is possible that there could also be sex differences in pain evoked by a control solution, e.g. isotonic saline, if different volumes would be used for men and women. On the other hand, sex differences in experimental pain are reported to increase in magnitude with greater stimuli intensity why this might not be the case. As stated previously, sex differences to experimental pain depend on many different factors, which all may affect the results [12, 31]. It would be interesting to further elaborate on this in future studies, e.g. by investigating brain activity after injections.
In order to determine hormonal influences on perceived pain intensity, blood test and hormonal analyses would preferably be taken. However, previous studies have shown that even if hormonal changes across the menstrual cycle may affect pain perception, the effect is small, and the inter-individual variation probably is of much greater importance [50].
Conclusions
This study shows that the previously reported higher pain levels in women were abolished when a lower dose of hypertonic saline was injected into the masseter muscle of the women than men. This indicates that the sex differences reported to hypertonic-induced muscle pain may be a dose issue. Further studies are required to validate these results.
Acknowledgement
This study was supported by Karolinska Institute. The volunteers are greatly acknowledged for their participation.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Regional Ethical Review Board in Stockholm approved the study (reg nr 2015/1659-31/2). All participants received verbal and written information about the study. Informed consent was obtained from all individual participants included in the study prior to participation.
Funding
This study was funded by Karolinska Institutet.
Conflicts of interest
The authors declare that they have no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 15, Oct 2019Accepted: Fri 08, Nov 2019
Published: Wed 20, Nov 2019
Copyright
© 2023 Negin Yekkalam. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2019.05.02
Author Info
Ekaterina Coello Mikaela Eklund Hajer Jasim Malin Ernberg Negin Yekkalam Nikolaos Christidis
Corresponding Author
Negin YekkalamDivision of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institute, and Scandinavian Center for Orofacial Neuroscience, SE 14104 Huddinge, Sweden
Figures & Tables
Table 1: Pain variables assessed after injection of hypertonic saline solution (58.5 mg/mL) into the masseter muscle of healthy participants. The men received an injection of 0.5 ml and the women 0.3 ml. Variables are presented as median (interquartile range).
|
Pain measures |
Men (n=25) |
Women (n=31) |
P-value |
|
Max pain intensity (VAS 0-100) |
68.0 (34.0) |
84.0 (31.5) |
0.171 |
|
Pain duration (s) |
300 (7.5) |
255 (180) |
<0.001 |
|
AUC (au) |
251100 (266400) |
133800 (184200) |
0.005 |
|
Pain after 300s (n) |
19 |
9 |
<0.001 |
|
Pain intensity at 300 s |
14.0 (54.0) |
0 (1.0) |
<0.001 |
|
Pain unpleasantness (VAS 0-100) |
69.0 (39.5) |
75.0 (46.0) |
0.786 |
|
Anxiety (VAS 0-100) |
26.0 (64.5) |
23.0 (53.0) |
0.649 |
|
Pain drawing area (Pixel) |
20355 (36341) |
18327 (16320) |
0.856 |
N=number of subjects, AUC = area under the curve, VAS = visual analogue scale
Table 2: Spearman correlation coefficients (rs) for associations between pain intensity (0-100), unpleasantness (0-100), anxiety (0-100), pain drawing area (pixel), and pain rating index (0-73) evoked by hypertonic saline injection into the masseter muscle of 25 healthy men and 31 healthy women.
|
|
|
Anxiety |
Unpleasantness |
Pain drawing area |
Pain Rating Index |
|
Pain intensity |
|
|
|
|
|
|
|
Men |
0.621 |
0.665 |
0.192 |
0.218 |
|
|
Women |
0.519 |
0.474 |
0.088 |
0.090 |
|
Anxiety |
|
|
|
|
|
|
|
Men |
|
0.641 |
0.285 |
0.274 |
|
|
Women |
|
0.426 |
0.345 |
0.306 |
|
Unpleasantness |
|
|
|
|
|
|
|
Men |
|
|
0.202 |
0.335 |
|
|
Women |
|
|
0.261 |
0.186 |
|
Pain drawing area |
|
|
|
|
|
|
|
Men |
|
|
|
0.284 |
|
|
Women |
|
|
|
0.279 |
Bold Figures denote significant differences after Bonferroni correction for multiple comparisons (p<0.01).



References
- Goldberg DS, McGee SJ (2011) Pain as a global public health priority. BMC Public Health 11: 770. [Crossref]
- Merskey H, Bogduk N (1994) Classification of chronic pain. 2nd edition: descriptions of chronic pain syndromes and definitions of pain terms prepared by the Task Force on Taxonomy of the International Association for the Study of Pain. Seattle:IASP Press.
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI et al. (2015) A classification of chronic pain for ICD-11 Pain 156:1003-1007. [Crossref]
- Hoffmann RG, Kotchen JM, Kotchen TA, Cowley T, Dasgupta M (2011) Temporomandibular disorders and associated clinical comorbidities. Clin J Pain 27: 268-274. [Crossref]
- Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV (2002) Oro-facial pain in the community: prevalence and associated impact. Community Dent Oral Epidemiol 30: 52-60. [Crossref]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D (2006) Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 10: 287-333. [Crossref]
- Yang Z, Zhao L, Xie X, Xu T, Zhang Y et al. (2017) The effectiveness of acupuncture for chronic pain with depression: A systematic review protocol. Medicine (Baltimore) 96: e8800. [Crossref]
- LeResche L (1997) Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med 8: 291-305. [Crossref]
- Lovgren A, Haggman-Henrikson B, Visscher CM, Lobbezoo F, Marklund S et al. (2016) Temporomandibular pain and jaw dysfunction at different ages covering the lifespan-A population-based study. Eur J Pain 20: 532-540. [Crossref]
- Dao TT, LeResche L (2000) Gender differences in pain. J Orofac Pain 14: 169-184. [Crossref].
- Yekkalam N, Wanman A (2014) Prevalence of signs and symptoms indicative of temporomandibular disorders and headaches in 35-, 50-, 65- and 75-year-olds living in Vasterbotten, Sweden. Acta Odontol Scand 272: 458-465. [Crossref]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd (2009) Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10: 447-485. [Crossref]
- Arendt-Nielsen L, Curatolo M, Drewes A (2007) Human experimental pain models in drug development: translational pain research. Curr Opin Investig Drugs 8: 41-53. [Crossref]
- Petersen-Felix S, Arendt-Nielsen L (2002) From pain research to pain treatment: the role of human experimental pain models. Best Pract Res Clin Anaesthesiol 16: 667-680. [Crossref]
- Svensson P, List T, Hector G (2001) Analysis of stimulus-evoked pain in patients with myofascial temporomandibular pain disorders. Pain 92: 399-409. [Crossref]
- Svensson P, Graven-Nielsen T (2001) Craniofacial muscle pain: review of mechanisms and clinical manifestations. J Orofac Pain 15: 117-145. [Crossref]
- Castrillon EE, Ernberg M, Cairns BE, Wang K, Sessle BJ et al. (2010) Interstitial glutamate concentration is elevated in the masseter muscle of myofascial temporomandibular disorder patients. J Orofac Pain 24: 350-360. [Crossref]
- Dawson A, Ghafouri B, Gerdle B, List T, Svensson P et al. (2013) Pain and intramuscular release of algesic substances in the masseter muscle after experimental tooth-clenching exercises in healthy subjects. J Orofac Pain 27: 350-360. [Crossref]
- Dowson A, Ghafouri B, Gerdle B, List T, Svensson P (2015) Effects of experimental tooth clenching on pain and intramuscular release of 5-HT and glutamate in patients with myofascial TMD. Clinc J Pain 31: 740-749. [Crossref]
- Louca S, Christidis N, Ghafouri B, Gerdle B, Svensson P et al. (2014) Serotonin, glutamate and glycerol are released after the injection of hypertonic saline into human masseter muscles – a microdialysis study. J Headache Pain 15: 89. [Crossref]
- Engmann L, Shaker A,White E, Bekir JS, Jacobs HS et al. (1998) Local side effects of subcutaneous and intramuscular urinary gonadotropins for ovarian stimulation in in vitro fertilization: a prospective, randomized study. Fertil Steril 69: 836-840. [Crossref]
- Ernberg M, Lundeberg T, Kopp S (2000) Effect of propranolol and granisetron on experimentally induced pain and allodynia/hyperalgesia by intramuscular injection of serotonin into the human masseter muscle. Pain 84: 339-346. [Crossref]
- Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P (2001) Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 86: 782-791. [Crossref]
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T et al (2003) Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain 101: 221-227. [Crossref]
- Frot M, Feine JS, Bushnell MC (2004) Sex differences in pain perception and anxiety A psychophysical study with topical capsaicin. Pain 108: 230-236. [Crossref]
- Christidis N, Ioannidou K, Milosevic M, Segerdahl M, Ernberg M (2008) Changes of hypertonic saline-induced masseter muscle pain characteristics, by an infusion of the serotonin receptor type 3 antagonist granisetron. J Pain 9: 892-901. [Crossref]
- Falla D, Arendt-Nielsen L, Farina D (2008) Gender-specific adaptations of upper trapezius muscle activity to acute nociceptive stimulation. Pain 138: 217-225. [Crossref]
- Louca S, Ernberg M, Christidis N (2013) Influence of intramuscular granisetron on experimentally induced muscle pain by acidic saline. J Oral Rehabil 40: 403-412. [Crossref]
- Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle B et al. (2008) Glutamate-evoked jaw muscle pain as a model of persistent myofascial TMD pain? Arch Oral Biol 53: 666-676. [Crossref]
- Ge HY, Madeleine P, Cairns BE, Arendt-Nielsen L (2006) Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: a potential experimental model of gender-specific differences. Clin J Pain 22: 37-44. [Crossref]
- Lei J, You HJ (2012) Variation of pain and vasomotor responses evoked by intramuscular infusion of hypertonic saline in human subjects: influence of gender and its potential neural mechanisms. Brain Res Bull 87: 564-570. [Crossref]
- Bartley EJ, Fillingim RB (2013) Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111: 52-58. [Crossref]
- Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA et al. (2006) Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 26: 5777-5785. [Crossref]
- Stephan CN (2010) The human masseter muscle and its biological correlates: A review of published data pertinent to face prediction. Forensic Sci Int 201: 153-159. [Crossref]
- Reis Durao AP, Morosolli A, Brown J, Jacobs R (2017) Masseter muscle measurement performed by ultrasound: a systematic review. Dentomaxillofac Radiol 46: 20170052. [Crossref]
- Brodsky MA, Swope DM, Grimes D (2012) Diffusion of botulinum toxins. Tremor Other Hyperkinet Mov(n y) 2: 6.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G et al. (2014) Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache 28: 6-27. [Crossref]
- Ferraz MB, Quaresma MR, Aquino LR, Atra E, Tugwell P et al. (1990) Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol 17: 1022-1024. [Crossref]
- Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle B et al. (2008) Glutamate-evoked jaw muscle pain as a model of persistent myofascial TMD pain? Arch Oral Biol 53: 666-676. [Crossref]
- Burckhardt CS, Bjelle A (1994) A Swedish version of the short-form McGill Pain Questionnaire. Scand J Rheumatol 23: 77-81. [Crossref]
- Melzack R (2005) The McGill pain questionnaire: from description to measurement. Anesthesiology 103: 199-202. [Crossref]
- Svensson P, Arendt-Nielsen L, Nielsen H, Larsen JK (1995) Effect of chronic and experimental jaw muscle pain on pain-pressure thresholds and stimulus-response curves. J Orofac Pain 9: 347-356. [Crossref]
- Riley JL 3rd, Robinson ME, Wise EA, Price DD (1998) A meta-analytic review of pain perception across the menstrual cycle. Pain 81: 225-235. [Crossref]
- Castrillon EE, Cairns BE, Wang K, Arendt-Nielsen L, Svensson P (2012) Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women. Pain 153:823-829. [Crossref]
- Vambheim S M, Flaten MA (2017) A systematic review of sex differences in the placebo and the nocebo effect. J Pain Res 10: 1831-1839. [Crossref]
- Graven-Nielsen T (2006) Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl: 122: 1-43. [Crossref]
- Okeson JP (2013) Management of Temporomandibular Disorders and Occlusion. 7th edn. Elsevier/Mosby, St Louis.
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T et al. (2003) Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain 101: 221-227. [Crossref]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF (2003) Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain 106: 253-261. [Crossref]
- Gazerani P, Andersen OK, Arendt-Nielsen L (2005) A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain: 118: 155-163. [Crossref]
- Iacovides, S, I Avidon, F, C Baker (2015) Does pain vary across the menstrual cycle? A review. Eur J Pain. 19: 1389-1405. [Crossref]
