Comparison of Harvesting and Processing Technique for Adipose Tissue Graft: Evaluation of Cell Viability
A B S T R A C T
Background: Clinical studies demonstrated the efficacy of therapies based on the autologous grafting of adult mesenchymal stem cells to accelerate the healing and regenerative processes of the skin and mesenchymal tissues; therefore, it is considered a valuable approach in the aesthetic rejuvenation treatment to give volumization and skin regeneration effects.
Objective: The aim of the project consisted of the control of cell viability of adipose tissue (AT) harvested using the two types of cannulas having 0.8 mm and 1 mm side port holes. The results were compared with tissue harvested with a standard liposuction technique and processed with a standard procedure consisting of enzymatic digestion (collagenase).
Methods: This study was performed on adipose tissues harvested from 7 patients (6 females and 1 male) with an average age of 48.5 years with 3 different techniques. We compared the cell vitality of every sample at T0 and T72.
Results: Lipoaspirate tissue-derived by 0.8- and 1 mm cannula from all samples proved to be vital and possess viable cells. The average absorbance was similar immediately after plating (T0) and 72 hours after (T72) for the two cannulas, 0.8- and 1 mm cannula. The two systems proved to equally harvest vital tissue. An increase in cell viability was observed in all samples for each condition (0.8-, 1 mm and enzymatic digestion).
Conclusion: This study proved that guided harvested adipose tissue with small cannulas with small side port holes yields a comparable amount of viable cells compared to adipose tissue harvested with a liposuction system and processed with enzymatic digestion (collagenase). This study confirms that the minimally invasive technique and minimal manipulation of the adipose tissue could yield a tissue with a good amount of viable cells. This micro fragmented adipose tissue is a promising source for regenerative treatments.
Keywords
Adipose-derived stem cells, autologous fat transfer, stromal-vascular fraction, clinical regeneration applications adipose tissue cell viability, adipose tissue manipulation, adipose tissue processing, adipose tissue harvesting
Introduction
Regenerative therapy based on the injection of micro fragmented adipose tissue is a promising treatment for degenerative diseases or disorders that cannot yet be successfully managed through conventional care; moreover, it is also a promising treatment in antiaging therapy. Regenerative therapy exploits the properties of the cells of stromal vascular fraction (SVF) naturally present in the adipose tissue. The most used cells in current cell-based approaches are the Mesenchymal Stem Cells (MSCs) which are multipotent stem cells present in almost every organ and tissue. Adipose tissue is a promising source of MSCs since Zuk et al. described it in 2001 as a stem cell population similar to the one from bone marrow [1-4]. Adipose-derived mesenchymal stem cells (ASCs) can be isolated through enzymatical digestion from the stromal vascular fraction (SVF), which contains a large number of cells composed of interrelated cell populations: adipocyte progenitors, pericytes, endothelial progenitor cells, and transit-amplifying cells [5]. ASCs have been shown to possess differentiation potential towards different lineages like osteogenic, chondrogenic, myogenic, hepatogenic and endothelial cells - both in vitro and in vivo [6, 7].
Moreover, like all MSCs, they exhibit antifibrotic and immunomodulatory characteristics and they stimulate angiogenesis and revascularization of fat grafts [8, 9]. Thanks to these characteristics, adipose tissue implantation has been used to improve skin trophism, accelerate the closure of complex wounds or ulcers, and enhancement of skin appearance after damage from radiotherapy [9, 10]. Recently M. Mantovani et al. proved the injection of micro fragmented adipose tissue as a promising therapy in GSM syndrome in gynaecology [11]. Therefore, the micro fragmented adipose tissue graft, naturally rich in cells from SVF and ADSCs, is considered a valuable approach in the aesthetic rejuvenation treatment to give volumization and skin regeneration effects [9, 12, 13]. Moreover, to obtain efficient engraftment and regenerative effect, superficial (subdermal plane) injection of smaller adipose tissue clusters is suggested [14, 15]. To date, we have two types of techniques to isolate SVF: enzymatic and mechanical. The enzymatic method is particularly indicated in SVF isolation since it disrupts the extracellular matrix (ECM) and the binding of adipocytes and other cells but is restricted by regulatory issues related to enzymatic procedures, especially within the European Community. Alternative mechanical methods were proposed by other authors who proved cell viability with mechanical procedures with mechanical manipulation of the adipose tissue [16].
In SEFFI (Superficial Enhanced Fluid Fat Injection) techniques, we proved that it is possible to obtain a good potential regenerative tissue with a good amount of viable cells with mechanical procedure moreover without any substantial manipulation; using micro-cannulas with very small side port holes (0.8 mm and 1 mm), we selected the clusters dimension during the guided harvesting procedure hence we did not need any substantial manipulation in order to thin the tissue [17-20]. In the light of these evidences, we tested cell viability of adipose tissue (AT) harvested using the three types of cannulas: 2 mm diameter cannula having 15 side port holes with 0.8 mm diameter (included in SEFFILLER™ device produced by SEFFILINE srl - Bologna, Italy) 2 mm diameter cannula having 16 side port holes with 1 mm diameter (included in SEFFICARE™, SEFFIGYN™, SEFFIHAIR™ SEFFICELLS™ devices produced by SEFFILINE srl - Bologna, Italy) and a standard liposuction cannula 4 mm diameter with 2 side port holes width 3mm height 5mm. The tissue harvested with the standard liposuction cannula was processed with a standard procedure consisting of enzymatic digestion (collagenase). The results of cell viability were compared.
Inclusion and Exclusion Criteria
This observational study was conducted under the Declaration of Helsinki’s guidelines. Before entering the study, all patients received detailed information regarding the procedure, purpose, and investigation's objective and provided written consent for participation and publication of data obtained. Inclusion and exclusion criteria are the following:
I Inclusion Criteria
i. Body Mass Index >25 kg/m2< 30 kg/m2
ii. Age <65 years old
II Exclusion Criteria
i. Diabetes mellitus type I and II
ii. Cardiovascular or neurologic disorders
iii. Patients in chronic drug therapy
iv. Smoke
v. Previous abdominal surgery (laparotomy)
Materials and Methods
In October 2021, we harvested adipose tissue from 7 consecutive patients (6 females and 1 male) with an average age of 48.5 years. The procedure was performed under local anaesthesia using the following cannulas (Figure 1):
i. Cannula 2mm, 15 side port holes, 0.8 mm diameter
ii. Cannula 2mm, 15 side port holes, 1 mm diameter
iii. Cannula 3mm, 2 side port holes, every hole width 2.5mm height 5mm (liposuction cannula)
Figure 1: The three cannulas we used for this study. From the top to the bottom: cannula 2 mm, 15 side port holes, 1 mm diameter; cannula 2 mm, 15 side port holes, 0.8 mm diameter; cannula 3 mm 2 side port holes, every hole width 2.5 mm height 5mm (liposuction cannula).
The guided harvesting procedures with a cannula with side port holes 0.8 mm and 1 mm were performed respectively with the medical devices SEFFILLER™ and SEFFICELLS™ and the procedure was performed according to their instructions for use (Figure 2). Devices are Produced by SEFFILINE srl, Bologna, Italy: the cannula with 15 side port holes, 1 mm diameter, is included in the SEFFICELLS™ device for the US market and in SEFFICARE™ and SEFFIGYN™ devices for the European market. The guide included in these devices is addressed to standardize the procedure and guarantee that tunneling is performed in the subcutaneous tissue adjacent to the dermis, particularly rich in cells of the stromal vascular fraction (SVF) without any risk of injuries to the deep structures (Figure 3) [21, 22]. The tissue harvested with liposuction technique is performed with cannula 3mm diameter; it was administrated with Klein’s tumescent solution and proceeded with aspiration after the infiltration. The harvesting procedure with liposuction cannula is performed with a standard no guided liposuction.
Figure 2: The harvesting procedures with cannulas with side port holes 0.8 mm and 1 mm are performed with local anaesthesia. The guided procedure: I) penetration of the tip of the cannula until the stop of the guide. II) Rotation and insertion of the cannula in the subcutaneous tissue. During the procedure, the blade of the guide touches the skin. III) The fluid harvested tissue inside the syringe. IV) The harvesting syringe with plunger lock. The procedure is according to their instructions for use.
Figure 3: The special guide allows the harvesting of tissue in the superficial subcutaneous layer. This layer is safe and it yields to collect micro fragmented adipose tissue richer in SVF cells.
We analysed seven samples. Adipose tissue using 0.8- and 1 mm side port holes cannulas were harvested and processed according to their instruction for use (produced by SEFFILINE srl Bologna Italy: SEFFILLER™ SEFFICELLS™ SEFFIGYN™ SEFFICARE™), were monitored for their cell viability using Presto Blue assay (Thermo Fisher Scientific). 100 μl of cell suspension were plated in a 96 well plate and 10 μl of Presto blue was added to each well. For every individual and two types of cannula triplicate were run. The cell viability alterations were analysed via absorbance spectroscopy and we reported the absorbance 570 nm after 10 min incubation (T0) at 37°C through multi-mode microplate readers (VICTOR Multilabel plate reader, PerkinElemer). Cell viability was evaluated at T0 and T72 and graphed.
Adipose tissue harvested using liposuction cannula was processed by enzymatic treatment as quality control of tissue. The lipoaspirates were digested at 37°C in DMEM with 0.25% weight per volume percent (w/v) collagenase type I (Sigma-Aldrich) and 1% fetal bovine serum (FBS) for 180 min at 37°C. Following digestion, we filtered the resulting suspension through a sterile 100 μm nylon mesh to remove undigested parts and centrifuged the remaining suspension at 1200 g for 10 min to extract a high-density pellet composed of the stromal vascular fraction (SVF). The SVF thus obtained was re-suspended in 1 ml DMEM-low glucose supplemented with 10% FBS and 1% penicillin-streptomycin (all Gibco, Thermo Fisher Scientific) and cells were counted by Crystal violet to exclude a-nucleated cells. Cells were then plated at a density of 10,000 cells/cm2 in a 96 well plate in growth medium with the addition of 10% of Presto blue and stored in a humidified incubator at 37°C with 5% CO2. Cell viability was assessed after 10 minutes (T0) and after 72 hours (T72).
Results
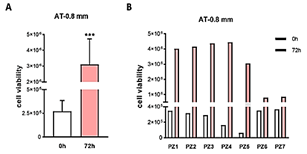
Lipoaspirate tissue-derived by 0.8- and 1 mm side port holes cannulas from all samples proved to be vital and possess viable cells. In adipose tissue harvested using a cannula of 0.8 mm side port holes, after 72 hours, adipose tissue resulted statistically more vital, meaning that released cells from the harvesting procedure and cells entrapped in the extracellular matrix are vital and metabolically active (Student’s t-test, *** p<0.001) (Figure 4). In adipose tissue harvested using a cannula of 1 mm side port holes after 72 hours, adipose tissue resulted statistically more vital, meaning that released cells from the harvesting procedure and cells entrapped in the extracellular matrix are vital and metabolically active (Student’s t-test, ** p<0.01) (Figure 5). In the adipose tissue harvested with liposuction technique using a cannula with 2 side port holes, every hole width 2.5 mm height 5mm, and enzymatically processed (collagenase). After 72 hours, the absorbance of SVF cells resulted statistically more metabolically active and proliferative compared to time 0 (Student’s t-test, **** p<0.0001) (Figure 6).
Figure 4: Cell viability of adipose tissue harvested using cannula of 0.8 mm side port holes. A) Average absorbance values are represented as an average for time point 0 (0h) and after 72 hours of incubation (72h). After 72 hours, AT resulted statistically more vital, meaning that released cells from the harvesting procedure and cells entrapped in the extracellular matrix are vital and metabolically active (Student’s t-test, *** p<0.001). B) Single value of each individual (from patient 1, PZ1, to patient 7, PZ7). PZ6 and PZ7 showed a lower level of cell viability after 72 hours compared to the other.
Figure 5: Cell viability of adipose tissue harvested using the cannula of 1 mm side port holes. A) Average absorbances values are represented as an average for time point 0 (0h) and after 72 hours of incubation (72h). After 72 hours, AT resulted statistically more vital, meaning that released cells from the harvesting procedure and cells entrapped in the extracellular matrix are vital and metabolically active (Student’s t-test, ** p<0.01). B) Single value of each individual (from patient 1, PZ1, to patient 7, PZ7). PZ5, PZ6 and PZ7 showed a lower level of cell viability.
Figure 6: Cell viability of stromal vascular fraction cells (SVF). SVF was derived by enzymatic treatment of adipose tissue harvested using a liposuction cannula and 10,000 cells/cm2 were plated in a 96 well plate for analysis. A) Average absorbance values are represented as an average for time point 0 (0h) and after 72 hours of incubation (72h). After 72 hours, the absorbance of SVF cells resulted as statistically more metabolically active and proliferative compared to time 0 (Student’s t-test, **** p<0.0001). B) Single value of each individual (from patient 1, PZ1, to patient 7, PZ7). Only PZ7 resulted in a lower level of cell viability after 72 hours compared to the other 4 individuals.
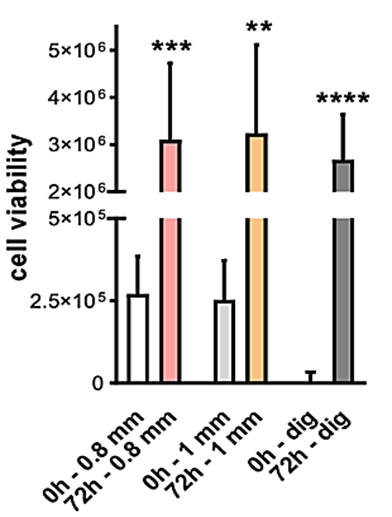
The average absorbance was similar immediately after plating (T0) and 72 hours after (T72) for the two cannulas, 0.8- and 1 mm cannula. The two systems proved to equally harvest vital tissue. An increase in cell viability was observed in all samples for each condition (0.8-, 1 mm and enzymatic digestion). The increase of absorbance signal is related to an increase in metabolic activity of cells, meaning that the adipose tissue and released cells (mesenchymal, pericytes and cells from the immune system) are vital and proliferate during the 72 hours of incubation. Moreover, absorbance at time 0 is higher in tissue compared to isolated SVF. However, absorbance after 72 hours is similar between the two cannula and enzymatic digestion method. Statistics were run compared to the time 0 of each group (Student’s t-test) (Figure 7).
Figure 7: Cell viability of AT and SVF from 7 individuals. SEFFILLER™ 0.8 mm, SEFFICELLS™ 1mm, liposuction cannula enzymatic digestion (collagenase).
Conclusion
Adipose tissue harvested using the three types of cannulas, 0.8 mm (included in SEFFILLER™ device), 1 mm side port holes (included in SEFFICELLS™, SEFFIGYN™ and SEFFICARE™ devices) and standard liposuction technique followed by gold-standard enzymatic digestion (collagenase), were analysed for their viability and it was confirmed that the tissue was vital and contained metabolically active cells. Indeed, viable and metabolically active cells reduce resazurin contained in the Presto blue kit, leading to the increase of absorbance value, which means that the cannula did not affect the cell viability of the tissue. The two types of cannulas (0.8- and 1mm side port holes) showed the same absorbance at time 0 and after 72 hours, meaning that the two systems are equivalent in the viability of the tissue. Moreover, values at 72 hours are similar compared to SVF cells derived by enzymatic digestion of the liposuction tissue, which confirmed the presence of viable and proliferative cells in the tissue itself and/or cells released by the mechanical procedure.
SVF cells showed a lower level of absorbance at time 0 because of a low plate density compared to the tissue itself, which possesses a complex structure having many cells within. The tissue harvested with 0.8 and 1mm side port holes showed a higher level of absorbance at T0 because of the single cells released from the mechanical action of the cannula. The enzymatic method (collagenase) is particularly indicated in SVF isolation since it disrupts the extracellular matrix (ECM) and the binding of adipocytes and other cells but is restricted by regulatory issues related to enzymatic procedures, especially within the European Community. This study confirmed that the tissue harvested with guided microcannula with small side port holes (0.8- and 1mm) and without any chemical or mechanical manipulation presents viable and proliferative cells. In the light of these evidences, we consider the adipose tissue harvested with guided micro-cannula without any substantial manipulation is a promising tissue in regenerative therapy. However, further studies are required to support and improve our findings.
Author Contributions
Alessandro Gennai and Silvia Zia: Performed procedure and data acquisition, made substantial contributions to conception and design of the study and performed data analysis and interpretation and draft of the article; Barbara Roda: Made substantial contributions to conception and design of the study; Andrea Zattoni, Pierluigi Reschiglian: Final approval of the version to be published; B. Bovani, M. Colli, F. Melfa, D. Piccolo, R. Russo: Made substantial contributions in selecting patients and performing procedure.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 12, Nov 2021Accepted: Fri 03, Dec 2021
Published: Mon 20, Dec 2021
Copyright
© 2023 Alessandro Gennai. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2021.02.03
Author Info
Alessandro Gennai B Bovani M Colli F Melfa D Piccolo R Russo Barbara Roda Andrea Zattoni Pierluigi Reschiglian Silvia Zia
Corresponding Author
Alessandro GennaiPlastic Surgeon, Private Practice, Studio Gennai, Bologna, Italy
Figures & Tables





References
1. Zuk PA, Zhu M,
Mizuno H, Huang J, Futrell JW et al. (2001) Multilineage cells from human
adipose tissue: implications for cell-based therapies. Tissue Eng 7:
211-228. [Crossref]
2. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI et al. (2002) Human
adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:
4279-4295. [Crossref]
3. Zuk PA (2010) The
adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell
21: 1783-1787. [Crossref]
4. Crisan M, Yap S,
Casteilla L, Chen CW, Corselli M et al. (2008) A perivascular origin for
mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:
301-313. [Crossref]
5. Tallone T, Realini C, Bohmler A, Kornfeld C, Vassalli G et al. (2011) Adult human
adipose tissue contains several types of multipotent cells. J Cardiovasc
Transl Res 4: 200-210. [Crossref]
6. Huang JI, Beanes
SR, Zhu M, Lorenz HP, Hedrick MH et al. (2002) Rat extramedullary adipose
tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr
Surg 109: 1033-1041. [Crossref]
7. Fraser JK,
Schreiber R, Strem B, Zhu M, Alfonso Z et al. (2006) Plasticity of human
adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin
Pract Cardiovasc Med 3: S33-S37. [Crossref]
8. Blaber SP, Webster
RA, Hill CJ, Breen EJ, Kuah D et al. (2012) Analysis of in vitro secretion
profiles from adipose-derived cell populations. J Transl Med 10: 172. [Crossref]
9. Coleman SR (2001)
Structural fat grafts: the ideal filler? Clin Plast Surg 28: 111-119. [Crossref]
10. Del Papa N, Di Luca G, Sambataro D, Zaccara E, Maglione W et al. (2015) Regional
implantation of autologous adipose tissue- derived cells induces a prompt
healing of long-lasting indolent digital ulcers in patients with systemic
sclerosis. Cell Transplant 24: 2297-2305. [Crossref]
11. Mantovani M, Gennai
A, Russo PR (2021) A new approach to regenerative medicine in gynecology. Int
J Gynecol Obstet. [Crossref]
12. Rigotti G, Marchi A, Galie M, Baroni G, Benati D et al. (2007) Clinical
treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing
process mediated by adipose-derived adult stem cells. Plast Reconstr Surg
119: 1409-1422. [Crossref]
13. Gennai A (2011) Endochirurgia Estetica Del Volto: SEE Editrice, Firenze. Acta Medica
Edizioni.
14. Gennai A, Saponaro A, Iozzo I (2009) El rol del lifting endoscópico
fronto-témporo-orbitario enel nuevo concepto de rejuvenecimiento facial: mini
invasivo, tensión moderada, restauración de volúmenes. Cirugia Plástica
Ibero-Latinoamericana
27-34.
15. Zeltzer AA, Tonnard
PL, Verpaele AM (2012) Sharp-needle intradermal fat grafting (SNIF). Aesthet
Surg J 32: 554-561. [Crossref]
16. Senesi L, De Francesco F, Farinelli L, Manzotti S, Gagliardi G et al. (2019) Mechanical
and Enzymatic Procedures to Isolate the Stromal Vascular Fraction From Adipose
Tissue: Preliminary Results. Front Cell Dev Biol 7: 88. [Crossref]
17. Gennai A,
Bernardini FP (2015) R3 facial rejuvenation through minimal incisions vertical
endoscopic lifting (MIVEL) and superficial enhanced fluid fat injection
(SEFFI): endoscopic repositioning, tissue regeneration, volume restoration. Aesthetic
Med 1: 54-60.
18. Bernardini FP, Gennai A, Izzo L, Zambelli A, Repaci E et al. (2015) Superficial
Enhanced Fluid Fat Injection (SEFFI) to correct volume defects and skin aging
of the face and periocular region. Aesthet Surg J 35: 504-515. [Crossref]
19. Bernardini FP,
Gennai A (2016) Fluid Fat Injection for Volume Restoration and Skin
Regeneration of the Periocular Aesthetic Unit. JAMA Facial Plast Surg
18: 68-70. [Crossref]
20. Tesauro P,
Trivisonno A, Gennai A, Marliani A, Clauser L (2020) Hair Transplantation in
Cicatricial Alopecia: The Role of Autologous Fat Transfer. Int J Regen Med
3: 2-8.
21. Trivisonno A, Di Rocco G, Cannistra C, Finocchi V, Farr ST et al. (2014) Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet Surg J. 34: 601-613. [Crossref]
22. Di Taranto G, Cicione C, Visconti G, Isgrò MA, Barba M et al. (2015) Qualitative and quantitative differences of adipose- derived stromal cells from superficial and deep subcutaneous lipoaspirates: a matter of fat. Cytotherapy 17: 1076-1089. [Crossref]
