Comparative Study of PD-L1 Status between Surgically Resected Specimens and Matched Biopsies of Gastric Cancer Reveal Major Discordances: A Potential Issue for Anti-PD-L1 Therapeutic Strategies
A B S T R A C T
Background: Inhibitors of programmed death-ligand 1 (PD-L1) have potential therapeutic value in gastric cancer. We investigated PD-L1 expression patterns in paired biopsy and resection specimens.
Patients and Methods: Thirty-nine formalin-fixed, paraffin-embedded paired samples were assessed using PD-L1 22C3 pharmDx immunohistochemistry technique. Combined positive score (CPS) was calculated as the ratio of PD-L1 stained cells (tumor cells, lymphocytes, and macrophages) to the total number of viable tumor cells, multiplied by 100. The CPS ≥1 indicated PD-L1 positivity.
Results: PD-L1 positivity was evident for 33 (84.6%) of 39 resection cases; all displayed low positivity (1≤CPS<50). Only 10 (30.3%) of 33 positive cases in the resection specimens had simultaneous PD-L1 positivity in the paired biopsy specimens; two cases displayed high positivity (CPS 50 and 70) and eight displayed low positivity (1≤CPS≤50). Among the 29 negative cases with biopsy specimens, 23 (79.3%) displayed PD-L1 low positivity in the paired resection specimens and only six had concordant negativity in both specimens with poor agreement (concordance rate 41.0%, k value = 0.118, correlation coefficient 0.234; p=0.152). All the high microsatellite instability cases had concordant PD-L1 positivity in resection and biopsy specimens.
Conclusions: There was relatively poor agreement of PD-L1 expression between biopsy and resected tumor specimens. The biopsy specimens underestimated the PD-L1 status observed for the total resected samples. This indicates the necessity of obtaining multiple biopsies from different areas of the tumor to enhance the validity and reliability of PD-L1 analysis.
Keywords
PD-L1, immunotherapy, immunohistochemistry, gastric cancer
Introduction
Gastric cancer is the fifth most malignant disease worldwide and the third most common cause of cancer-associated death [1]. The prognosis of advanced gastric cancer is still not favourable, despite of the pronounced progress in treatment options, including targeted therapy against human epidermal growth factor receptor 2 (HER2) [2]. Therefore, more efficient therapies for advanced stages of gastric cancers are needed. Programmed death-ligand 1 (PD-L1) is expressed in many malignant tumors and has a noticeable potential to be a target for immunotherapy. PD-L1 is a 40 kDa transmembrane protein and is a member of the B7 protein family. It is involved in the immunoregulatory system, acts as a programmed cell death-1 (PD-1_ ligand and is often overexpressed in many malignant tumors [3]. Activation of the PD-1/PD-L1 signaling pathway leads to a tolerable mechanism for malignant tumors [4, 5]. Inhibition of the PD-1/PD-L1 checkpoint is a strategy in cancer immunotherapy when antitumor immunity is ineffective against PD-L1-producing tumors [6-8]. An immune checkpoint inhibitor, targeting the PD-1 pathway, has proven to be efficacious in several cancers, including lung cancer and melanoma [9-11].
Recently, two anti-PD-1 immunotherapy molecules, pembrolizumab and nivolumab, were approved as standard treatment for advanced gastric cancer and the corresponding clinical trials are currently ongoing [12, 13]. Despite the promising therapeutic effect of immune checkpoint inhibitors, the results concerning the prognostic and predictive roles of PD-L1 expression have been inconsistent [14]. This is due to the various antibody clones, analysis criteria, and platforms used in the different studies [15]. Therefore, a standard PD-L1 immunohistochemistry detection strategy is necessary. The intratumoral heterogeneous expression of PD-L1 is also an important factor to consider in the interpretation of PD-L1 expression [14-16]. Due to these limitations, patient selection is crucial to the success of immunotherapy, using immune checkpoint inhibitors [17]. To select patients, the PD-L1 expression status should be evaluated by immunohistochemistry using biopsy or resection specimens. PD-L1 testing is usually carried out on biopsy specimens in cases of lung cancer. However, the use of biopsy tissue might not be representative of the expression of PD-L1 in the whole tumor, which can deliver false-positive or false-negative results and lead to unnecessary treatment or insufficient treatment [18]. The discordance between resection and biopsy specimens has been addressed in lung cancer cases. However, the correlation of PD-L1 expression between biopsy and corresponding resected specimens in gastric cancer has not been yet reported [15, 18, 19]. The aim of the present study was to characterize the PD-L1 expression pattern and to assess the reliability of the profiles obtained from biopsy and resection specimens in representing the PD-L1 expression status in gastric carcinomas.
Materials and Methods
I Patients and Samples
Between January 2014 and October 2015, a consecutive cohort of 438 patients with advanced gastric adenocarcinoma was treated with palliative chemotherapy at Yonsei Cancer Center, Yonsei University College of Medicine. Among those, 39 cases with available biopsy and corresponding resected specimens were selected. Patient information was retrospectively collected by reviewing the medical records for evaluation of clinicopathologic characteristics and survival outcome. Staging was determined using the 8th edition of the American Joint Committee on Cancer guideline and Dukes-MAC-like staging system [20, 21].
II Immunohistochemistry and PD-L1 Evaluation
Immunohistochemical staining was performed using the PD-L1 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA) and the link 48 system (Dako, Carpinteria, CA). The specimens were stained with an anti-PD-L1 22C3 mouse monoclonal primary antibody with the EnVision FLEX visualization system (Agilent Technologies) along with negative control reagents [22]. Evaluation of HER2, EBV, and mismatch repair protein was performed based on previous studies as shown in (Supplementary Figure 1) [23-25]. In brief, IHC was performed on a Ventana XT automated staining instrument (Ventana Medical Systems, Tucson, AZ, USA). Target-specific antibodies were used according to the manufacturer’s instructions. The following clones were applied: MutL homolog 1 (MLH1, ready to use, clone M1, Roche, Basel, Switzerland), MutS protein homolog 2 (MSH2, ready to use, clone G219-1129, Roche), MutS homolog 6 (MSH6, 1:100, clone 44, Cell Marque, Rocklin, CA, USA), and postmeiotic segregation increased 2 (PMS2, 1:40, clone MRQ28, Cell Marque). A loss of mismatch repair (MMR) protein expression was defined as none of the neoplastic cells showing nuclear staining, whereas normal expression was defined as the presence of nuclear expression in tumor cells, irrespective of the proportion or intensity. Epstein-Barr virus-encoded small RNAs (EBER) in situ hybridization (ISH) was performed using a Ventana Benchmark ISH system and ISH iView kit (Ventana Medical Systems, Tucson, AZ, USA). A tumor was considered as EBER-negative, if EBER staining was undetected or it was localized only in benign-appearing lymphoid cells and EBER-positive, if the signal was localized to malignant epithelial cells.
The expression level was evaluated by four pathologists (YYR, HMJ, MJK, and SHK). We separately counted PD-L1 positive tumor cells and PD-L1 positive tumor-associated mononuclear inflammatory cells, including lymphocytes and macrophages, which are present within the tumor nest or adjacent stroma. The total number of PD-L1 positive cells is a numerator in combined positive score (CPS), which is defined as a ratio of PD-L1-positive cells [tumor cells, macrophages, lymphocytes] to the total number of tumor cells, with that value multiplied by 100 [13, 22, 26]. We did not include PD-L1 positive immune cells that are associated with normal structure, neutrophils, eosinophils, plasma cells, ganglion cells, or stromal cells, including fibroblasts, as recommended in the PD-L1 IHC 22C3 pharmDX interpretation manual and as performed in KEYNOTE-012 [17].
III Statistical Analyses
Pearson's chi-square test (or Fisher’s exact test for cases with an n value of <10) was used for categorical variables. We evaluated the concordance rate between resection and biopsy specimens, as well as Cohen’s k coefficient. All the P-values were two-sided, and p<0.05 was considered as statistically significant. Statistical analysis was conducted using SPSS (version 21.0; SPSS, Inc., Chicago, IL).
Results
I Clinicopathological Characteristics
The 39 paired cases comprised 38 gastric carcinomas and one gastroesophageal junction carcinoma. The majority of patients were men (79.5%). Ages ranged from 30 to 80 years (Table 1). The majority of carcinomas were poorly differentiated or undifferentiated (n=26, 66.7%), Epstein-Barr virus (EBV)-negative (n=25, 89.7%), and microsatellite stable/microsatellite instability-low (MSS/MSI-L, n=35, 89.7%). Thirty-five cases were categorized to stage III and IV. Most of the MSS/MSI-L cases in resection specimens expressed PD-L1 (n=29, 82.9%). Interestingly, all the MSI-high cases expressed concordant PD-L1 positivity (CPS range 3-10). The majority of the PD-L1 positive resection specimens were of the intestinal type without statistical significance. There was no significant difference with respect to age, pathologic tumor-node-metastasis (TNM) stage, Duke-MAC-like stage, histologic classification, and EBV status.
Table 1: Clinical and pathological characteristics of patients and associations between PD-L1 positivity in resection and biopsy and clinicopathologic characteristics.
|
Parameters |
Case no. |
PD-L1 expression (CPS) Resection |
PD-L1 expression (CPS) Biopsy |
||||
|
CPS≥1 |
CPS<1 |
P-value |
CPS≥1 |
CPS<1 |
P-value |
||
|
Gender Male Female |
31 8 |
26 (83.9%) 7 (87.5%) |
5 (16.1%) 1 (12.5%) |
0.8 |
10 (32.3%) 0 (0%) |
21 (67.7%) 8 (100.0%) |
0.06 |
|
Mean age (range) |
|
57.8 (30-74) |
62.2 (59-80) |
|
58.1 (44-70) |
58.7 (30-80) |
|
|
AJCC Stage I/II III/IV |
14 25 |
12 (85.7%) 21 (84.0%) |
2 (14.3%) 4 (16.0%) |
0.89 |
4 (28.6%) 6 (24.0%) |
10 (71.4%) 19 (76.0%) |
0.75 |
|
T Stage T2 T3 T4a T4b |
3 17 18 1 |
2 (66.7%) 14 (85.7%) 16 (84.0%) 1 (100.0%) |
1 (33.3%) 3 (14.3%) 2 (16.0%) 0 (0%) |
0.74
|
1 (33.3%) 3 (28.6%) 6 (33.3%) 0 (0%) |
2 (66.7%) 14 (71.4%) 12 (66.7%) 1 (100.0%) |
0.67
|
|
N Stage N0 N1 N2 N3a N3b |
5 11 9 4 10 |
4 (80.0%) 8 (72.7%) 9 (100.0%) 4 (100.0%) 8 (80.0 %) |
1 (20.0%) 3 (27.3%) 0 (0%) 0 (0%) 2 (20.0 %) |
0.43
|
2 (40.0%) 3 (27.3%) 2 (22.2%) 1 (25.0%) 2 (20.0%) |
3 (60.0%) 8 (72.7%) 7 (77.8%) 3 (75.0%) 8 (80.0%) |
0.94
|
|
Dukes-MAC like stage B2 C1 C2 D |
4 2 14 19 |
3 (75.0%) 1 (50.0%) 12 (85.7%) 17 (89.5 %) |
1 (25.0%) 1 (50.0%) 2 (14.3%) 2 (10.5 %) |
0.48
|
1 (75.0%) 1 (50.0%) 2 (14.3%) 6 (31.6%) |
3 (25.0%) 1 (50.0%) 12 (85.7%) 13 (68.4%) |
0.59
|
|
WHO classification WD/MD PD/UND SRRC GCLS |
10 26 2 1 |
9 (90.0%) 23 (88.5%) 0 (0%) 1 (100.0%) |
1 (10.0%) 3 (11.5%) 2(100.0%) 0 (0%) |
0.01
|
3 (30.0%) 7 (27.0%) 0 (0%) 0 (0%) |
7 (70.0%) 19 (73.0%) 2 (100.0%) 1 (100.0%) |
0.67 |
|
EBV Positive Negative MSI MSI-H MSS/MSI-L |
4 35
4 35 |
4 (100.0%) 29 (82.9%)
4 (100.0%) 29 (82.9%) |
0 (0%) 6 (17.1%)
0 (0%) 6 (17.1%) |
1.00
1.00 |
2 (50%) 8 (22.9%)
4 (100.0%) 6 (17.1%) |
2 (50%) 27 (77.1%)
0 (0%) 29 (82.9%) |
0.27
0.003 |
|
Total |
39 |
33 (84.6%) |
6 (15.4%) |
|
29 (74.4%) |
10 (25.6%) |
|
II Pattern of PD-L1 Expression in GC
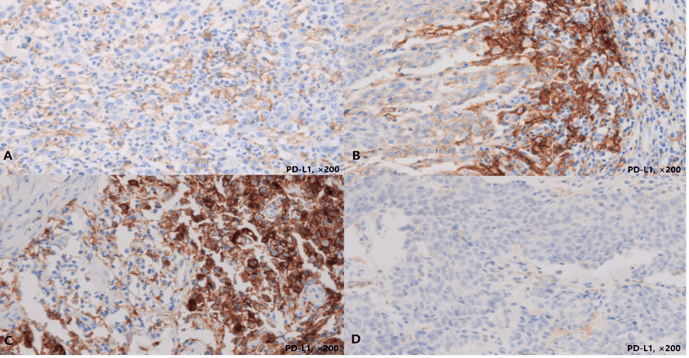
Thirty-three (84.6%) of the 39 resection cases displayed PD-L1 positivity (CPS≥1). All of these displayed low positivity (1≤CPS<50). In biopsy cases, only 10 (25.6%) samples displayed PD-L1 positivity (CPS≥1), and two of these cases had CPS≥50 (Table 2). Regarding the expression patterns, the majority of the negative cases (17/29, 58.6%) in the biopsy specimens were completely negative, while only 6% (2/33) negative cases in the resection specimens displayed completely negative staining. Overall, the positive and negative predictive value of PD-L1 in biopsies was 100 and 20.7%, respectively. Figure 1 shows partial or complete linear membrane PD-L1 staining mainly in tumor cells (Figure 1A), and both in tumor cell and tumor-associated mononuclear inflammatory cells (Figure 1B) among resection cases. Figure 1C shows significantly high expression of PD-L1 and (Figure 1D) shows focal low partial linear membrane staining only in tumor cells for representative biopsy cases.
Table 2: Comparison of PD-L1 expression between cancer resection and biopsy specimens by immunohistochemistry.
|
|
|
Resection |
Correlation coefficient |
|||
|
|
|
Negative (CPS<1) |
Low positive 1≤CPS<50 |
High positive (CPS≥50) |
Total |
|
|
Biopsy |
Negative (CPS<1) |
6 (15.4%) |
23 (59.0%) |
0 (%) |
29 (74.4%) |
0.234 |
|
Low positive (1≤CPS<50) |
0 (0%) |
8 (20.5%) |
0 (0%) |
8 (20.5%) |
||
|
High positive (CPS≥50) |
0 (0%) |
2 (5.1%) |
0 (0%) |
2 (5.1%) |
||
|
|
Total |
6 (15.4%) |
33 (84.6%) |
0 (0%) |
39 (100.0%) |
|
*CPS (Combined positive score): Number of PD-L1–positive cells [tumor cells, macrophages, lymphocytes] divided by the total number of tumor cells, multiplied by 100.
Figure 1: Immunohistochemistry of PD-L1 in gastric carcinomas in resection and biopsy specimens.
Representative images of the diffuse expression resection case (A, CPS 30, ×200) and focal expression resection case (B, CPS 5, ×200), showing PD-L1 expression in both tumor cells and tumor-associated mononuclear inflammatory cells. Diffuse high expression in a biopsy sample (C, CPS 75, ×200), focal low expression in PD-L1 positive biopsy sample (D, CPS 5, ×200), without PD-L1 expression in the immune cells.
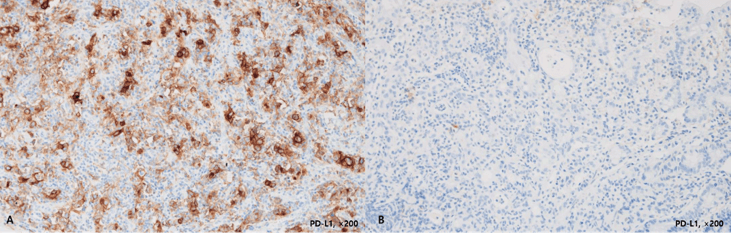
Figure 2: Discordance of PD-L1 expression between matched biopsy and resection specimens.
Representative image resection of a PD-L1 positive case (A, CPS 35, ×200) and paired biopsy specimen without confidential PD-L1 expression (B, CPS<1, ×200).
III Discordance of PD-L1 Expression between Biopsy and Resection Specimens
Only 10 (30.3%) of 33 positive cases in resection specimens had simultaneous PD-L1 positivity in paired biopsy specimens. Of these, two displayed high positivity (CPS 50 and 70) and the remaining eight cases displayed low positivity (1≤CPS≤50). Among the 29 negative cases in the biopsy specimens, 23 (79.3%) displayed low PD-L1 positivity in the paired resection specimens and only six displayed concordant negativities in both specimens (Figure 2). The overall resection-biopsy concordance rate was 41.0% (k value 0.118. correlation coefficient 0.234; p=0.152).
IV PD-L1 Expression in Tumor and Inflammatory Cells
Of the 33 (84.6%) PD-L1 positive cases (CPS≥1) among resection samples, 22 cases (56.4%) showed PD-L1 positivity only in tumor cells. The remaining 11 (28.2%) cases showed PD-L1 positivity in both tumor cells and tumor infiltrating lymphocytes, resulting in overall PD-L1 positivity with CPS≥1. However, all the PD-L1 positive cases in biopsy samples were positive only in tumor cells (Table 3).
Table 3: Influence of immune cell PD-L1 positivity on evaluation of PD-L1 expression in gastric carcinomas.
|
|
Number of cases |
|
|
|
Resection (N=39) |
Biopsy (N=39) |
|
TC + only |
22 (56.4%) |
10 (25.6%) |
|
TC+/IC+ |
11 (28.2%) |
0 |
|
Total (CPS≥1) |
33 (84.6%) |
10 (25.6%) |
*TC +: Tumor cell PD-L1 positivity.
*IC +: Immune cell PD-L1 positivity.
Discussion
The aim of our study was to characterize the PD-L1 expression pattern in gastric cancers to determine whether PD-L1 immunohistochemistry is a reliable and concordant hallmark, regardless of sample status. PD-L1 positivity was markedly discordant in matched biopsy and gastrectomy samples. The discordance was mainly due to false-negative issues of biopsy specimens. No meaningful association was found between the patient clinicopathological characteristics and PD-L1 expression. In the present study, the immunohistochemical expression of PD-L1 in biopsy and surgically resected specimens did not correlate significantly, indicating that the evaluation of PD-L1 expression status using biopsy specimens might not adequately represent the PD-L1 status of the patient.
The tumor microenvironment is important in the context of the therapeutic effect of the PD-L1 inhibitor in terms of T-cell repletion [27]. PD-L1 is expressed in both tumor and tumor-infiltrating immune cells in gastroesophageal carcinomas. PD-L1 expression in the immune cells is also an essential issue. The differences in the cut-off value and in the antibody assays, developed by different drug companies, are the main concerns in the immunotherapy of lung cancers. The expression pattern of PD-L1 in the tumor infiltrating lymphocytes, including CD8+ T cells, their association with clinical outcome, and their predictive relevance were recently reported [6, 26, 28]. A recent phase 2 clinical trial provided convincing evidence of the safety and efficacy of anti-PD-1 immunotherapy in advanced gastric and gastroesophageal junction cancer. In that study, CPS harbouring PD-L1 positivity in immune cells was used as a criterion to evaluate the PD-L1 expression status [13]. The importance of immune cells in terms of the therapeutic effect of anti-PD-1/PD-L1 agents is unclear, and the specifics of anti-PD-1/PD-L1 therapy are ambiguous. However, the level of PD-L1 expression in tumor-associated immune cells becomes an important issue, when a particular therapy must be selected for the patient. Studies examining the discordance of PD-L1 expression between resection and biopsy samples have mainly focused on lung cancers, especially non-small cell lung cancers [18, 29].
The frequency of PD-L1 positivity in tumor infiltrating lymphocytes was higher in resection samples than in biopsy samples. Furthermore, PD-L1 negative cases in biopsy specimens demonstrated immune cell positivity in matched resection specimens. Although immune cell PD-L1 positivity seemed to be more frequent in resection samples, only tumor cell positive cases (TC+ and TC+/IC+) were defined as CPS≥1, implying that cells other than immune cells are involved. Therefore, low PD-L1 positivity in tumor cells may be the main reason for the underestimation of biopsy specimens, and results of PD-L1 testing based only on biopsy specimens could lead to incorrect diagnosis for the expression status of PD-L1 and, subsequently, to insufficient treatment. Likewise, even though all EBV-positive cases showed PD-L1 positivity in the resection specimens, only half of them were PD-L1 positive in biopsy samples. This discrepancy could be also explained by the intratumoral heterogeneity of PD-L1 expression in gastric cancer. Therefore, the heterogeneous expression of PD-L1, even in immune cells, is a limitation for the exclusive use of a biopsy specimen to evaluate PD-L1 expression.
Interestingly, all the MSI-high cases had concordant PD-L1 positivity in resection and biopsy specimens. Therefore, immunostaining of PD-L1 may be useful regardless of tumor specimens. However, interpretation of the association between PD-L1 expression, MSI status, and tumor type is limited due to the small number of non-intestinal type cases. MSI-high tumors produce tumor neoantigens that can evade the host immune response through the upregulation of immune checkpoints, and MSI-high gastroesophageal cancers show a more favourable response to PD-L1 inhibitors [3, 30]. Thus, MSI-high status could be substituted with a composite predictive biomarker for PD-L1 expression level in gastroesophageal cancers.
Likewise, our results indicate that the heterogeneity of PD-L1 expression in gastric cancer and biopsy specimens is not an appropriate strategy to evaluate PD-L1 expression in selecting patients for anti-PD-1 immunotherapy. The poor agreement between two types of PD-L1 testing indicates that biopsy specimens might tend to undervalue the PD-L1 expression status, reflected in the resection samples. Heterogenous expression can result in false-negative results with respect to the PD-L1 expression status. If the biopsy were taken in a PD-L1 negative area, the patient would be considered as negative for PD-L1 expression, even if other regions of the tissue express PD-L1. The evaluation should be performed using resection specimens, except in inoperable stage VI cases, or using multiple biopsy samples from different sites of the tumor to reduce false-negative results due to intra-tumoral heterogeneity in PD-L1 expression.
Several limitations should be considered during evaluation of the results of this study. The limitations are as follows: 1) the small number of included cases, and 2) testing of only one PD-L1 antibody. This selection bias may lead to relatively higher PD-L1 positivity. The main strength of our study is the careful assessment of full sections of tissue samples. Furthermore, as shown in the Blueprint study, three PD-L1 assays (22C3, 28-8, and SP263) showed comparable analytical performance for assessment of PD-L1 expression [31]. We used the 22C3 PD-L1 antibody, a companion diagnostic marker for approved anti-PD-1 antibodies, using the same protocol (instrument platform, staining procedure, and scoring methods) to provide a practical guideline. Finally, further investigations, incorporating more cases of gastric and gastroesophageal carcinomas, are needed to validate our study.
PD-L1 expression is heterogeneous in terms of anatomic location and time, and, as shown herein, in the size of specimens [18]. The heterogeneity of PD-L1 expression could partly explain the contradictory value of its role as a predictive marker. Even though both biopsy and resection specimens cannot completely represent the PD-L1 expression status in gastric carcinomas, the value of PD-L1 expression as a prognostic predictor is still worth evaluating further to explore and overcome the associated limitations. With the development of biomarkers, such as MSI-H status or the immune profile of tumor microenvironment, for optimizing patient selection, PD-1/PD-L1 inhibitors would be a more valuable therapeutic option for advanced gastric cancers. The application of genomics for the immunotherapy of gastric cancer in the era of precision medicine deserves particular consideration.
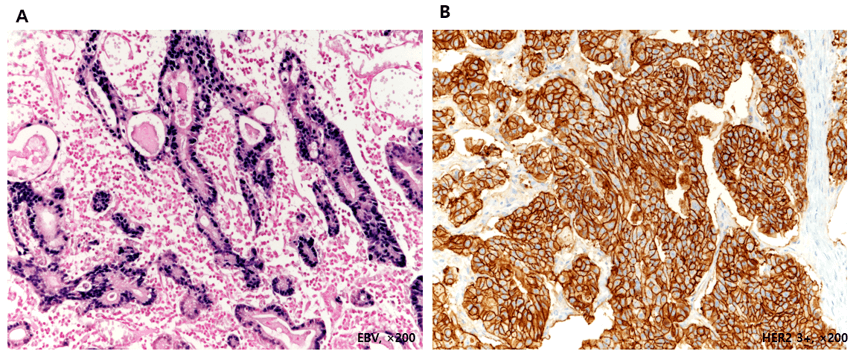
Supplementary Figure 1: Representative positive images of the EBV(A) and HER2 3+ expression (B)
Acknowledgements
This study was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2017 (2017-32-0021, Hyo Song Kim) and by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1A2B6003707, Hyo Song Kim).
Conflicts of Interest
None.
Ethical Standards
The study was approved by Yonsei University College of Medicine institutional review board (IRB 4-2014-0349).
Article Info
Article Type
Original ArticlePublication history
Received: Sat 11, Jan 2020Accepted: Wed 29, Jan 2020
Published: Fri 31, Jan 2020
Copyright
© 2023 Soo Hee Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.01.06
Author Info
Hyae Min Jeon Hyo Song Kim Kum Hee Yun Min Ju Kim Min Kyung Jeon Soo Hee Kim Ye Young Rhee
Corresponding Author
Soo Hee KimPathology Center, Seegene Medical Foundation, Seoul, Korea
Figures & Tables
Table 1: Clinical and pathological characteristics of patients and associations between PD-L1 positivity in resection and biopsy and clinicopathologic characteristics.
|
Parameters |
Case no. |
PD-L1 expression (CPS) Resection |
PD-L1 expression (CPS) Biopsy |
||||
|
CPS≥1 |
CPS<1 |
P-value |
CPS≥1 |
CPS<1 |
P-value |
||
|
Gender Male Female |
31 8 |
26 (83.9%) 7 (87.5%) |
5 (16.1%) 1 (12.5%) |
0.8 |
10 (32.3%) 0 (0%) |
21 (67.7%) 8 (100.0%) |
0.06 |
|
Mean age (range) |
|
57.8 (30-74) |
62.2 (59-80) |
|
58.1 (44-70) |
58.7 (30-80) |
|
|
AJCC Stage I/II III/IV |
14 25 |
12 (85.7%) 21 (84.0%) |
2 (14.3%) 4 (16.0%) |
0.89 |
4 (28.6%) 6 (24.0%) |
10 (71.4%) 19 (76.0%) |
0.75 |
|
T Stage T2 T3 T4a T4b |
3 17 18 1 |
2 (66.7%) 14 (85.7%) 16 (84.0%) 1 (100.0%) |
1 (33.3%) 3 (14.3%) 2 (16.0%) 0 (0%) |
0.74
|
1 (33.3%) 3 (28.6%) 6 (33.3%) 0 (0%) |
2 (66.7%) 14 (71.4%) 12 (66.7%) 1 (100.0%) |
0.67
|
|
N Stage N0 N1 N2 N3a N3b |
5 11 9 4 10 |
4 (80.0%) 8 (72.7%) 9 (100.0%) 4 (100.0%) 8 (80.0 %) |
1 (20.0%) 3 (27.3%) 0 (0%) 0 (0%) 2 (20.0 %) |
0.43
|
2 (40.0%) 3 (27.3%) 2 (22.2%) 1 (25.0%) 2 (20.0%) |
3 (60.0%) 8 (72.7%) 7 (77.8%) 3 (75.0%) 8 (80.0%) |
0.94
|
|
Dukes-MAC like stage B2 C1 C2 D |
4 2 14 19 |
3 (75.0%) 1 (50.0%) 12 (85.7%) 17 (89.5 %) |
1 (25.0%) 1 (50.0%) 2 (14.3%) 2 (10.5 %) |
0.48
|
1 (75.0%) 1 (50.0%) 2 (14.3%) 6 (31.6%) |
3 (25.0%) 1 (50.0%) 12 (85.7%) 13 (68.4%) |
0.59
|
|
WHO classification WD/MD PD/UND SRRC GCLS |
10 26 2 1 |
9 (90.0%) 23 (88.5%) 0 (0%) 1 (100.0%) |
1 (10.0%) 3 (11.5%) 2(100.0%) 0 (0%) |
0.01
|
3 (30.0%) 7 (27.0%) 0 (0%) 0 (0%) |
7 (70.0%) 19 (73.0%) 2 (100.0%) 1 (100.0%) |
0.67 |
|
EBV Positive Negative MSI MSI-H MSS/MSI-L |
4 35
4 35 |
4 (100.0%) 29 (82.9%)
4 (100.0%) 29 (82.9%) |
0 (0%) 6 (17.1%)
0 (0%) 6 (17.1%) |
1.00
1.00 |
2 (50%) 8 (22.9%)
4 (100.0%) 6 (17.1%) |
2 (50%) 27 (77.1%)
0 (0%) 29 (82.9%) |
0.27
0.003 |
|
Total |
39 |
33 (84.6%) |
6 (15.4%) |
|
29 (74.4%) |
10 (25.6%) |
|
Table 2: Comparison of PD-L1 expression between cancer resection and biopsy specimens by immunohistochemistry.
|
|
|
Resection |
Correlation coefficient |
|||
|
|
|
Negative (CPS<1) |
Low positive 1≤CPS<50 |
High positive (CPS≥50) |
Total |
|
|
Biopsy |
Negative (CPS<1) |
6 (15.4%) |
23 (59.0%) |
0 (%) |
29 (74.4%) |
0.234 |
|
Low positive (1≤CPS<50) |
0 (0%) |
8 (20.5%) |
0 (0%) |
8 (20.5%) |
||
|
High positive (CPS≥50) |
0 (0%) |
2 (5.1%) |
0 (0%) |
2 (5.1%) |
||
|
|
Total |
6 (15.4%) |
33 (84.6%) |
0 (0%) |
39 (100.0%) |
|
*CPS (Combined positive score): Number of PD-L1–positive cells [tumor cells, macrophages, lymphocytes] divided by the total number of tumor cells, multiplied by 100.
Table 3: Influence of immune cell PD-L1 positivity on evaluation of PD-L1 expression in gastric carcinomas.
|
|
Number of cases |
|
|
|
Resection (N=39) |
Biopsy (N=39) |
|
TC + only |
22 (56.4%) |
10 (25.6%) |
|
TC+/IC+ |
11 (28.2%) |
0 |
|
Total (CPS≥1) |
33 (84.6%) |
10 (25.6%) |
*TC +: Tumor cell PD-L1 positivity.
*IC +: Immune cell PD-L1 positivity.
Representative images of the diffuse expression resection case (A, CPS 30, ×200) and focal expression resection case (B, CPS 5, ×200), showing PD-L1 expression in both tumor cells and tumor-associated mononuclear inflammatory cells. Diffuse high expression in a biopsy sample (C, CPS 75, ×200), focal low expression in PD-L1 positive biopsy sample (D, CPS 5, ×200), without PD-L1 expression in the immune cells.
Representative image resection of a PD-L1 positive case (A, CPS 35, ×200) and paired biopsy specimen without confidential PD-L1 expression (B, CPS<1, ×200).
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90. [Crossref]
- Blum MA, Takashi T, Suzuki A, Ajani JA (2013) Management of localized gastric cancer. J Surg Oncol 107: 265-270. [Crossref]
- Lin EM, Gong J, Klempner SJ, Chao J (2018) Advances in immuno-oncology biomarkers for gastroesophageal cancer: Programmed death ligand 1, microsatellite instability, and beyond. World J Gastroenterol 24: 2686-2697. [Crossref]
- Cho J, Chang YH, Heo YJ, Kim S, Kim NK et al. (2018) Four distinct immune microenvironment subtypes in gastric adenocarcinoma with special reference to microsatellite instability. ESMO Open 3: e000326. [Crossref]
- Ni L, Dong C (2017) New checkpoints in cancer immunotherapy. Immunol Rev 276: 52-65. [Crossref]
- Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N et al. (2017) Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 66: 794-801. [Crossref]
- Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN et al. (2014) Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 94: 107-116. [Crossref]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443-2454. [Crossref]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711-723. [Crossref]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O et al. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515: 563-567. [Crossref]
- Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M et al. (2010) Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 116: 1757-1766. [Crossref]
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y et al. (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390: 2461-2471. [Crossref]
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T et al. (2018) Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 4: e180013. [Crossref]
- Mino-Kenudson M (2016) Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol Med 13: 157-170. [Crossref]
- McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V et al. (2016) Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2: 46-54. [Crossref]
- Mu CY, Huang JA, Chen Y, Chen C, Zhang XG (2011) High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 28: 682-688. [Crossref]
- Muro K, Chung HC, Shankaran V, Geva R, Catenacci D et al. (2016) Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 17: 717-726. [Crossref]
- Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S et al. (2016) Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 27: 147-153. [Crossref]
- Kim H, Kwon HJ, Park SY, Park E, Chung JH (2017) PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: a comparative study. Oncotarget 8: 98524-98532. [Crossref]
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE et al. (2017) The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 67: 93-99. [Crossref]
- Gurzu S, Sugimura H, Orlowska J, Szederjesi J, Szentirmay Z et al. (2017) Proposal of a Dukes-MAC-like staging system for gastric cancer. J Investig Med 65: 316-322. [Crossref]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS et al. (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018-2028. [Crossref]
- Bae YS, Kim H, Noh SH, Kim H (2015) Usefulness of Immunohistochemistry for Microsatellite Instability Screening in Gastric Cancer. Gut Liver 9: 629-635. [Crossref]
- Kim HS, Shin SJ, Beom SH, Jung M, Choi YY et al. (2016) Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: implications for individualized therapy. Oncotarget 7: 44608-446020. [Crossref]
- Laghi L, Bianchi P, Malesci A (2008) Differences and evolution of the methods for the assessment of microsatellite instability. Oncogene 27: 6313-6321. [Crossref]
- Wang W, Wang K, Chen Z, Chen L, Guo W et al. (2018) Immuno-classification characterized by CD8 and PD-L1 expression is associated with the clinical outcome of gastric cancer patients. Oncotarget 9: 12164-12173. [Crossref]
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B et al. (2017) T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545: 60-65. [Crossref]
- Ju X, Shen R, Huang P, Zhai J, Qian X et al. (2017) Predictive relevance of PD-L1 expression with pre-existing TILs in gastric cancer. Oncotarget 8: 99372-99381. [Crossref]
- Kitazono S, Fujiwara Y, Tsuta K, Utsumi H, Kanda S et al. (2015) Reliability of Small Biopsy Samples Compared With Resected Specimens for the Determination of Programmed Death-Ligand 1 Expression in Non-Small-Cell Lung Cancer. Clin Lung Cancer 16: 385-390. [Crossref]
- Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM et al. (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5: 43-51. [Crossref]
- Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC et al. (2018) PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 13: 1302-1311. [Crossref]
