CIDP Masquerading as Mononeuritis Multiplex: The Value of MR Neurography
A B S T R A C T
Background: We present two patients with the Lewis-Sumner variant of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), charting the diagnostic challenge posed by their clinical presentation and electrodiagnostic findings. The learning points center on the use of magnetic resonance imaging (MRI) in establishing a definitive diagnosis when clinical and neurophysiology data do not otherwise add up.
Cases: The first patient presented with slowly progressive asymmetric distal weakness of the lower limbs with wasting, weakness, areflexia and numbness on examination. The second patient experienced stepwise asymmetric hand/forearm weakness with deformity and areflexia, plus mild distal sensory impairment. Neurophysiological studies for both patients were initially most suggestive of mononeuritis multiplex, with no evidence of demyelination.
Conclusion: The possibility of asymmetric or multifocal CIDP, the Lewis-Sumner variant, should not be forgotten in suspected mononeuritis multiplex and the value of MRI in such cases is discussed.
Keywords
Inflammatory polyneuropathy, Lewis-Sumner syndrome, MADSAM, clinical neurophysiology, neuroimmunology
Introduction
Variants of CIDP often present a diagnostic challenge, especially where there exist many mimics of what is already a heterogeneous disorder. Serial assessment is frequently needed, and trials of therapeutic options may be required where the diagnosis is insecure. Typically, CIDP diagnosis is based on a mix of clinical, neurophysiological and sometimes laboratory data. The European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) criteria considers MRI showing gadolinium enhancement and/or hypertrophy of the cauda equina, lumbosacral or cervical nerve roots, or the brachial or lumbosacral plexus, as supportive when neurophysiology is inconclusive; MRI in this instance is classified as a level C recommendation, with a reported sensitivity between 44 to 82% [1, 2]. We present two patients with ambiguous neurophysiology where a negative response to steroids for suspected peripheral nerve vasculitis led to consideration of the Lewis-Sumner variant of CIDP, also termed multifocal acquired demyelinating sensory and motor neuropathy (MADSAM), which was confirmed using MRI.
Patient Information
Case 1
A 56-year-old woman presented with a 2-year history of asymmetric predominantly distal limb weakness. She had developed type II diabetes four years earlier and the following year polymyalgia rheumatica. She developed limb weakness in the months following right leg bypass surgery with toe amputation for peripheral vascular disease (PVD). She became depressed and lost weight after the surgery but also noticed difficulty in weight-bearing on the right leg with progressive foot drop, worse on the right. Six months post-surgery, she reached a nadir, mobilizing very little due to arm and leg weakness. MRI of whole spine was unremarkable. There was an improvement in mood, weight and strength over the next year, but worsening slightly in the months before presentation. She was mobile with a rollator and her family helped with dressing and readying for bed. The examination noted asymmetric distal limb wasting and weakness, with a flail right foot. There was lower limb areflexia and sensory loss: absent vibration and pinprick loss in sciatic distributions. The initial concern was of a severe mononeuritis multiplex, presumed vasculitis. CK, TSH, ACE, HIV, HBV, HCV, ESR, complement, immunoglobulins, RA latex, ANCA, ANA, ENA serum/urine electrophoresis, neuronal antibodies and cryoglobulins were normal. Neurophysiology identified asymmetric sensorimotor axonal neuropathy, consistent with a confluent mononeuritis multiplex (Table 1).
Due to concerns regarding foot healing given PVD, the left superficial radial nerve was selected for biopsy, as despite a lack of upper limb sensory signs, neurophysiology had identified a diminished response from this nerve. Upon reassessment at 3 months, it was clear that she was declining fast: there was a new weakness of shoulder abduction and greatly worsened hip flexion, so prednisolone 1 mg/kg/day (60 mg daily) was initiated for suspected peripheral nerve vasculitis whilst awaiting biopsy results. There was a modest improvement after a month and although biopsy results could not confirm vasculitis, there was perivascular lymphocyte cuffing and patchy axonal loss consistent with it, and pulsed cyclophosphamide was given alongside reducing daily prednisolone. There were further gains in strength, but with each pulse neuropathic pains eased for several days only to return, and she relapsed when prednisolone was reduced below 20 mg daily. Ten pulses of cyclophosphamide were ultimately provided, but she declined despite this and steroids were increased to 30 mg, then to 40 mg before recovery.
Lack of sustained response to this treatment argued against vasculitis, so tests were repeated. Lumbar puncture found only a marginally elevated protein of 0.59 g/L. Neurophysiology showed axonal changes (Table 1) plus active denervation in mostly distal muscles. 3T MRI of brachial and lumbosacral plexuses (Figure 1) revealed thickening and T2 hyperintensity of the femoral nerves bilaterally as they exited the pelvis and along their course, suggestive of neuritis involving the sciatic and femoral nerves.
Table 1: Neurophysiology results for both patients.
|
Nerve (N) / other details |
Case 1 |
Case 2 |
|||||||
|
NCS 1 |
NCS 2 |
NCS 1 |
NCS 2 |
||||||
|
R |
L |
R |
L |
R |
L |
R |
L |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RADIAL N: wrist-forearm SNAP |
9.9 uV |
5.0 uV |
NR |
- |
15.1 uV |
4.3 uV |
13.6 mV |
4.8 mV |
|
|
MEDIAN N: F3-wrist SNAP |
4.5 uV |
1.7 uV |
10.2 mV |
10.6 mV |
NR |
2.6 uV |
NR |
NR |
|
|
ULNAR N: F5-wrist SNAP |
0.9 uV |
NR |
NR |
NR |
NR |
2.5 uV |
NR |
1.8 uV |
|
|
SURAL N: calf-ankle SNAP |
NR |
NR |
NR |
NR |
2.1 uV |
2.5 uV |
3.5 uV |
NR |
|
|
SUP PERONEAL N: calf-ankle SNAP |
NR |
NR |
NR |
NR |
NR |
1.5 uV |
6.6 uV |
NR |
|
|
|
|
|
|
|
|
|
|
|
|
|
MEDIAN N: DML |
3.6 ms |
3.0 ms |
3.3 ms |
3.2 ms |
6.7 ms |
3.3 ms |
NR |
3.8 ms |
|
|
MEDIAN N: wrist CMAP (APB) |
6.1 mV |
9.9 mV |
8.4 mV |
13.0 mV |
0.3 mV |
8.1 mV |
NR |
10.8 mV |
|
|
MEDIAN N: elbow-wrist CV |
49 m/s |
53 m/s |
43 m/s |
50 m/s |
NR |
50 m/s |
NR |
51 m/s |
|
|
MEDIAN N: elbow CMAP (APB) |
5.5 mV |
12.6 mV |
7.2 mV |
10.2 mV |
NR |
7.1 mV |
NR |
8.9 mV |
|
|
MEDIAN N: axilla-elbow CV |
- |
- |
- |
58 m/s |
- |
52 m/s |
- |
14 m/s |
|
|
MEDIAN N: axilla CMAP (APB) |
- |
- |
- |
8.9 mV |
- |
4.4 mV |
- |
3.4 mV |
|
|
|
|
|
|
|
|
|
|
|
|
|
ULNAR N: DML |
NR |
3.2 ms |
NR |
5.0 ms |
3.0 ms |
2.5 ms |
4.0 ms |
3.4 ms |
|
|
ULNAR N: wrist CMAP (ADM) |
NR |
0.5 mV |
NR |
1.7 mV |
3.0 mV |
11.8 mV |
1.0 mV |
9.7 mV |
|
|
ULNAR N: elbow-wrist CV |
- |
51 m/s |
- |
51 m/s |
61 m/s |
53 m/s |
48 m/s |
51 m/s |
|
|
ULNAR N: elbow CMAP (ADM) |
- |
0.4 mV |
- |
1.9 mV |
2.1 mV |
8.8 mV |
1.4 mV |
7.6 mV |
|
|
ULNAR N: around elbow CV |
- |
48 m/s |
- |
69 m/s |
56 m/s |
56 m/s |
45 m/s |
48 m/s |
|
|
ULNAR N: above elbow CMAP (ADM) |
- |
0.4 mV |
- |
2.0 mV |
2.3 mV |
8.2 mV |
1.0 mV |
7.2 mV |
|
|
ULNAR N: axilla-above elbow CV |
- |
- |
- |
- |
48 m/s |
57 m/s |
47 m/s |
38 m/s |
|
|
ULNAR N: axilla CMAP (ADM) |
- |
- |
- |
- |
2.2 mV |
6.1 mV |
1.4 mV |
5.3 mV |
|
|
ULNAR N: F-wave |
NR |
31 ms |
- |
- |
21.7 ms |
37.5 ms |
NR |
39.4 ms |
|
ADM: Abductor Digiti Minimi; APB: Abductor
Pollicis Brevis; CMAP: Compound Muscle Action Potential; CV: Conduction
Velocity; DML: Distal Motor Latency; NCS: Nerve Conduction Study; SNAP: Sensory
Nerve Action Potential. There was severe asymmetric distal axonal damage
evident in case 1, but no demyelination (except for a prolonged left ulnar
distal motor latency on the 2nd study). In case 2, the prolonged
right median distal motor latency on the first study is attributable to axonal
loss, not demyelination (bold type), but there was a prolonged left ulnar F
wave (bold type); more consistent demyelination was seen in the 2nd
study plus clear left median conduction block (bold type). Lower limb motor
studies were performed but contributed less, so they were not displayed here.
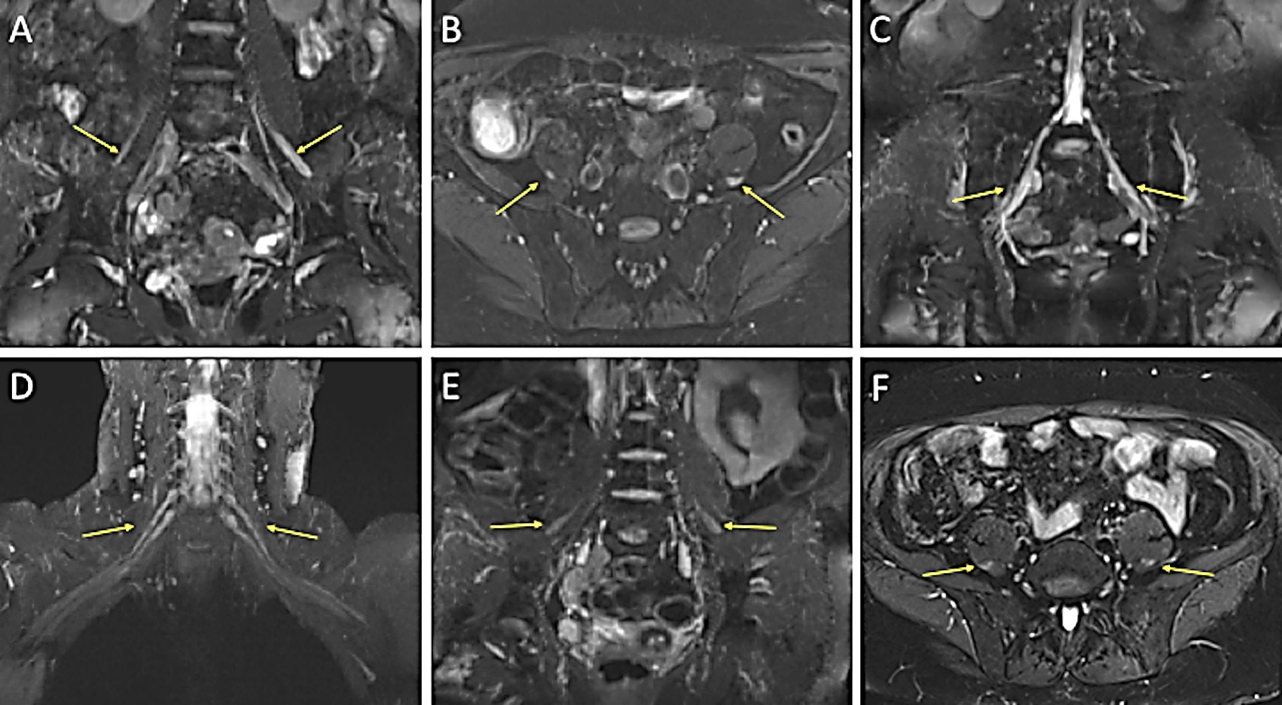
Figure 1: Figure 1 displaying thickened nerves on MRI in Patient 1 (images A, B and C) and Patient 2 (images D, E and F). These display additional high signal in all images apart from image D. A) Coronal STIR Space and B) axial SPAIR images of the lumbosacral spine with arrows highlighting thickened femoral nerves bilaterally, more so on the left. C) Coronal STIR Space showing thickened S1 nerves bilaterally. D) Coronal T2 STIR Space of the brachial plexus with slightly thickened nerve roots bilaterally, particularly distally. E) Coronal T2 STIR Space and F) axial SPAIR images highlighting thickened femoral nerves bilaterally.
(STIR: Short Tau Inversion Recovery; SPAIR: Spectral Attenuated Inversion Recovery).
There was no definite enhancement of these nerves on the post-contrast images. This result indicated CIDP despite repeat neurophysiology failing to show demyelination. Dropping prednisolone to 35 mg daily led to a motor decline and she was treated with IVIg without benefit. Prednisolone was restored to 40 mg daily with good effect and mycophenolate mofetil (MFM) was introduced (despite a lack of evidence in CIDP) with the hope it might allow a reduction in steroids. Over the course of the next year, there was sustained improvement in objective strength and function plus subjectively in quality of life. Prednisolone was successfully reduced to 22.5 mg daily, suggesting some benefit from MFM; subsequent attempts to lower prednisolone prompted worsening symptoms, although a dose of 20 mg daily was reached following very gradual dose reduction. Further reductions by 1 mg per month are planned.
Case 2
A 66-year-old woman was assessed after noticing reduced muscle bulk and weakness of both hands and forearms. This began in the right hand, leading to carpal tunnel release, as neurophysiology suggested focal median nerve entrapment. She soon noticed similar symptoms on the left side with associated cramping and muscle twitches. By the time of assessment, there was a drop of the medial two fingers of the right hand. The patient had a past medical history of B12 deficiency and breast cancer. Examination revealed some dysarthria with mild right tongue wasting. There was wasting in the small muscles of the hands, more pronounced on the right, with clawing of the medial two fingers. On the left, weakness of pronation and wrist/finger extension were evident. There was areflexia but intact sensation. The initial suspicion was of multiple entrapment neuropathies, despite negative hereditary neuropathy with liability to pressure palsy (HNPP) genetics. Anterior horn cell pathology and mononeuritis multiplex were considered, although she did not progress as one might suspect, and had a normal left superficial radial nerve fascicular biopsy. Blood tests were normal including CRP, ESR, ANA, ENA, ANCA, immunoglobulins and serum/urine electrophoresis. Soon after initial assessment, the left hand dramatically weakened over a week before stabilizing, but she was otherwise stable for two years aside from numbness in the feet. She then developed right and then left foot drop followed by left elbow weakness over a few months.
On reassessment, profound weakness of left elbow/wrist/finger extension, bilateral grip, right first dorsal interosseous (FDI) and left abductor pollicis (APB) was noted with additional weakness in other upper limb muscle groups and of knee flexion and ankle dorsiflexion, plus left hip flexion and knee extension weakness. HbA1c, HIV, HBV, HCV, ACE, cryoglobulins, glycolipid/neuronal antibodies, chest X-ray and repeat paraprotein screen were unremarkable. An evolving mononeuritis multiplex was suspected, and she was treated with 1 mg/kg/day prednisolone. Neurophysiology originally noted chronic right median/ulnar and left radial nerve denervation, with reduced lower limb sensory responses, although there was a prolonged left ulnar F-wave (Table 1). Repeat studies following her more global decline showed more convincing demyelination: prolonged left ulnar and peroneal F-wave latencies were noted with the absence of the left median and right peroneal F-waves despite reasonable CMAP amplitudes; furthermore, there was proximal left median and ulnar slowing with evidence of conduction block (Table 1).
She declined dramatically in just 2 weeks on prednisolone 70 mg daily, so she was admitted to the hospital for further investigation and treatment of suspected CIDP. Whilst steroids are the first-line treatment for CIDP, it is well recognised that some patients decline on steroids, particularly those with a motor-predominant presentation; this would not be expected in those with vasculitis. Lumbar puncture was unremarkable (protein only 0.27 g/L). Repeat left radial nerve biopsy revealed mild axonal loss with evidence of regeneration but no evidence of vasculitis. 3T MRI brachial and lumbosacral plexuses (Figure 1) found diffuse abnormal nerve thickening and signal hyperintensity, confirming CIDP. The patient responded well to IVIg and was substantially stronger by six months, with an ongoing requirement for 80 g every 10/11 days to maintain strength.
Discussion
Asymmetric or multifocal CIDP, the Lewis-Sumner variant, should not be forgotten in the differential diagnosis of suspected mononeuritis multiplex. The first case did not satisfy EFNS/EAN criteria for CIDP diagnosis because at least 2 demyelinating neurophysiological features are required, even when supportive criteria are present, but on balance was judged to be CIDP given the MRI findings, negative nerve biopsy and atypical (for vasculitis) pattern of response to steroids [1]. The second case met EFNS/EAN criteria for definite CIDP given the demyelinating features present on the second study combined with supportive criteria (MRI findings and clinical response to immunotherapy). Paranodal antibodies were not checked as these have not been associated with multifocal presentations.
These cases highlight the value of MR neurography but also how neurophysiology can be misleading, a particular difficulty if the presentation is atypical. The second case did eventually show some demyelination on neurophysiology, but only because of neurophysiology that looked more proximally than might normally occur, and as the presentation of this case was atypical for CIDP, further tests were appropriate. The finding of nerve thickening with high signal on 3T MRI was able to secure the diagnosis. Gadolinium enhancement was not found in the first case and was not examined in the second: our radiologists prefer to avoid the use of gadolinium to minimise risk and time. They recommend contrast where there is suspicion of infection or malignancy, but for inflammatory disorders, consider thickening and signal change more useful findings with little added value from the finding (or not) of contrast enhancement [2]. It is important to discuss MRI protocols and subsequent interpretation, plus the radiologist should be experienced in viewing plexus MRIs, especially as objective criteria for abnormality which are yet to be developed.
Key Points
i. Apparent mononeuritis multiplex may prove to be an atypical presentation of inflammatory polyneuropathy, and vice versa.
ii. Be prepared to revise the diagnosis and/or arrange further tests when events challenge what may have been the best interpretation initially.
iii. Neurophysiology can miss demyelination, especially proximally, and repeat studies may help with challenging cases.
iv. MRI is a useful adjunct to neurophysiology and laboratory tests in cases where inflammatory polyneuropathy is suspected despite ambiguous neurophysiology.
Author Contributions
JH had full access to all the neurophysiological data and takes responsibility for the integrity of the data and the accuracy of the data analysis; Drafting the manuscript: SR, SM; Critical revision of the manuscript for important intellectual content: All authors.
Acknowledgement
Many thanks to both patients and to Dr. Bhojak, consultant neuroradiologist, The Walton Centre, for advice regarding the figures.
Funding
None.
Competing Interests
None.
Consent
Both patients gave written consent.
Ethical Approval
Not required. Approval was not obtained as we present case studies reflecting routine care, however informed written consent for text and image publication was obtained.
Article Info
Article Type
Case ReportPublication history
Received: Mon 26, Apr 2021Accepted: Wed 12, May 2021
Published: Wed 26, May 2021
Copyright
© 2023 James Holt. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2021.02.01
Author Info
Selina Robertson Simon McCrory James Holt
Corresponding Author
James HoltNeurology Department, The Walton Centre NHS Foundation Trust, Liverpool, UK
Figures & Tables
Table 1: Neurophysiology results for both patients.
|
Nerve (N) / other details |
Case 1 |
Case 2 |
|||||||
|
NCS 1 |
NCS 2 |
NCS 1 |
NCS 2 |
||||||
|
R |
L |
R |
L |
R |
L |
R |
L |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RADIAL N: wrist-forearm SNAP |
9.9 uV |
5.0 uV |
NR |
- |
15.1 uV |
4.3 uV |
13.6 mV |
4.8 mV |
|
|
MEDIAN N: F3-wrist SNAP |
4.5 uV |
1.7 uV |
10.2 mV |
10.6 mV |
NR |
2.6 uV |
NR |
NR |
|
|
ULNAR N: F5-wrist SNAP |
0.9 uV |
NR |
NR |
NR |
NR |
2.5 uV |
NR |
1.8 uV |
|
|
SURAL N: calf-ankle SNAP |
NR |
NR |
NR |
NR |
2.1 uV |
2.5 uV |
3.5 uV |
NR |
|
|
SUP PERONEAL N: calf-ankle SNAP |
NR |
NR |
NR |
NR |
NR |
1.5 uV |
6.6 uV |
NR |
|
|
|
|
|
|
|
|
|
|
|
|
|
MEDIAN N: DML |
3.6 ms |
3.0 ms |
3.3 ms |
3.2 ms |
6.7 ms |
3.3 ms |
NR |
3.8 ms |
|
|
MEDIAN N: wrist CMAP (APB) |
6.1 mV |
9.9 mV |
8.4 mV |
13.0 mV |
0.3 mV |
8.1 mV |
NR |
10.8 mV |
|
|
MEDIAN N: elbow-wrist CV |
49 m/s |
53 m/s |
43 m/s |
50 m/s |
NR |
50 m/s |
NR |
51 m/s |
|
|
MEDIAN N: elbow CMAP (APB) |
5.5 mV |
12.6 mV |
7.2 mV |
10.2 mV |
NR |
7.1 mV |
NR |
8.9 mV |
|
|
MEDIAN N: axilla-elbow CV |
- |
- |
- |
58 m/s |
- |
52 m/s |
- |
14 m/s |
|
|
MEDIAN N: axilla CMAP (APB) |
- |
- |
- |
8.9 mV |
- |
4.4 mV |
- |
3.4 mV |
|
|
|
|
|
|
|
|
|
|
|
|
|
ULNAR N: DML |
NR |
3.2 ms |
NR |
5.0 ms |
3.0 ms |
2.5 ms |
4.0 ms |
3.4 ms |
|
|
ULNAR N: wrist CMAP (ADM) |
NR |
0.5 mV |
NR |
1.7 mV |
3.0 mV |
11.8 mV |
1.0 mV |
9.7 mV |
|
|
ULNAR N: elbow-wrist CV |
- |
51 m/s |
- |
51 m/s |
61 m/s |
53 m/s |
48 m/s |
51 m/s |
|
|
ULNAR N: elbow CMAP (ADM) |
- |
0.4 mV |
- |
1.9 mV |
2.1 mV |
8.8 mV |
1.4 mV |
7.6 mV |
|
|
ULNAR N: around elbow CV |
- |
48 m/s |
- |
69 m/s |
56 m/s |
56 m/s |
45 m/s |
48 m/s |
|
|
ULNAR N: above elbow CMAP (ADM) |
- |
0.4 mV |
- |
2.0 mV |
2.3 mV |
8.2 mV |
1.0 mV |
7.2 mV |
|
|
ULNAR N: axilla-above elbow CV |
- |
- |
- |
- |
48 m/s |
57 m/s |
47 m/s |
38 m/s |
|
|
ULNAR N: axilla CMAP (ADM) |
- |
- |
- |
- |
2.2 mV |
6.1 mV |
1.4 mV |
5.3 mV |
|
|
ULNAR N: F-wave |
NR |
31 ms |
- |
- |
21.7 ms |
37.5 ms |
NR |
39.4 ms |
|
ADM: Abductor Digiti Minimi; APB: Abductor
Pollicis Brevis; CMAP: Compound Muscle Action Potential; CV: Conduction
Velocity; DML: Distal Motor Latency; NCS: Nerve Conduction Study; SNAP: Sensory
Nerve Action Potential. There was severe asymmetric distal axonal damage
evident in case 1, but no demyelination (except for a prolonged left ulnar
distal motor latency on the 2nd study). In case 2, the prolonged
right median distal motor latency on the first study is attributable to axonal
loss, not demyelination (bold type), but there was a prolonged left ulnar F
wave (bold type); more consistent demyelination was seen in the 2nd
study plus clear left median conduction block (bold type). Lower limb motor
studies were performed but contributed less, so they were not displayed here.
(STIR: Short Tau Inversion Recovery; SPAIR: Spectral Attenuated Inversion Recovery).
References
1.
Van den Bergh
PYK, Hadden RDM, Bouche P, Cornblath DR, Hahn A et al. (2010) European
Federation of Neurological Societies/Peripheral Nerve Society guideline on
management of chronic inflammatory demyelinating polyradiculoneuropathy: report
of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first
revision. Eur J Neurol 17: 356-363. [Crossref]
2.
Lozeron P, Lacour MC, Vandendries C,
Théaudin M, Cauquil C et al. (2016) Contribution of plexus MRI in the diagnosis
of atypical chronic inflammatory demyelinating polyneuropathies. J Neurol
Sci 360: 170-175. [Crossref]
