Chronic Unpredictable Stress Alters Brain Tryptophan Metabolism and Impairs Working Memory in Mice without Causing Depression-Like Behaviour
A B S T R A C T
Chronic stress is a well-known risk factor in major depressive disorder and disrupts the kynurenine and serotonin pathways of tryptophan metabolism. Here, we characterize the temporal central and peripheral changes in tryptophan metabolism and concomitant depressive-like behavioural phenotype induced during the progression of chronic unpredictable stress (CUS). Mice were exposed to 0, 10, 20, or 30 days of CUS followed by a panel of behavioural assays to determine depressive-like phenotypes. Immediately after behavioural testing, plasma and brain tissue were collected for metabolic analysis. While anhedonia-like and anxiety-like behaviours were unaffected by stress, nesting behaviour and cognitive deficits became apparent in response to CUS exposure. While CUS caused a transient reduction in circulating quinolinic acid, no other tryptophan metabolites significantly changed in response to CUS. In the brain, tryptophan, kynurenine, picolinic acid, and 5-hydroxyindoleacetic acid concentrations were significantly elevated in CUS-exposed mice compared with non-stress control animals, while kynurenic acid, xanthurenic acid, and serotonin decreased in CUS-exposed mice. Metabolic turnover of serotonin to the major metabolite 5-hydroxyindoleacetic acid was markedly increased in response to CUS. These results suggest that CUS impairs hippocampal-dependent working memory and enhances nascent nesting behaviour in C57BL/6J male mice, and these behaviours are associated with increased brain kynurenine pathway metabolism leading to accumulation of picolinic acid and a significant reduction in serotonin levels.
Keywords
Kynurenine, serotonin, picolinic acid, depression, stress
Introduction
Depression is a major public health concern affecting approximately 300 million people globally [1]. Chronic stress is a well-characterized risk factor in the development of depression [2]. Depressed individuals have elevated levels of cortisol in their saliva and plasma, as well as dysregulated feedback inhibition of the HPA axis [3, 4]. Further, antidepressant treatment reduces HPA activity in animals and depressed humans [5]. Chronic unpredictable stress (CUS), social defeat, restraint, and other stressors are common pre-clinical models used in the investigation of the pathogenesis of depression [6].
A key component of the physiological response to chronic stress is the interaction with the immune system [7-10]. Pro-inflammatory markers in the central nervous system (CNS) have been highly implicated in the pathogenesis of depression [11-14]. Mounting evidence suggests that chronic stress up-regulates the expression of pro-inflammatory cytokines in the CNS through activation of resident microglia [15-17]. Il-1β, in particular, has been shown to be strongly up-regulated in response to chronic mild stress in mice, while genetic or pharmacologic blockade of Il-1β receptor ameliorates the stress-induced depressive-like behavioural response [18, 19]. Over the past two decades, alterations in the kynurenine pathway of tryptophan metabolism have been implicated in the pathogenesis of inflammation-induced depression, and preclinical models have largely recapitulated these findings [12, 20]. However, relatively less is known pertaining to the potential pathogenic role of tryptophan metabolism along the kynurenine pathway.
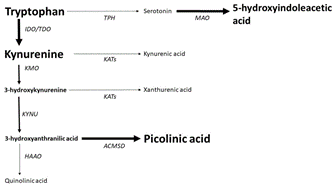
Tryptophan is metabolized to kynurenine through the indolamine enzymes (indolamine-2,3-dioxygenase and tryptophan dioxygenase; IDO1, IDO2, and TDO) (Figure 1). Downstream kynurenine metabolism then diverges into two branches to produce the neuroactive metabolites kynurenic acid (KA) and quinolinic acid (QA). Under baseline conditions, the majority of kynurenine is converted to KA through the enzyme kynurenic acid transferase (KAT). However, this balance is shifted during an inflammatory response, resulting in up-regulated expression of IDO and kynurenine monooxygenase (KMO) in microglial cells. KMO initiates the oxidative branch of the kynurenine metabolism pathway by metabolizing kynurenine into 3-hydroxykynurenine (3-HK), the precursor to QA. Elevated levels of QA in the brain have been associated with depressive symptoms in humans and inflammation results in a robust increase in kynurenine metabolism that mediates depressive-like behaviours and cognitive impairment [21-23]. This metabolic change is characterized by a shift towards oxidative kynurenine metabolism, particularly in the hippocampus, while serotonin levels are largely unaffected [24, 25]. Kynurenine metabolism via the cortisol-dependent enzyme TDO has been implicated in mediating KP metabolism during stress, but experimental data are limited [26]. Gibney et al. found that the non-selective TDO inhibitor attenuated depressive-like behaviour in rats exposed to repeated restraint stress, while Dostal et al. found that acute restraint stress in mice up-regulated TDO in an IDO1-dependent manner to increased kynurenine levels, but no phenotypic measures were reported in the latter study [27, 28].
Figure 1: Chronic unpredictable stress shifts brain tryptophan metabolism towards oxidative kynurenine metabolites and up-regulates serotonin turnover.
IDO: Indolamine-2,3-Dioxygenase; TDO: Tryptophan Dioxygenase; TPH: Tryptophan Hydroxylase; MAO: Monoamine Oxygenase; KMO: Kynurenine Monooxygenase; KAT: Kynurenine Aminotransferase; KYNU: Kynureninase; HAAO: 3-Hydroxyanthranilate Oxidase; ACMSD: Amino-β-Carboxymuconate-Semialdehyde-Decarboxylase
In this study, central and peripheral kynurenine pathway metabolites and depressive-like behavioural changes were measured in C57Bl/6J mice following varying durations of CUS exposure. Here, we report that CUS exposure failed to precipitate a depressive-like phenotype, but nascent nesting behaviour was increased and working memory impaired by CUS exposure. These behavioural phenotypes were paralleled by increased levels of tryptophan and certain downstream kynurenine pathway metabolites (kynurenine and picolinic acid) in the brain, while central kynurenic acid and xanthurenic acid and peripheral quinolinic acid were reduced. Furthermore, brain serotonin concentration levels were significantly decreased following prolonged stress exposure, while 5-HIAA was greatly increased. These results suggest that the tryptophan in the brain is shunted towards the kynurenine metabolism pathway and coupled with increased serotonin turnover, brain serotonin levels are markedly reduced. Together, these observations suggest that dysregulated tryptophan metabolism pathways are a possible mechanistic link between chronic stress and depressive-like phenotypes.
Methods
I Mice
8-10-week-old male C57BL/6J mice (The Jackson Laboratory; Bar Harbor, ME; stock # 000664) were housed in groups of 2-4 mice/cage and maintained on a 12:12h reverse light cycle (lights off at 11:00). Food and water were available ad libitum. Body weights were recorded throughout the experiment to track physiological responses to stress. All animal care and use were carried out in accord with the Guide for the Care and Use of Laboratory Animals, 8th edition (NRC) and approved by the Institutional Animal Care and Use Committee at The University of Texas Health San Antonio.
Mice were exposed to 0, 10, 20, or 30 days of chronic unpredictable stress (n=8/group). On the last day of stress exposure, a battery of behavioural assays was performed. Behavioural analysis of mice exposed to 0 days of CUS was performed in conjunction with Groups 1-3. Immediately following behavioural assessments, mice were euthanized via carbon-dioxide asphyxiation and exsanguination. Blood was collected for plasma analysis. Mice were then perfused with heparinized saline. Brain tissue and spleens were collected and snap-frozen in liquid nitrogen. All tissue and plasma were stored at -80°C. Brain tissue was homogenized with mortar and pestle on dry ice to be divided into equal samples for metabolite and protein analysis (described below; n=4-6/group).
II Chronic Unpredictable Stress Paradigm
All procedures were initiated during the dark phase of the light cycle, unless otherwise noted, and were carried out in the room the CUS-exposed mice were housed in. Mice exposed to 0 days of stress were housed in a separate room on the same light cycle. The stressors were applied randomly to maximize unpredictability; each stressor was only repeated once every 10 days (Table 1) and at varying times during the dark phase.
Table 1: Schedule for the experimental chronic unpredictable
stress paradigm and behavioural assessment.
|
Chronic Unpredictable Stress Experimental Schedule |
|
|
Day 1 |
Cage tilt & Wet bedding |
|
Day 2 |
Cold swim, Restraint |
|
Day 3 |
Cage agitation |
|
Day 4 |
Strobe light |
|
Day 5 |
Social defeat |
|
Day 6 |
Predator odor |
|
Day 7 |
Warm cage |
|
Day 8 |
Restraint & Tail pinch, Isolation |
|
Day 9 |
Isolation Begin saccharin
preference testing |
|
Day 10 |
Isolation & Tail pinch Behaviour testing and
Tissue collection |
|
Day 11 |
Strobe light |
|
Day 12 |
Cold swim, Restraint |
|
Day 13 |
Predator odor |
|
Day 14 |
Social defeat |
|
Day 15 |
Cage agitation |
|
Day 16 |
Cage tilt & Wet bedding |
|
Day 17 |
Warm cage |
|
Day 18 |
Restraint & Tail pinch, Isolation |
|
Day 19 |
Isolation Begin saccharin
preference testing |
|
Day 20 |
Isolation, Tail pinch Behaviour testing and
Tissue collection |
|
Day 21 |
Social defeat |
|
Day 22 |
Warm cage |
|
Day 23 |
Cold swim, Restraint |
|
Day 24 |
Cage tilt & Wet bedding |
|
Day 25 |
Predator odor |
|
Day 26 |
Cage agitation |
|
Day 27 |
Restraint & Tail pinch |
|
Day 28 |
Strobe light, Isolation |
|
Day 29 |
Isolation Begin saccharin preference testing |
|
Day 30 |
Isolation, Tail pinch Behaviour testing and Tissue collection |
i Cage Agitation
The mice were placed in their home cages on an orbital shaker set to 70 RPM. The orbital shaker was set on a timer for one hour on/off intervals for 24 hours.
ii Cold Swim
4-liter cylindrical clear containers were filled with tap water and ice to maintain temperatures of 17-19°C. Mice floated or swam in the cold water for 10 minutes. After 10 minutes, mice were removed from the cold water and dabbed with paper towels.
iii Restraint
50-ml Falcon conical tubes were modified by removing the tip to allow airflow and 3mm holes were drilled in the caps to allow access to their tails. Mice were held in these tubes with the cap screwed on behind them for 2 hours.
iv Restraint with a Tail Pinch
Mice were held for 2 hours in 50-ml tubes as described. In the last 2 minutes of restraint, plastic clothespins were clipped onto the base of their tails, exposed through the caps.
v Predator Odor
The gauze was soaked with 2ml of coyote urine (Coyote Pee Brand Coyote Urine;https://www.predatorpeestore.com/coyote-urine-16-oz-33-day-dispenser-combo_moreinfo.html) and secured to the inside of the micro-isolator tops of the mouse home cages for 12 hours. After 12 hours, the mice were placed into fresh cages with new micro-isolator tops and the gauze was disposed of outside the animal housing facility.
vi Cage Tilt
Mice were placed in their home cages on a shelf tipped to 45° for 24 hours.
vii Wet Bedding
Approximately 300-400ml of water was added to home cages to sufficiently soak bedding with no standing water. After 24 hours, mice were moved to cages with fresh bedding.
viii Strobe Light
A strobe light was pointed at the home cages of the mice for 12 hours during the dark phase of the light cycle.
ix Warm Cage
The micro-isolator top of the home cages was removed and a 75watt heat bulb was pointed over the cages so that the temperature was maintained at approximately 35° (underneath bulb) to 26° (periphery of the cage). The heat bulbs were turned on for one-hour on-off intervals for 12 hours.
x Reverse Light Cycle
For 12 hours during the dark phase of the light cycle, 120 lux bulbs were pointed at the home cages of the mice.
xi Modified Social Defeat
Experimental mice were placed in the home cage of 2 adult male CD1 mice (Charles River; strain code 022) for 5 minutes. One hour later, experimental mice were placed in the home cage of 2 different CD1 male mice for 5 minutes.
III Behaviour Analysis
i Saccharin Preference
Three days prior to CUS exposure, mice were trained to differentiate water and 0.04% saccharin using a two-bottle choice testing paradigm. Bottles were placed above the food hopper and their location was alternated daily to control for a potential place preference. On the last day of CUS, the two bottles were returned to the cages. Saccharin preference was calculated as (saccharin intake)/(water + saccharin intake)*100 and interpreted as a measure of anhedonia-like behaviour.
ii Nesting
Nesting behaviour was assessed in the home cage of individually housed mice following the zone clearance method [29]. Nestlet pieces were divided into 6 equal pieces and placed in each corner of the home cage (“zones”; Figure 2B). Mice instinctively gather up the nestlet pieces to build one larger nest. The number of nestlet pieces that have been cleared from their respective zones is counted every 6 minutes for a maximum score of 5 zones cleared in 42 minutes (Figure 2B).
iii Locomotor Activity and Open Field Test
Mice were placed in a dimly lit 40 x 40 cm open field (OF) chamber for 5 minutes to assess exploratory locomotor activity and anxiety-like behaviour. The activity was video recorded and Ethovision XT 8.5 analysis software (Noldus, Leesburg, VA) was used to assess the total distance traveled and time spent in the central or outer areas of the chamber.
iv Y-Maze
Working memory was evaluated using a two-trial Y-maze task. In the exposure trial, one arm of the Y-maze was blocked off so that the mouse was unable to enter (Figure 2G). Mice were placed in one arm of the maze and allowed to explore for 12 minutes. One hour later, the block was removed, and mice were returned to the maze to explore all open arms for 5 minutes. The testing trial activity was video recorded and analysed using Ethovision 8.5 analysis software (Noldus, Leesburg, VA) to determine the distance traveled and time spent in each arm.
IV Metabolite Analysis
Thawed plasma samples were diluted with 5x 0.2% acetic acid and 1mM internal standards. Frozen brain tissue was diluted 3x with 0.2% acetic acid and 1mM internal standards and homogenized at 4° with 1.4mm zirconium ceramic oxide beads (Omni International) in an Omni International Bead Ruptor 24 Homogenizer (Kennesaw, GA; pulse duration: 45s, pulse number: 2, rest interval: 15s). The diluted brain homogenate and plasma were then transferred to Millipore Amicon Ultra Filters and centrifuged at 13500xg for 1 hour at 4°C. Liquid chromatography/mass spectrometry (LCMS) was performed on a Thermo Fisher Scientific Q Exactive mass spectrometer (Waltham, MA) with online separation by a Thermo Fisher Scientific Dionex Ultimate 3000 HPLC in the Mass Spectrometry Core Facility at the University of Texas Health San Antonio. Data were analysed using Xcalibur 2.2 software (Thermo Fisher Scientific).
V Statistical Analysis
All data were analysed using GraphPad Prism 8.2.0 (San Diego, CA). Data were considered significant if p < 0.05. Outlier data were identified using Chauvenet’s outlier test and omitted from the analysis. Behavioural assays and metabolite concentrations were analysed by group using one-way ANOVA followed by Dunnett’s post-hoc test. In the absence of significant main effects, post-hoc analysis was performed according to a priori hypothesis.
Results
I Chronic Unpredictable Stress Drives Distinct Depressive-Like Behavioural Changes in C57Bl/6J Mice
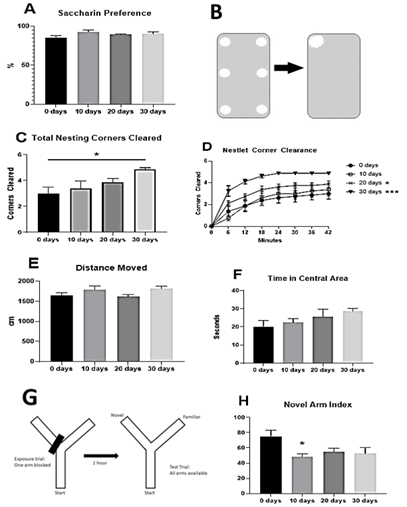
Mice were tested in saccharin preference, nesting, open field test, and working memory Y-maze after 0, 10, 20, or 30 days of CUS exposure. CUS exposure failed to cause a reduction in saccharin preference in the acute 2-bottle choice task (Figure 2A). Nesting behaviour was increased in mice exposed to longer durations of CUS compared to low or no stress controls (Figures 2B-2D). Mice exposed to 30 days CUS gathered nestlet pieces from more corners of the cage by the end of the assay and did so more rapidly. Distance moved and time spent in the central area of the open field test did not significantly differ between groups (Figures 2E & 2F), suggesting that stress did not increase lethargy or drive an anxiety-like phenotype. The Novel Arm Index of the working memory Y-maze task was significantly reduced after 10 days of CUS, however, indicating a reduction in working memory (p=0.015; Figure 2H). The Novel Arm Index remained reduced for 20 and 30 days of CUS but did not reach significance in these groups.
Figure 2: Chronic unpredictable stress increases nesting behaviour and induces working memory deficits. Mice were assessed for depressive-like behavioural changes following 0, 10, 20, or 30 days of chronic unpredictable stress (CUS). A) Saccharin preference was measured for 2 hours following CUS and no effect was detected. B) 6 small nestlet pieces were arranged around the edges of the home cages of the mice to determine the effects of stress on nesting behaviour. Every 6 minutes, the nestlet pieces cleared from each corner of the cage were counted for a maximum score of 5 at each time point. C) Mice exposed to 30 days of CUS cleared significantly more corners at the end of the nesting assay than control mice. D) Nestlets were gathered significantly quicker by mice exposed to 20 and 30 days of CUS than 0 and 10 days of stress. E & F) Locomotor activity and anxiety-like behaviour were measured using the open field test. The distance moved around the chamber and time spent in the central area were unaffected by the duration of stress. G & H) Mice were placed in the Y-maze with one arm blocked for 12 minutes. One hour later, mice were placed back in the Y-maze for 5 minutes with the block removed. Working memory was determined by the Novel Arm Index (Time spent in Novel arm/(Time spent in Familiar + Start arms)). Novel Arm Index was significantly reduced after 10 days of CUS, indicating a deficit in working memory. The Novel Arm Index remained reduced for 20 and 30 days of CUS but did not reach significance.
Data represent sample means (s.e.m.) n = 8 samples per group.
*: Post hoc comparison with 0 days CUS control group, p < 0.05.
*: p<0.05; **: p<0.01; ***: p<0.001.
II Chronic Unpredictable Stress Increases Central Tryptophan Levels and Shifts the Peripheral Kynurenine Pathway Away from the Oxidative Branch
Brains and plasma were analysed by LCMS to determine if increasing exposure to CUS induces a shift in downstream tryptophan and kynurenine metabolism. Complete statistical analysis is reported in (Table 2). There was a significant main effect of CUS duration on central tryptophan, XT, and KYNA, and peripheral QA. Central QA was not reliably detected in any of the brain samples in this study. CUS caused a significant shift in the balance in downstream kynurenine metabolism. Complete statistical analysis of ratio data is outlined in (Table 3). In the brain, KYNA was elevated in relation to kynurenine after 10 and 20 days of CUS. Peripheral QA was significantly decreased over kynurenine and KYNA after 10 and 20 days of CUS exposure. These data suggest that CUS drives a shift towards KYNA and away from KMO-dependent metabolism.
Table 2: Brain and plasma kynurenine metabolite analysis
following chronic unpredictable stress.
|
|
0 days CUS |
10 days CUS |
20 days CUS |
30 days CUS |
Main effects |
|
Brain metabolites (μM) |
|||||
|
Tryptophan |
344.9 (49.50) |
692.2 (114.5) † |
630.9 (93.02) |
356.7 (42.87) |
*p<0.05 |
|
Kynurenine |
0.15 (0.027) |
0.41 (0.068) †††† |
0.33 (0.073) |
0.29 (0.098) |
n.s. |
|
3-HK |
0.036 (0.013) |
0.026 (0.0140) |
0.018 (0.0022) |
0.04 (0.018) |
n.s. |
|
XT |
0.023 (0.00097) |
0.004 (0.0011) †† |
0.013 (0.0027) |
0.022 (0.0057) |
**p<0.01 |
|
3-HAA |
0.02 (0.013) |
0.0038 (0.0038) |
0.0056 (0.0035) |
0.021 (0.0078) |
n.s. |
|
PA |
1.072 (0.11) |
2.55 (0.35) †† |
2.22 (0.36) † |
2.01 (0.77) |
n.s. |
|
QA |
n.d. |
n.d. |
n.d. |
n.d. |
n/a |
|
KYNA |
0.033 (0.0036) |
0.014 (0.0024) |
0.021 (0.0017) |
0.045 (0.013) |
*p<0.05 |
|
Plasma metabolites
(μM) |
|||||
|
Tryptophan |
955.3 (67.80) |
887.3 (38.68) |
1053 (66.07) |
966.7 (70.37) |
n.s. |
|
Kynurenine |
1.110 (0.08282) |
1.149 (0.07760) |
1.432 (0.1713) |
1.175 (0.1713) |
n.s. |
|
3-HK |
0.058 (0.029) |
0.057 (0.015) |
0.069 (0.02) |
0.052 (0.0094) |
n.s. |
|
XT |
0.053 (0.0015) |
0.048 (0.0049) |
0.051 (0.0035) |
0.054 (0.0034) |
n.s. |
|
3-HAA |
n.d. |
n.d. |
n.d. |
n.d. |
n/a |
|
PA |
1.47 (0.082) |
1.13 (0.087) |
1.31 (0.089) |
1.26 (0.1) |
n.s. |
|
QA |
0.39 (0.12) |
0.15 (0.021) † |
0.19 (0.018) † |
0.23 (0.026) |
*p<0.05 |
|
KYNA |
0.049 (0.0028) |
0.048 (0.0052) |
0.064 (0.007) |
0.056 (0.0049) |
n.s. |
3-HK: 3-Hydroxykynurenine; XT:
Xanthurenic Acid; 3-HAA: 3-Hydroxyanthranilic
Acid; PA: Picolinic Acid; QA: Quinolinic Acid; KYNA: Kynurenic Acid.
Kynurenine
pathway metabolites were quantified (μM) by LC/MS in the whole brain and plasma
samples from male C67Bl/6J mice following 0, 10, 20, or 30 days of chronic unpredictable
stress. Concentration values represent sample means (s.e.m.) n = 4-6 samples
per group.
*:
Main effect of CUS, p < 0.05.
†:
Post hoc comparison with 0 days CUS control group.
*,†:
p<0.05; **,††: p<0.01.
Table 3: Tryptophan metabolism pathways balance shifts
following chronic unpredictable stress.
|
|
0 days CUS |
10 days CUS |
20 days CUS |
30 days CUS |
Main effects |
|
Brain metabolite ratios |
|||||
|
5-HT/TRP |
0.07345 (0.0284) |
0.02002 (0.00375) †† |
0.0163 (0.0027†† |
0.02424 (0.00308) † |
*p<0.05 |
|
KYN/TRP |
0.41 (0.0215) |
0.6 (0.0453) †† |
0.52 (0.0494) |
0.96 (0.5) |
n.s. |
|
KYNA/KYN |
0.27 (0.071) |
0.037 (0.0087) †† |
0.079 (0.016) † |
0.19 (0.058) |
**p<0.01 |
|
|
|
|
|
|
|
|
Total oxidative kynurenines (μM) |
1.186 (0.112) |
2.585 (0.352) † |
2.247 (0.367) |
2.072 (0.788) |
n.s. |
|
KAT-dependent kynurenines (μM) |
0.05669 (0.00411) |
0.01825 (0.00307) † |
0.03460 (0.0418) |
0.06787 (0.0185) |
*p<0.05 |
|
Ratio of oxidative KYNs/KATs |
21.26 (2.7) |
151.4 (19.22) †††† |
68.65 (14.78) |
28.88 (4.07) |
****p<0.0001 |
5-HT: Serotonin; TRP: Tryptophan; KYN:
Kynurenine; KYNA: Kynurenic Acid; KAT: Kynurenine Aminotransferase.
Kynurenine
pathway metabolism was analysed by comparing concentrations (μM) to calculate a
ratio value. Total oxidative kynurenines include 3-hydroxykynurenine,
3-hydroxyanthranilic acid, picolinic acid, quinolinic acid. Total KATs include
kynurenic acid and xanthurenic acid.
Ratio
data represent sample means (s.e.m.) n = 4-6 samples per group.
*:
Main effect of CUS, p < 0.05.
†:
Dunnett’s post hoc comparison with 0 days CUS control group.
*,†:
p<0.05; **,††: p<0.01.
III Chronic Stress Drives Serotonin Turnover in the Brain
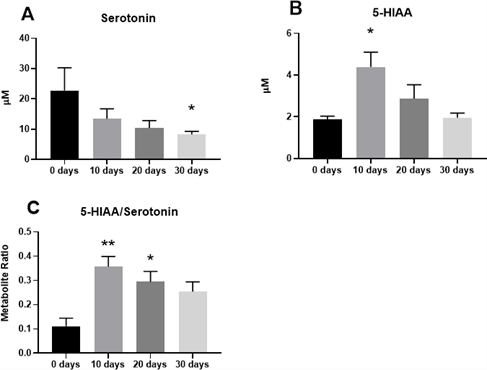
There was a significant reduction of serotonin in the brain following 30 days of CUS compared to 0 days controls (Figure 3). 5-HIAA was significantly increased at 10 days of CUS. The ratio of serotonin to tryptophan was significantly reduced for the duration of the study, while the ratio of 5-HIAA to serotonin remained elevated. These data indicate that CUS lowers central serotonin, possibly by up-regulating turnover to 5-HIAA.
Discussion
Chronic stress is a known risk factor for the development of depressive symptoms. The focus of this study was to characterize the change over time in downstream tryptophan metabolism pathways in conjunction with the depressive-like behavioural effects induced by stress. Here, we compiled several common stressors known to induce robust behavioural phenotypes or inflammatory processes into a chronic unpredictable stress (CUS) protocol [30-34]. We found that CUS significantly increased nesting behaviour and impaired working memory function (Figure 3). These behavioural effects occurred in tandem with an elevation in central tryptophan, kynurenine, picolinic acid (PA), and 5-hydroxyindoleacetic acid (5-HIAA). Central serotonin, xanthurenic acid (XT), and kynurenic acid (KYNA) levels were decreased. Peripherally, only quinolinic acid (QA) levels were reduced, while all others we measured remained steady.
The strain of mice used in this study (C57Bl/6J) is known to be resilient to the behavioural effects of stress compared to other strains [35-39]. In line with this, our behavioural assays did not reveal a robust depressive-like phenotype driven by our CUS protocol. In particular, we did not see the expected effects of CUS on anhedonia-like behaviour, a core component of the depressive-like phenotype, or on anxiety-like behaviour. This absence of a stress effect may be due to the insensitivity of the 2-hour saccharin preference test, which our lab typically performs over 24 hours. Further, while the open field test is widely recognized for its ability to detect anxiogenic effects by a reduction of the time spent in the central area and increase in thigmotaxis behaviour, studies have shown opposing effects of stress in this assay [40, 41]. However, there was an impairment of working memory as early as 10-days of CUS, which remained impaired throughout stress exposure. We also saw a profound increase in nesting behaviour induced by stress. Nesting is an innate behaviour and serves as an indicator of well-being in mice [42]. The nesting clearance procedure was established by Negus et al. to measure pain-induced functional behavioural impairment; we hypothesized that chronic stress would likewise reduce nesting behaviour [29]. Instead, we saw that prolonged stress exposure increased nesting behaviour, as measured by the speed with which mice gathered the nestlet materials and the number of nestlets collected into one corner at the end of the assay (Figure 3). This increased activity, despite the absence of stress effects on locomotor activity, suggests that stress drives comfort-seeking behaviour. In this regard, nesting behaviour may serve as an alternative anxiety-like phenotype readout.
Figure 3: Serotonin turnover in the brain is up-regulated by chronic stress.
Tryptophan and serotonin metabolite levels and ratios were measured after 0, 10, 20, or 30 days of chronic unpredictable stress (CUS). A) Serotonin concentration (μM) was significantly reduced in the brain at 20 and 30 days of CUS. B) 5-hydroxyindoleacetic acid (5-HIAA) concentration (μM) was significantly increased by 10 days of CUS and returned to baseline levels by 20 days of CUS. C) The 5-HIAA/serotonin ratio was up-regulated at 10 and 20 days of CUS.
Concentration values and ratio data represent sample means (s.e.m.) n = 4-6 samples per group.
*: Dunnett’s post hoc comparison with 0 days CUS control group, p < 0.05.
*: p<0.05; **: p<0.01; ***: p<0.001.
It has been hypothesized that inflammation-induced depression is driven by a reduction of tryptophan in the brain due to increased kynurenine metabolism, thus limiting the tryptophan supply available for serotonin synthesis [43-46]. Stress similarly activates up-regulation of pro-inflammatory cytokines and drives imbalances in the kynurenine metabolism pathway [43, 47-51]. However, compared with inflammation, stress increases tryptophan concentrations in the brain [52-55]. This rise in tryptophan is accompanied by an up-regulation of serotonin turnover: serotonin is depleted while 5-HIAA concentrations rise, although conflicting results have also been reported with varying stressors and brain regions [48, 52-57]. Here we report similar trends in increased central tryptophan concentrations and serotonin turnover as a result of a modified CUS protocol (Table 2 & Figure 2). It has been proposed that stress drives the elevation of tryptophan in the brain by increased trafficking of tryptophan from the plasma in order to supply the source necessary for this increased serotonin turnover. Pre-treatment with valine, a competitor for the large amino acid transporter that allows tryptophan to cross the blood-brain barrier, prevents a restraint stress-induced elevation in tryptophan and downstream serotonin turnover in the brain [58]. Here, we show that peripheral tryptophan levels remain steady despite increased concentrations in the brain after stress (Table 2).
Acute stress up-regulates the enzyme IDO, resulting in an increased tryptophan-kynurenine turnover [27, 28]. We show here that while the concentration of kynurenine in the brain is increased after 10 days of CUS, the ratio of kynurenine to tryptophan did not reach significance. Unexpectedly, there was a main effect of stress-reducing central KYNA and XT and peripheral QA. The only downstream metabolite of the kynurenine pathway measured here that increased in the brain was PA. Interestingly, increased activity of ACMSD, the enzyme that converts 5-hydroxyanthranilic acid (5-HAA) to PA, is implicated in depression in humans: increased production of PA is suggested to be protective against suicidality by reducing the availability of the precursor 5-HAA for QA metabolism [59]. The elevated kynurenine concentrations we measured here are shunted towards picolinic acid production, possibly driving the lack of depressive-like behaviours in these stress-exposed mice.
Acknowledgement
This work was supported by Merit Review Award I01BX003195 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Service (to JCO) and Translational Sciences Training Award TL1 TR002647 from the NIH National Center for Advancing Translational Sciences (to GAP). The content is the sole responsibility of the authors and does not necessarily represent the views of the U.S. Department of Veterans Affairs, United States Government, National Center for Advancing Translational Studies, or the National Institutes of Health.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 12, Aug 2021Accepted: Sat 28, Aug 2021
Published: Sat 25, Sep 2021
Copyright
© 2023 Jason C. O’Connor. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2021.03.03
Author Info
Grace A. Porter Jason C. O’Connor
Corresponding Author
Jason C. O’ConnorAudie L. Murphy VA Hospital, South Texas Veterans Health System, San Antonio, Texas, USA
Figures & Tables
Table 1: Schedule for the experimental chronic unpredictable
stress paradigm and behavioural assessment.
|
Chronic Unpredictable Stress Experimental Schedule |
|
|
Day 1 |
Cage tilt & Wet bedding |
|
Day 2 |
Cold swim, Restraint |
|
Day 3 |
Cage agitation |
|
Day 4 |
Strobe light |
|
Day 5 |
Social defeat |
|
Day 6 |
Predator odor |
|
Day 7 |
Warm cage |
|
Day 8 |
Restraint & Tail pinch, Isolation |
|
Day 9 |
Isolation Begin saccharin
preference testing |
|
Day 10 |
Isolation & Tail pinch Behaviour testing and
Tissue collection |
|
Day 11 |
Strobe light |
|
Day 12 |
Cold swim, Restraint |
|
Day 13 |
Predator odor |
|
Day 14 |
Social defeat |
|
Day 15 |
Cage agitation |
|
Day 16 |
Cage tilt & Wet bedding |
|
Day 17 |
Warm cage |
|
Day 18 |
Restraint & Tail pinch, Isolation |
|
Day 19 |
Isolation Begin saccharin
preference testing |
|
Day 20 |
Isolation, Tail pinch Behaviour testing and
Tissue collection |
|
Day 21 |
Social defeat |
|
Day 22 |
Warm cage |
|
Day 23 |
Cold swim, Restraint |
|
Day 24 |
Cage tilt & Wet bedding |
|
Day 25 |
Predator odor |
|
Day 26 |
Cage agitation |
|
Day 27 |
Restraint & Tail pinch |
|
Day 28 |
Strobe light, Isolation |
|
Day 29 |
Isolation Begin saccharin preference testing |
|
Day 30 |
Isolation, Tail pinch Behaviour testing and Tissue collection |
Table 2: Brain and plasma kynurenine metabolite analysis
following chronic unpredictable stress.
|
|
0 days CUS |
10 days CUS |
20 days CUS |
30 days CUS |
Main effects |
|
Brain metabolites (μM) |
|||||
|
Tryptophan |
344.9 (49.50) |
692.2 (114.5) † |
630.9 (93.02) |
356.7 (42.87) |
*p<0.05 |
|
Kynurenine |
0.15 (0.027) |
0.41 (0.068) †††† |
0.33 (0.073) |
0.29 (0.098) |
n.s. |
|
3-HK |
0.036 (0.013) |
0.026 (0.0140) |
0.018 (0.0022) |
0.04 (0.018) |
n.s. |
|
XT |
0.023 (0.00097) |
0.004 (0.0011) †† |
0.013 (0.0027) |
0.022 (0.0057) |
**p<0.01 |
|
3-HAA |
0.02 (0.013) |
0.0038 (0.0038) |
0.0056 (0.0035) |
0.021 (0.0078) |
n.s. |
|
PA |
1.072 (0.11) |
2.55 (0.35) †† |
2.22 (0.36) † |
2.01 (0.77) |
n.s. |
|
QA |
n.d. |
n.d. |
n.d. |
n.d. |
n/a |
|
KYNA |
0.033 (0.0036) |
0.014 (0.0024) |
0.021 (0.0017) |
0.045 (0.013) |
*p<0.05 |
|
Plasma metabolites
(μM) |
|||||
|
Tryptophan |
955.3 (67.80) |
887.3 (38.68) |
1053 (66.07) |
966.7 (70.37) |
n.s. |
|
Kynurenine |
1.110 (0.08282) |
1.149 (0.07760) |
1.432 (0.1713) |
1.175 (0.1713) |
n.s. |
|
3-HK |
0.058 (0.029) |
0.057 (0.015) |
0.069 (0.02) |
0.052 (0.0094) |
n.s. |
|
XT |
0.053 (0.0015) |
0.048 (0.0049) |
0.051 (0.0035) |
0.054 (0.0034) |
n.s. |
|
3-HAA |
n.d. |
n.d. |
n.d. |
n.d. |
n/a |
|
PA |
1.47 (0.082) |
1.13 (0.087) |
1.31 (0.089) |
1.26 (0.1) |
n.s. |
|
QA |
0.39 (0.12) |
0.15 (0.021) † |
0.19 (0.018) † |
0.23 (0.026) |
*p<0.05 |
|
KYNA |
0.049 (0.0028) |
0.048 (0.0052) |
0.064 (0.007) |
0.056 (0.0049) |
n.s. |
3-HK: 3-Hydroxykynurenine; XT:
Xanthurenic Acid; 3-HAA: 3-Hydroxyanthranilic
Acid; PA: Picolinic Acid; QA: Quinolinic Acid; KYNA: Kynurenic Acid.
Kynurenine
pathway metabolites were quantified (μM) by LC/MS in the whole brain and plasma
samples from male C67Bl/6J mice following 0, 10, 20, or 30 days of chronic unpredictable
stress. Concentration values represent sample means (s.e.m.) n = 4-6 samples
per group.
*:
Main effect of CUS, p < 0.05.
†:
Post hoc comparison with 0 days CUS control group.
*,†:
p<0.05; **,††: p<0.01.
Table 3: Tryptophan metabolism pathways balance shifts
following chronic unpredictable stress.
|
|
0 days CUS |
10 days CUS |
20 days CUS |
30 days CUS |
Main effects |
|
Brain metabolite ratios |
|||||
|
5-HT/TRP |
0.07345 (0.0284) |
0.02002 (0.00375) †† |
0.0163 (0.0027†† |
0.02424 (0.00308) † |
*p<0.05 |
|
KYN/TRP |
0.41 (0.0215) |
0.6 (0.0453) †† |
0.52 (0.0494) |
0.96 (0.5) |
n.s. |
|
KYNA/KYN |
0.27 (0.071) |
0.037 (0.0087) †† |
0.079 (0.016) † |
0.19 (0.058) |
**p<0.01 |
|
|
|
|
|
|
|
|
Total oxidative kynurenines (μM) |
1.186 (0.112) |
2.585 (0.352) † |
2.247 (0.367) |
2.072 (0.788) |
n.s. |
|
KAT-dependent kynurenines (μM) |
0.05669 (0.00411) |
0.01825 (0.00307) † |
0.03460 (0.0418) |
0.06787 (0.0185) |
*p<0.05 |
|
Ratio of oxidative KYNs/KATs |
21.26 (2.7) |
151.4 (19.22) †††† |
68.65 (14.78) |
28.88 (4.07) |
****p<0.0001 |
5-HT: Serotonin; TRP: Tryptophan; KYN:
Kynurenine; KYNA: Kynurenic Acid; KAT: Kynurenine Aminotransferase.
Kynurenine
pathway metabolism was analysed by comparing concentrations (μM) to calculate a
ratio value. Total oxidative kynurenines include 3-hydroxykynurenine,
3-hydroxyanthranilic acid, picolinic acid, quinolinic acid. Total KATs include
kynurenic acid and xanthurenic acid.
Ratio
data represent sample means (s.e.m.) n = 4-6 samples per group.
*:
Main effect of CUS, p < 0.05.
†:
Dunnett’s post hoc comparison with 0 days CUS control group.
*,†:
p<0.05; **,††: p<0.01.
IDO: Indolamine-2,3-Dioxygenase; TDO: Tryptophan Dioxygenase; TPH: Tryptophan Hydroxylase; MAO: Monoamine Oxygenase; KMO: Kynurenine Monooxygenase; KAT: Kynurenine Aminotransferase; KYNU: Kynureninase; HAAO: 3-Hydroxyanthranilate Oxidase; ACMSD: Amino-β-Carboxymuconate-Semialdehyde-Decarboxylase
Data represent sample means (s.e.m.) n = 8 samples per group.
*: Post hoc comparison with 0 days CUS control group, p < 0.05.
*: p<0.05; **: p<0.01; ***: p<0.001.
References
1. Ferrari AJ,
Charlson FJ, Norman RE, Patten SB, Freedman G et al. (2013) Burden of
depressive disorders by country, sex, age, and year: findings from the global
burden of disease study 2010. PLoS Med 10: e1001547. [Crossref]
2. Pariante CM, Miller
AH (2001) Glucocorticoid receptors in major depression: relevance to
pathophysiology and treatment. Biol Psychiatry 49: 391-404. [Crossref]
3. Nemeroff CB, Vale
WW (2005) The neurobiology of depression: inroads to treatment and new drug
discovery. J Clin Psychiatry 66: 5-13. [Crossref]
4. Pariante CM (2006)
The glucocorticoid receptor: part of the solution or part of the problem? J
Psychopharmacol 20: 79-84. [Crossref]
5. Pariante CM,
Lightman SL (2008) The HPA axis in major depression: classical theories and new
developments. Trends Neurosci 31: 464-468. [Crossref]
6. Mineur YS, Belzung
C, Crusio WE (2006) Effects of unpredictable chronic mild stress on anxiety and
depression-like behavior in mice. Behav Brain Res 175: 43-50. [Crossref]
7. Turnbull AV, Rivier
CL (1999) Regulation of the hypothalamic-pituitary-adrenal axis by cytokines:
actions and mechanisms of action. Physiol Rev 79: 1-71 [Crossref]
8. Dhabhar FS, McEwen
BS (1997) Acute stress enhances while chronic stress suppresses cell-mediated
immunity in vivo: a potential role for leukocyte trafficking. Brain Behav
Immun 11: 286-306. [Crossref]
9. Iwakabe K, Shimada
M, Ohta A, Yahata T, Ohmi Y et al. (1998) The restraint stress drives a shift
in Th1/Th2 balance toward Th2-dominant immunity in mice. Immunol Lett 62:
39-43. [Crossref]
10. Moynihan JA (2003)
Mechanisms of stress-induced modulation of immunity. Brain Behav Immun
17: S11-S16. [Crossref]
11. Schiepers OJG,
Wichers MC, Maes M (2005) Cytokines and major depression. Prog Neuropsychopharmacol
Biol Psychiatry 29: 201-217. [Crossref]
12. Dantzer R, O’Connor
JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and
depression: when the immune system subjugates the brain. Nat Rev Neurosci
9: 46-56. [Crossref]
13. Koo JW, Russo SJ,
Ferguson D, Nestler EJ, Duman RS (2010) Nuclear factor-kappaB is a critical
mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl
Acad Sci U S A 107: 2669-2674. [Crossref]
14. Anisman H, Merali Z
(1999) Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med
Biol 461: 199-233 [Crossref]
15. Walker FR, Nilsson
M, Jones K (2013) Acute and chronic stress-induced disturbances of microglial
plasticity, phenotype and function. Curr Drug Targets 14: 1262-1276. [Crossref]
16. Johnson JD, Barnard
DF, Kulp AC, Mehta DM (2019) Neuroendocrine Regulation of Brain Cytokines After
Psychological Stress. J Endocr Soc 3: 1302-1320. [Crossref]
17. Hellwig S, Brioschi
S, Dieni S, Frings L, Masuch A et al. (2016) Altered microglia morphology and
higher resilience to stress-induced depression-like behavior in
CX3CR1-deficient mice. Brain Behav Immun 55: 126-137. [Crossref]
18. Goshen I, Kreisel
T, Zidon OBM, Licht T, Weidenfeld J, Hur TB et al. (2008) Brain interleukin-1
mediates chronic stress-induced depression in mice via adrenocortical
activation and hippocampal neurogenesis suppression. Mol Psychiatry 13:
717-728. [Crossref]
19. Koo JW, Duman RS
(2008) IL-1 is an essential mediator of the antineurogenic and anhedonic
effects of stress. Proc Natl Acad Sci U S A 105: 751-756. [Crossref]
20. Miller A. H. (2013) Conceptual confluence: the
kynurenine pathway as a common target for ketamine and the convergence of the
inflammation and glutamate hypotheses of depression. Neuropsychopharmacology,
38: 1607-1608. [Crossref]
21. Parrott JM,
O’Connor JC (2015) Kynurenine 3-Monooxygenase: An Influential Mediator of
Neuropathology. Front Psychiatry 6: 116. [Crossref]
22. Salazar A, Rivera
BLG, Redus L, Parrott JM, O’Connor JC (2012) Indoleamine 2,3-dioxygenase
mediates anhedonia and anxiety-like behaviors caused by peripheral
lipopolysaccharide immune challenge. Horm Behav 62: 202-209. [Crossref]
23. Parrott JM, Redus
L, Coelho DS, Morales J, Gao X et al. (2016) Neurotoxic kynurenine metabolism
is increased in the dorsal hippocampus and drives distinct depressive behaviors
during inflammation. Transl Psychiatry 6: e918. [Crossref]
24. Ohgidani M, Kato
TA, Sagata N, Hayakawa K, Shimokawa N et al. (2016) TNF-α from hippocampal
microglia induces working memory deficits by acute stress in mice. Brain
Behav Immun 55: 17-24. [Crossref]
25. Wohleb ES, Hanke
ML, Corona AW, Powell ND, Stiner La'Tonia M et al. (2011) β-Adrenergic receptor
antagonism prevents anxiety-like behavior and microglial reactivity induced by
repeated social defeat. J Neurosci 31: 6277-688. [Crossref]
26. Patki G, Solanki N,
Atrooz F, Allam F, Salim S (2013) Depression, anxiety-like behavior and memory
impairment are associated with increased oxidative stress and inflammation in a
rat model of social stress. Brain Res 1539: 73-86. [Crossref]
27. Kiank C, Zeden JP,
Drude S, Domanska G, Fusch G et al. (2010) Psychological stress-induced,
IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice
and humans. PLoS One 5: e11825. [Crossref]
28. Brooks AK, Lawson
MA, Smith RA, Janda TM, Kelley KW et al. (2016) Interactions between
inflammatory mediators and corticosteroids regulate transcription of genes
within the Kynurenine Pathway in the mouse hippocampus. J Neuroinflammation
13: 98. [Crossref]
29. Negus SS,
Neddenriep B, Altarifi AA, Carroll FI, Leitl MD et al. (2015) Effects of
ketoprofen, morphine, and kappa opioids on pain-related depression of nesting
in mice. Pain 156: 1153-1160. [Crossref]
30. Schweizer MC,
Henniger MSH, Sillaber I (2009) Chronic mild stress (CMS) in mice: of
anhedonia, 'anomalous anxiolysis' and activity. PLoS One 4: e4326. [Crossref]
31. Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E et al. (2005) Anhedonia
and motivational deficits in rats: impact of chronic social stress. Behav
Brain Res 162: 127-134. [Crossref]
32. Liu YY, Zhou XY,
Yang LN, Wang HY, Zhang YQ et al. (2017) Social defeat stress causes
depression-like behavior with metabolite changes in the prefrontal cortex of
rats. PLoS One 12: e0176725. [Crossref]
33. Dugan AM, Parrott
JM, Redus L, Hensler JG, O’Connor JC (2015) Low-Level Stress Induces Production
of Neuroprotective Factors in Wild-Type but Not BDNF+/- Mice: Interleukin-10
and Kynurenic Acid. Int J Neuropsychopharmacol 19: pyv089. [Crossref]
34. Shintani F, Nakaki
T, Kanba S, Sato K, Yagi G et al. (1995) Involvement of interleukin-1 in
immobilization stress-induced increase in plasma adrenocorticotropic hormone
and in release of hypothalamic monoamines in the rat. J Neurosci 15:
1961-1970. [Crossref]
35. Razzoli M, Carboni
L, Andreoli M, Ballottari A, Arban R (2011) Different susceptibility to social
defeat stress of BalbC and C57BL6/J mice. Behav Brain Res 216: 100-108.
[Crossref]
36. Razzoli M, Carboni L, Andreoli M, Michielin F, Ballottari A et al. (2011)
Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment
in the mouse. Pharmacol Biochem Behav 97: 566-576. [Crossref]
37. Hayley S, Borowski
T, Merali Z, Anisman H (2001) Central monoamine activity in genetically
distinct strains of mice following a psychogenic stressor: effects of predator
exposure. Brain Res 892: 293-300. [Crossref]
38. Anisman H, Hayley
S, Kelly O, Borowski T, Merali Z (2001) Psychogenic, neurogenic, and systemic
stressor effects on plasma corticosterone and behavior: mouse strain-dependent
outcomes. Behav Neurosci 115: 443-454. [Crossref]
39. Belzung C, Griebel
G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a
review. Behav Brain Res 125: 141-149. [Crossref]
40. Prut L, Belzung C
(2003) The open field as a paradigm to measure the effects of drugs on
anxiety-like behaviors: A review. Eur J Pharmacol 463: 3-33. [Crossref]
41. Lee EH, Tsai MJ,
Chai CY (1986) Stress selectively influences center region activity of mice in
an open field. Physiol Behav 37: 659-62. [Crossref]
42. Jirkof P (2014)
Burrowing and nest building behavior as indicators of well-being in mice. J
Neurosci Methods 234: 139-146. [Crossref]
43. Won E, Kim YK
(2016) Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway
in the Etiology of Depression. Curr Neuropharmacol 14: 665-673. [Crossref]
44. Capuron L,
Neurauter G, Musselman DL, Lawson DH, Nemeroff CB et al. (2003)
Interferon-alpha-induced changes in tryptophan metabolism. relationship to
depression and paroxetine treatment. Biol Psychiatry 54: 906-914. [Crossref]
45. Capuron L, Ravaud
A, Neveu PJ, Miller AH, Maes M et al. (2002) Association between decreased
serum tryptophan concentrations and depressive symptoms in cancer patients
undergoing cytokine therapy. Mol Psychiatry 7: 468-473. [Crossref]
46. Myint AM, Kim YK
(2003) Cytokine-serotonin interaction through IDO: a neurodegeneration
hypothesis of depression. Med Hypotheses 61: 519-525. [Crossref]
47. O’Connor KA,
Johnson JD, Hansen MK, Frank JLW, Maksimova E et al. (2003) Peripheral and
central proinflammatory cytokine response to a severe acute stressor. Brain
Res 991: 123-132. [Crossref]
48. Pawlak D, Takada Y,
Urano T, Takada A (2000) Serotonergic and kynurenic pathways in rats exposed to
foot shock. Brain Res Bull 52: 197-205. [Crossref]
49. Lapin IP (2003)
Neurokynurenines (NEKY) as common neurochemical links of stress and anxiety. Adv
Exp Med Biol 527:121-125. [Crossref]
50. Fuertig R,
Azzinnari D, Bergamini G, Cathomas F, Sigrist H et al. (2016) Mouse chronic
social stress increases blood and brain kynurenine pathway activity and fear
behaviour: Both effects are reversed by inhibition of indoleamine
2,3-dioxygenase. Brain Behav Immun 54: 59-72. [Crossref]
51. Liu YN, Peng YL, Liu L, Wu TY, Zhang Y et al. (2015)
TNFα mediates stress-induced depression by upregulating indoleamine
2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur
Cytokine Netw 26: 15-25 [Crossref]
52. Curzon G, Joseph
MH, Knott PJ (1972) Effects of immobilization and food deprivation on rat brain
tryptophan metabolism. J Neurochem 19: 1967-1974. [Crossref]
53. Fontenot MB, Kaplan
JR, Manuck SB, Arango V, Mann JJ (1995) Long-term effects of chronic social
stress on serotonergic indices in the prefrontal cortex of adult male
cynomolgus macaques. Brain Res 705: 105-108. [Crossref]
54. Dunn AJ (1988)
Changes in plasma and brain tryptophan and brain serotonin and
5-hydroxyindoleacetic acid after footshock stress. Life Sci 42:
1847-1853. [Crossref]
55. Miura H, Ozaki N,
Shirokawa T, Isobe K (2008) Changes in brain tryptophan metabolism elicited by
ageing, social environment, and psychological stress in mice. Stress 11:
160-169. [Crossref]
56. Amat J, Amat PM,
Watkins LR, Maier SF (1998) Escapable and inescapable stress differentially and
selectively alter extracellular levels of 5-HT in the ventral hippocampus and
dorsal periaqueductal gray of the rat. Brain Res 797: 12-22. [Crossref]
57. Kirby LG,
Chou-Green JM, Davis K, Lucki I (1997) The effects of different stressors on
extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res
760: 218-230. [Crossref]
58. Kennett GA, Joseph MH (1981) The functional importance of increased brain tryptophan in the serotonergic response to restraint stress. Neuropharmacology 20: 39-43. [Crossref]
59. Brundin L, Sellgren CM, Lim CK, Grit J, Pålsson E et al. (2016) An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry 6: e865. [Crossref]
