Cholecystocutaneous Fistula Associated with Necrotizing Fasciitis of the Abdominal Wall: A Case Report
A B S T R A C T
Cholecystocutaneous fistula represents an extremely rare complication of calculous cholecystopathy. In the past 50 years, less than 50 cases have been reported. The most frequent site of spontaneous cholecystocutaneous fistula is the right hypochondrium, followed by the left hypochondrium, periumbilical, right lumbar, right iliac fossa and gluteal areas. The association with necrotizing fasciitis that represents a serious infection, characterized by extensive and rapidly progressive necrosis, affecting the subcutaneous plane and reaching the muscular fascia provides a high mortality rate and extensive procedures are required. Herein we present a case of a 64-years-old, female, admitted to the emergency department with complaint of diffuse, severe abdominal pain, associated with a tense and painful lesion in the abdominal wall with the diagnose of cholecystocutaneous fistula associated with necrotizing fasciitis that despite an aggressive surgical approach developed a multisystem failure and died 24 hours after admission.
Keywords
Acute cholecystitis, cutaneous fistula, postoperative complications, necrotizing fasciitis, sepsis
Introduction
Cholecystocutaneous fistula represents an extremely rare complication of calculous cholecystopathy. In the past 50 years, less than 50 cases have been reported. It is defined as the communication of the gallbladder with the external environment through the loss of continuity of the abdominal wall, usually due to chronic inflammatory process of the gallbladder. The most frequent site of spontaneous cholecystocutaneous fistula is the right hypochondrium, followed by the left hypochondrium, periumbilical, right lumbar, right iliac fossa and gluteal areas [1].
The first reports of this condition were described, in 1670, by Thilesus, followed by reports by Bonnet in 1876, Courvosier in 1890 and Naunyn in 1897. Represents a rare complication that occurs in less than 10% of patients with cholelithiasis. In recent years, probably due to the earlier diagnosis and surgical resolution of cholecystopathy, a drastic reduction in the number of reported cases were observed [1-3]. Table 1 summarizes the case reports from the world literature within the 21st century. Necrotizing fasciitis represents a serious infection, characterized by extensive and rapidly progressive necrosis, affecting the subcutaneous plane and reaching the muscular fascia. Its mortality ranges from 13-76%, being influenced by early diagnosis as well as by underlying morbid conditions. Its association with external biliary fistula is an extremely rare condition [4].
Etiology
The cholecystocutaneous fistula represents a condition secondary to inflammation, most often chronic, of the gallbladder. The vast majority of the reported cases are related to calculous cholecystopathy, however, it is known that other conditions that generate inflammatory process of the gallbladder, such as acalculous cholecystitis and neoplastic process, can also be related to etiology. It is known that Salmonella typhi infection is a predisposing factor to chronic inflammation of the gallbladder and consequently to the formation of cholecystocutaneous fistulas [5]. As for pathophysiology, the mechanism related to the development of the fistula begins with the increase in the intraluminal pressure in the gallbladder resulting from obstruction of the cystic duct. As consequence, a reduction in blood flow is observed, followed by necrosis and perforation, resulting acutely as peritonitis, sub acutely as abscess or in a chronic form with the formation of fistulas [5, 6].
Table 1: Case reports from the world literature within the 21st century.
|
Cases Reporting Table |
|||||
|
AUTHOR |
YEAR |
COUNTRY |
AGE |
GENDER |
TECNIQUE |
|
Sulakshane et al. [9] |
2018 |
India |
67 |
M |
Open |
|
Rinzivillo et al. [10] |
2017 |
Italia |
76 |
M |
Open |
|
Maynard et al. [11] |
2016 |
United Kingdom |
68 |
F |
Open |
|
Jayasinghe et al. [12] |
2016 |
United Kingdom |
>70 |
F |
Open |
|
Neto et al. [13] |
2016 |
Brazil |
94 |
F |
Open |
|
Moreno et al. [5] |
2015 |
Chile |
64 |
F |
Open |
|
Guardado –B et al. [3] |
2015 |
Mexico |
30 |
F |
Open |
|
Álvarez et al. [14] |
2014 |
Argentina |
79 |
F |
Open |
|
Dixon et al. [15] |
2014 |
United Kingdom |
94 |
F |
Open |
|
Pripotnev e Petrakos [7] |
2014 |
Canada |
85 |
F |
Open |
|
Kim et al. [16] |
2013 |
Australia |
72 |
F |
Open |
|
Jayant et al. [17] |
2013 |
India |
42 |
F |
Open |
|
Kapoor et al. [19] |
2013 |
India |
45 |
M |
Open |
|
Sodhi et al. [18] |
2012 |
India |
66 |
F |
Open |
|
Ozdemir et al. [20] |
2012 |
India |
45, 65 |
M |
Open |
|
Ugalde Serrano et al. [21] |
2012 |
Spain |
83 |
M |
Open |
|
Andersen e Friss Andersen [22] |
2012 |
Denmark |
89 |
F |
Open |
|
Ioannidis et al. [23] |
2011 |
Greece |
71 |
M |
Open |
|
Cheng et al. [24] |
2011 |
China |
21 |
F |
Open |
|
Gordon et al. [25] |
2011 |
United States |
83 |
F |
Open |
|
Sayed et al. [26] |
2010 |
United Kingdom |
85 |
F |
Open |
|
Pezzilli et al. [27] |
2010 |
Italy |
90 |
F |
Open |
|
Metsemakers et al. [28] |
2010 |
Belgium |
69 |
M |
Open |
|
Aguilar et al. [2] |
2010 |
Spain |
83 |
F |
Open |
|
Khan et al. [29] |
2010 |
Ireland |
76 |
M |
Open |
|
Hawari et al. [30] |
2010 |
United Kingdom |
84 |
M |
Open |
|
Speranzini et al. [1] |
2009 |
Brazil |
63 |
M |
Open |
|
Murphy et al. [31] |
2008 |
United Kingdom |
80 |
M |
Open |
|
Ijaz et al. [34] |
2008 |
United Kingdom |
80 |
F |
Open |
|
Chatterjee et al. [32] |
2007 |
India |
45 |
F |
Open |
|
Cruz et al. [6] |
2006 |
Brazil |
81 |
M |
Open |
|
Wong et al. [4] |
1997-2002 |
Singapore |
27-84 |
M, F |
Open |
|
Malik et al. [33] |
2007 |
United Kingdom |
76 |
F |
Laparoscopic |
Case Report
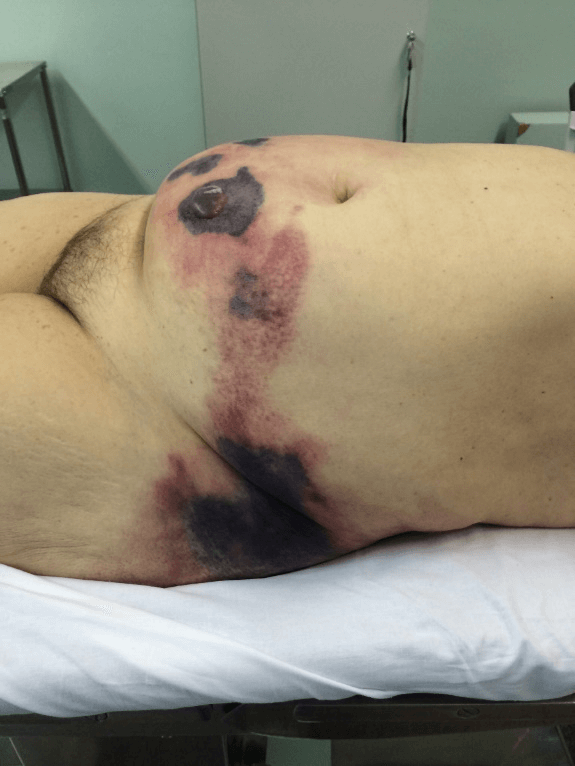
RAF, 64-years-old, female, admitted to the emergency department with complaint of diffuse, severe abdominal pain, associated with a tense and painful lesion in the abdominal wall. The patient sustained also a psychiatric disorder, using risperidone and promethazine, and was unable to specify the aspect and duration of the symptoms. During physical examination, the patient was in poor general condition, dehydrated, tachycardic, with increased respiratory rate, normotensive, anicteric and afebrile. The abdominal examination demonstrated the presence of an extensive lesion compromising most the abdominal wall, with the presence of phlogistic signs (pain, heat and hyperemia) in addition to crackling and areas of necrosis with blistering (Figure 1).
Laboratory tests were performed with the following results: leukocyte count of 16,200, with 20% of rods and 40% of segmented ones, PCR 371.1, Creatinine 4.9, Urea 210, venous lactic acid 95.0, INR 1.7, TTPA 37.6, arterial blood gases with pH 7.18, pCO2 26.0, pO2 53.5, HCO3 10.7, BE -16.1, O2 saturation 75.2, other laboratory tests within normal ranges. Despite the severity of the clinical condition during admission at the ER, the patient presented with blood pressure at normal levels, and was submitted to an abdominal CT scan that revealed blurring of part of the subcutaneous fatty planes, noting gaseous components on the right side. Empiric antibiotics were promptly initiated following institutional protocols using metronidazole and ceftriaxone prior to surgery.
Figure 1: Appearance of the abdominal wall with signs of necrosis.
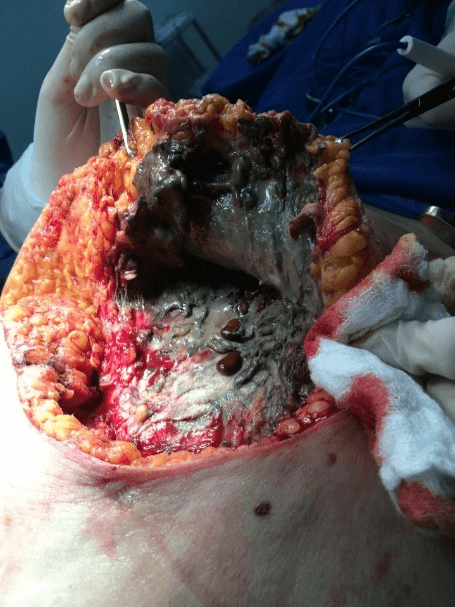
In view of the findings, the patient underwent extensive debridement of the abdominal wall, removing all the necrotic tissues from the upper right quadrant and lateral region of the wall bilaterally, with the discharge of a large amount of purulent-looking secretion with characteristic odor, with extensive involvement of subcutaneous tissue. During debridement in the lower right quadrant of the abdomen, multiple gallstone calculi were identified. When extending the debridement to the upper right quadrant, a fistulous orifice was found with the gallbladder. The purulent fluid was collected from the abdominal cavity, which later identified the presence of Escherichia coli. During the intraoperative period, the patient developed hemodynamic instability requiring high doses of vasoactive drugs (Figure 2).
Figure 2: Intraoperative appearance of the tissue with necrosis and the presence of gallstones.
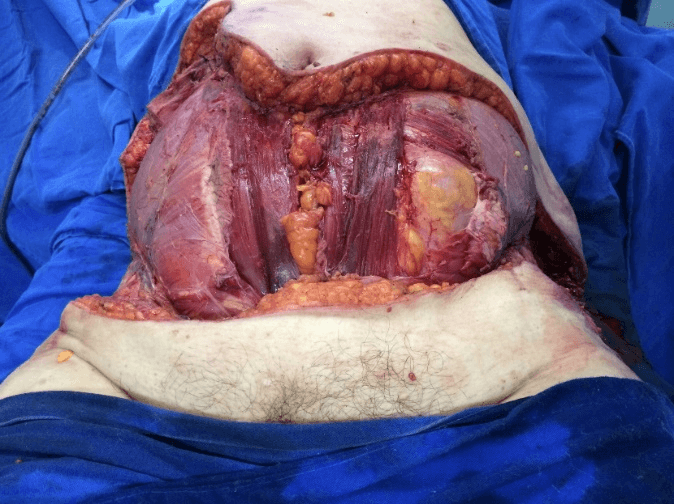
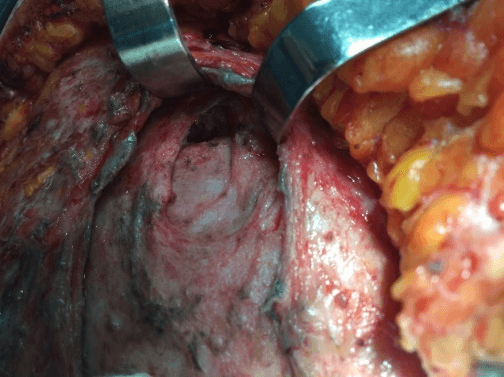
After removal of necrotic tissues, the patient was then stabilized and prepared for a new approach after 12 hours to expand the initial debridement of necrotizing fasciitis, (Figure 3), with cholecystectomy, (Figure 4). The anatomopathological study of the abdominal wall showed skin and soft tissues with a chronic suppurative inflammatory process and, in another segment, fibro-adipose and skeletal muscle tissue with chronic non-specific suppurative inflammatory reaction and necrosis. Anatomopathological examination of the gallbladder revealed chronic calculous cholecystitis.
Figure 3: Final aspect of debridement.
Figure 4: Surgical access to the upper right quadrant to perform cholecystectomy.
The patient in the postoperative period in the intensive care unit evolved with severe septic shock and hemodynamic instability with the need for increasing doses of vasopressors yet presenting with refractory hypotension. The patient progressed to cardiorespiratory arrest and death 24 hours after the surgical procedure.
Discussion
Cholecystocutaneous fistulas represents a very uncommon complication due to an inflammatory or infectious process involving the gallbladder. Biliary fistulas can be divided into internal and external. Internals are more common, with 75% involving the duodenum and 15% the colon. External fistulas are infrequent and rarely occur spontaneously due to inflammation of the biliary tract or liver abscesses. Most of the time, they occur as postoperative complications of liver, biliary tract or trauma-related surgery [1]. This condition was first described in 1670 by Thilesius. In 1890 Courvosier described 499 cases of gallbladder perforation associated with acute cholecystitis, of which 169 had cholecystocutaneous fistulas. In 1897, Bonnet reported another 122 cases of spontaneous cholecystocutaneous fistulas. In 1986, Naunyn described 184 cases, one of the largest series in the last 30 years. However, despite previous reports, the number of occurrences of this condition has significantly decreased in past years. This decrease in incidence is probably due to early diagnosis, the availability of antibiotic therapy and due to early surgical treatment for acute cholecystitis as well as gallbladder empyema [7].
This contrast in the incidence of the disease is reflected when looking at large series studies published before the 20th century, compared to recent literature, in which spontaneous external fistulas occur mainly in women between the fifth and seventh decade of life and may be related to the increase in conditions such as cholecystitis and gallbladder cancer in this age group. The most affected site is the upper right quadrant (48%), as shown in the case described, followed by the umbilical area (27%) and finally the right inguinal area and buttocks. The pathophysiology of the disease is related to increased pressure in the gallbladder with a consequent decrease in local blood flow, necrosis and perforation. Perforation can occur acutely, leading to peritonitis, subacute when observing the formation of abscesses or chronic with the formation of fistulas. It is known that there is a greater relationship with acute acalculous cholecystitis, with Salmonella typhi infection being a predisposing factor for the occurrence of the fistulas. The incidence of perforation in acalculous cholecystitis is lower, corresponding to 0.6 to 1% of cases [5, 6].
Generally, patients have an insidious clinical picture, complaining of recurrent biliary colic and in more advanced cases worsening of abdominal discomfort, bulging in the abdominal wall and the presence of bile content leaking through the skin. Although the signs and symptoms of spontaneous cholecystocutaneous fistula are evident, it is necessary to carry out studies with imaging tests such as ultrasound, tomography or fistulogram to make the definitive diagnosis [3]. The treatment of this disease is based on cholecystectomy, resection of the fistula and repair of the defect in the abdominal wall, in addition to the management of possible complications resulting from the disease. The option of an abbreviated surgery (damage control) as the initial approach for our patient was due to the presence of intraoperative hemodynamic instability, and after stabilization in the ICU, the patient was returned to the operating room to complete the procedure.
It is important to highlight that an extensive removal of necrotic tissues (surgical debridement), the collection of material for culture as well as the introduction of antibiotics with a wide spectrum of action, mainly for gram negative and anaerobic agents are fundamental steps for the treatment. The patient will only return to the operating room for a new procedure after hemodynamic stability and recovery of physiological parameters [8]. Despite being a rare complication of calculous cholecystopathy, all the acute care and general surgeons must have a high index suspicion. With the rapid diagnosis and early surgical treatment, there was a reduction in the incidence of such fistulas, but it is still possible to find it due to the high incidence of calculous cholecystopathy in the general population. The association of necrotizing fasciitis with this condition should be promptly diagnosed and treated, given the high morbidity and mortality associated with these cases.
Conclusion
Although cholecystocutaneous fistulas are infrequent and their incidence has decreased over the years, early diagnosis associated with adequate and accurate intervention is of fundamental importance. Once the diagnosis is made, surgical treatment associated with antibiotic therapy and meticulous post-surgical monitoring are essential to have a favourable and positive prognosis with reduced morbidity and mortality.
Article Info
Article Type
Case ReportPublication history
Received: Wed 29, Apr 2020Accepted: Wed 13, May 2020
Published: Fri 15, May 2020
Copyright
© 2023 Marcelo Ribeiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2020.02.07
Author Info
Victoria Accioly Russowsky Marcelo Ribeiro Murillo Lima Favaro Stephanie Santin Vinicius Cunha Rodrigues
Corresponding Author
Marcelo RibeiroFull Professor of General and Trauma Surgery. Chief of Trauma Surgery Hospital Moriah and Professor of Surgery at the Post Graduation Program at IAMSPE, São Paulo, Brazil
Figures & Tables
Table 1: Case reports from the world literature within the 21st century.
|
Cases Reporting Table |
|||||
|
AUTHOR |
YEAR |
COUNTRY |
AGE |
GENDER |
TECNIQUE |
|
Sulakshane et al. [9] |
2018 |
India |
67 |
M |
Open |
|
Rinzivillo et al. [10] |
2017 |
Italia |
76 |
M |
Open |
|
Maynard et al. [11] |
2016 |
United Kingdom |
68 |
F |
Open |
|
Jayasinghe et al. [12] |
2016 |
United Kingdom |
>70 |
F |
Open |
|
Neto et al. [13] |
2016 |
Brazil |
94 |
F |
Open |
|
Moreno et al. [5] |
2015 |
Chile |
64 |
F |
Open |
|
Guardado –B et al. [3] |
2015 |
Mexico |
30 |
F |
Open |
|
Álvarez et al. [14] |
2014 |
Argentina |
79 |
F |
Open |
|
Dixon et al. [15] |
2014 |
United Kingdom |
94 |
F |
Open |
|
Pripotnev e Petrakos [7] |
2014 |
Canada |
85 |
F |
Open |
|
Kim et al. [16] |
2013 |
Australia |
72 |
F |
Open |
|
Jayant et al. [17] |
2013 |
India |
42 |
F |
Open |
|
Kapoor et al. [19] |
2013 |
India |
45 |
M |
Open |
|
Sodhi et al. [18] |
2012 |
India |
66 |
F |
Open |
|
Ozdemir et al. [20] |
2012 |
India |
45, 65 |
M |
Open |
|
Ugalde Serrano et al. [21] |
2012 |
Spain |
83 |
M |
Open |
|
Andersen e Friss Andersen [22] |
2012 |
Denmark |
89 |
F |
Open |
|
Ioannidis et al. [23] |
2011 |
Greece |
71 |
M |
Open |
|
Cheng et al. [24] |
2011 |
China |
21 |
F |
Open |
|
Gordon et al. [25] |
2011 |
United States |
83 |
F |
Open |
|
Sayed et al. [26] |
2010 |
United Kingdom |
85 |
F |
Open |
|
Pezzilli et al. [27] |
2010 |
Italy |
90 |
F |
Open |
|
Metsemakers et al. [28] |
2010 |
Belgium |
69 |
M |
Open |
|
Aguilar et al. [2] |
2010 |
Spain |
83 |
F |
Open |
|
Khan et al. [29] |
2010 |
Ireland |
76 |
M |
Open |
|
Hawari et al. [30] |
2010 |
United Kingdom |
84 |
M |
Open |
|
Speranzini et al. [1] |
2009 |
Brazil |
63 |
M |
Open |
|
Murphy et al. [31] |
2008 |
United Kingdom |
80 |
M |
Open |
|
Ijaz et al. [34] |
2008 |
United Kingdom |
80 |
F |
Open |
|
Chatterjee et al. [32] |
2007 |
India |
45 |
F |
Open |
|
Cruz et al. [6] |
2006 |
Brazil |
81 |
M |
Open |
|
Wong et al. [4] |
1997-2002 |
Singapore |
27-84 |
M, F |
Open |
|
Malik et al. [33] |
2007 |
United Kingdom |
76 |
F |
Laparoscopic |
References
- Speranzini MB, Pezzolo S, Martinelli RB, Ducatti LSS, Lemes MPL et al. (2009) Fístula colecistocutânea espontânea: uma rara complicação da doença calculosa da vesícula biliar. Arq Bras Ciên Saúde 34: 201-204.
- Aguilar T, Porras L, Garcia M (2010) Fístula colecistocutánea. Una rara complicación de la colelitiasis. Gastroenterol Hepatol 33: 553-554.
- Guardado-Bermúdez F, Aguilar-Jaimes A, Ardisson-Zamora FJ, Guerrero-Silva LA, Villanueva-Rodriguez E et al. (2015) Spontaneous cholecystocutaneous fistula. Cirugía y Cirujanos 83: 61-64.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL (2003) Necrotizing Fasciitis: Clinical Presentation, Microbiology, and Determinants of Mortality. J Bone Joint Surg Am 85: 1454-1460. [Crossref]
- Moreno A, Fuente M, Rodriguez E, Cruz F (2015) Fístula colecistocutánea espontânea. Rev Chil Cir 67: 413-415.
- Cruz Jr RJ, Nahas J, de Figueiredo LF (2006) Spontaneous cholecystocutaneous fistula: a rare complication of gallbladder disease. Sao Paulo Med J 124: 234-236. [Crossref]
- Pripotnev S, Petrakos A (2014) Cholecystocutaneous fistula after percutaneous gallbladder drainage. Case Rep Gastroenterol 8: 119-122. [Crossref]
- Edelmuth RCL, Buscariolli YS, Ribeiro Jr MAF (2013) Cirurgia para controle de danos: estado atual. Rev Col Bras Cir 40: 142-151.
- Sulakshane S, Thakare V, Dumbre R (2018) Consequences of gallbladder inflammation: spontaneous cholecystocutaneous fistula: a case report 5.
- Rinzivillo NMA, Danna R, Leanza V, Lodato M, Marchese S et al. (2017) Case Report: Spontaneous cholecystocutaneous fistula, a rare cholethiasis complication. F1000 Research 6: 1768. [Crossref]
- Maynard W, McGlone ER, Deguara J (2016) Unusual aetiology of abdominal wall abscess: cholecystocutaneous fistula presenting 20 years after open subtotal cholecystectomy. BMJ Case Rep 2016: bcr2015213326. [Crossref]
- G Jayasinghe, J Adam, Y Abdul-Aal (2016) Unusual presentation of gallbladder perforation. Int J Surg Case Rep 18: 42-44. [Crossref]
- Neto CS, Alves AS, da Silva MCSP, Pinto SM, de Figueredo LO et al. (2016) Fístula colecistocutânea. Relatos Casos Cir :1-3.
- Álvarez F, Meraldi A, Emery NC, Bogetti D, Young P (2014) Spontaneous cholecystocutaneous fistula in an elderly woman. Rev Med Chil 142: 1076-1077. [Crossref]
- Dixon S, Sharma M, Holtham S (2014) Cholecystocutaneous fistula: an unusual complication of a para-umbilical hernia repair. BMJ Case Rep 2014: bcr2013202417. [Crossref]
- Kim M, Beenen E, Fergusson J (2014) Spontaneous cholecystocutaneous fistula by gallstone erosion into abdominal wall. ANZ J Surg 84: 888-889. [Crossref]
- Jayant M, Kaushik R, Attri AK (2014) Spontaneous cholecystocutaneous abscess. Indian J Gastroenterol 33: 498. [Crossref]
- Sodhi K, Athar M, Kumar V, Sharma ID, Husain N (2012) Spontaneous cholecysto-cutaneous fistula complicating carcinoma of the gall bladder: a case report. Indian J Surg 74: 191-193. [Crossref]
- Kapoor Y, Singh G, Khokhar M (2013) Spontaneous cholecystocutaneous fistula-not an old-time story. Indian J Surg 75: 188-191. [Crossref]
- Ozdemir Y, Yucel E, Sucullu I, Filiz I, Gulec B et al. (2012) Spontaneous cholecystocutaneous fistula as a rare complication of gallstones. Bratisl Lek Listy 113: 445-447. [Crossref]
- Ugalde Serrano P, Solar García L, Miyar de León A, González-Pinto Arrillaga I, González González J (2013) Cholecystocutaneous fistula as a first sign of presentation of a gallbladder adenocarcinoma. Cir Esp 91: 396-397. [Crossref]
- Andersen P, Friis-Andersen H (2012) Spontaneous cholecystocutaneous fistula presenting in the right breast. Ugeskr Laeger 174: 1235-1236. [Crossref]
- Ioannidis O, Paraskevas G, Kotronis A, Chatzopoulos S, Konstantara A et al. (2012) Spontaneous cholecystocutaneous fistula draining from an abdominal scar from previous surgical drainage. Ann Ital Chir 83: 67-69. [Crossref]
- Cheng HT, Wu CI, Hsu YC (2011) Spontaneous cholecystocutaneous fistula managed with percutaneous transhepatic gallbladder drainage. Am Surg 77: E285-E286. [Crossref]
- Gordon PE, Miller DL, Rattner DW, Conrad C (2011) Image of the Month-Quiz Case. Arch Surg 146: 487-488.
- Leela Sayed, Sam Sangal, Guy Finch (2010) Spontaneous cholecystocutaneous fistula: a rare presentation of gallstones. J Surg Case Rep 5: 5. [Crossref]
- Pezzilli R, Barakat B, Corinaldesi R, Cavazza M (2010) Spontaneous Cholecystocutaneous Fistula. Case Rep Gastroenterol 4: 356-360. [Crossref]
- Metsemakers WJ, Quanten I, Vanhoenacker F, Spiessens T (2010) Spontaneous cholecystocutaneous abscess. JBR-BTR 93: 198-200. [Crossref]
- Khan AA, Azhar MZ, Khan AA, Rasheed A, Khan KN (2005) Spontaneous Cholecystocutaneous Fistula. J Coll Physicians Surg Pak 15: 726-727. [Crossref]
- Hawari M, Wemyss-Holden S, Parry GW (2010) Recurrent Chest Wall Abscesses Overlying a Pneumonectomy Scar: An Unusual Presentation of a Cholecystocutaneous Fistula. Interact Cardiovasc Thorac Surg 10: 828-829. [Crossref]
- Murphy JA, Vimalachandran CD, Howes N, Ghaneh P (2008) Anterior abdominal wall abscess secondary to subcutaneous gallstones. Case Rep Gastroenterol 2: 219-223. [Crossref]
- Chatterjee S, Choudhuri T, Ghosh G, Ganguly A (2007) Spontaneous Cholecystocutaneous Fistula in a Case of Chronic CAlculous Cholecystitis--a Case Report. J Indian Med Assoc 105: 644, 646, 656. [Crossref]
- Malik AH, Nadeem M, Ockrim J (2007) Complete laparoscopic management of cholecystocutaneous fistula. Ulster Med J 76: 166-167. [Crossref]
- Ijaz S, Lidder S, Mohamid W, Thompson HH (2008) Cholecystocutaneous fistula secondary to chronic calculous cholecystitis. Case Rep Gastroenterol 2: 71-75. [Crossref]
