Challenges in the Diagnosis of Giant Cell Arteritis Before Visual Loss
A B S T R A C T
Background: Giant cell arteritis (GCA) is the most common type of systemic vasculitis affecting the elderly. Ophthalmic presentations of GCA in particular can be difficult to identify prior to permanent visual loss occurring.
Methods: Here, we present 3 challenging cases as a retrospective series to highlight the variable presentations of GCA with ophthalmic involvement, but GCA was not suspected due to atypical presentation.
Results: Unfortunately, all 3 cases went on to develop visual loss in the affected eye due to a delay in diagnosis or treatment. The authors wish to highlight the challenges posed to the referring clinicians, when patients had systemic/ocular co-morbidities, which delayed the suspicion of GCA.
Conclusion with a Practical Point: Our cases highlight the variable presentations of this condition as well as the devastating ophthalmic implications that GCA can have. A high index of suspicion must be maintained; particularly in elderly patients with atypical presentations.
Keywords
Giant cell arteritis, temporal arteritis, ophthalmoplegia, vasculitis, inflammation
Introduction
Giant cell arteritis (GCA), characterised by inflammation of medium and large sized blood vessels, is the most common type of systemic vasculitis affecting the elderly [1-3]. The intense vessel inflammation and resulting occlusion means that prompt identification and treatment is vital, particularly to prevent severe complications such as blindness or stroke. Ophthalmic presentations of GCA can be difficult to identify prior to permanent visual loss occurring. Whilst the typical symptom of amaurosis fugax may be present before visual loss occurs, there are many instances where this is not the case and represents a particular challenge to clinicians from both medical and ophthalmology backgrounds.
This difficulty in diagnosing GCA accurately (with a mean time to diagnosis of 35 days) gives it a high likelihood of causing permanent visual loss in one or both eyes [4]. Visual symptoms at presentation vary from 26-35% with approximately 20% of patients developing some degree of visual loss prior to steroid initiation and 8% report monocular blindness at 6 months [1, 2, 5, 6]. This has led to the development of fast track GCA pathways across various centres like ours, aimed to reduce the incidence and outcome of visual loss by identifying these patients early. Here, we present a series of challenging cases highlighting the variable presentations of GCA with ophthalmic involvement.
Methods
This was a retrospective, non-consecutive case series of 3 patients who had an atypical presentation of GCA studying the challenges posed to the referring clinicians, due to systemic or ocular co-morbidities resulting in delayed diagnosis and treatment. All patients were later referred to the Coventry fast-track GCA pathway for further management. The Coventry fast-track GCA pathway was established at University Hospital Coventry and Warwickshire NHS trust in 2013, to allow a streamlined process for the management of GCA on the basis of specified criteria for referral as outlined in (Addendum 1) [7]. Over a period of 4 years (2014-2017), 652 patients were referred to this pathway for suspect GCA. The 3 cases described went on to lose vision due to the delay in suspecting GCA and referral to the local fast track pathway. This case series adhered to the tenets of the Declaration of Helsinki as amended in 2008.
Case Report 1
A 79-year-old gentleman with a background of a stable left orbit marginal zone lymphoma and a left Warthin’s parotid tumor presented with sudden onset of visual loss in his right eye and inability to move his eye on waking up that morning. Prior to this, he was being extensively investigated in a different hospital by the medical team for headaches, weight loss, fever and night sweats which had been ongoing for 6-8 weeks. There were concerns his symptoms were due to a possible metastatic spread of his left orbital lymphoma and his investigations included full body computed tomography (CT) imaging, magnetic resonance imaging (MRI) head, blood cultures and lumbar puncture; however, no underlying cause for his symptoms was found. He was discharged from the previous unit and was awaiting outpatient follow up in clinic for pyrexia of unknown origin.
On presentation, his visual acuity was perception of light in the right eye and 6/12 in the left. He had complete Ophthalmoplegia, partial ptosis and a total afferent pupillary defect in the right eye. The anterior segment examination was otherwise normal. Right fundus revealed a pale chalky disc, narrowed arteries with box carrying of the vessels and a pale retina with no cherry red spot (Figure 1A). This was felt to represent an anterior ischaemic optic neuropathy (AION) with an Ophthalmic artery occlusion. The left eye anterior segment and fundal examination were normal. Examination of the temple revealed firm, nodular, pulsatile, non-tender temporal arteries.
Figure 1A: Widefield colour fundus photograph of right fundus showing AION and Central Retinal Artery Occlusion.
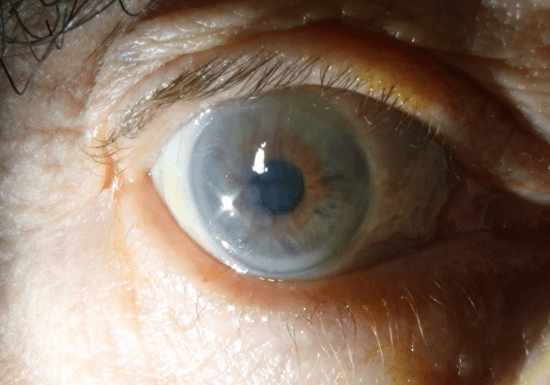
Figure 1B: Anterior segment of the right eye showing corneal oedema secondary to ocular ischaemia.
At this point there was a strong suspicion for GCA, and the patient was investigated accordingly including ESR and CRP, which were raised at 67 and 89 respectively. Temporal artery USS was inconclusive. He was given intravenous (IV) Methylprednisolone over 3 days before being stepped down to 60mg oral prednisolone. The complete Ophthalmoplegia was felt to be unusual for GCA, hence CT orbits and venogram were arranged to exclude Orbital Apex Syndrome or Cavernous Sinus pathology and was reported as normal (except for the pre-existing left orbit marginal zone lymphoma). Temporal artery biopsy (TAB), done 10 days after starting steroids, subsequently confirmed GCA. During the admission, his eye movements and ptosis gradually improved however he developed signs of anterior segment ischaemia including corneal oedema (Figure 1B), Descemet membrane folds, atonic pupil, AC activity and hypotony (IOP 3) in the right eye. A review 3 months later revealed complete resolution of the Ophthalmoplegia and anterior segment ischaemia in the right eye. There was no progression of visual loss in the right eye and the patient continues to maintain vision in his left eye.
Case Report 2
A 71-year-old lady presented with history of headache, neck pain and double vision for 2 weeks. She was admitted in the acute medical unit, where she was investigated with a CT scan to rule out a neurological cause for her symptoms. CT head revealed small vessel disease but there were no acute intracranial abnormalities observed. The lumbar puncture showed no abnormalities. Blood tests revealed no abnormalities apart from a borderline raised CRP of 21. She was then referred to the ophthalmic team for double vision and was found to have visual acuity of 6/7.5 in both eyes. Her anterior segment and fundal examination were normal. She had full colour vision and equally reacting pupils. Orthoptic assessment showed restricted up gaze in her left eye. Hence, an MRI/MRA scan was requested to rule out any cause for third nerve palsy.
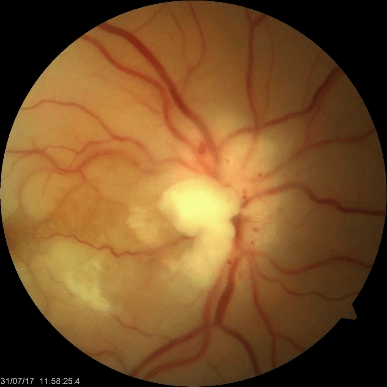
Figure 2A: Right fundus photograph showing pale, swollen optic disc suggestive of AION.
Figure 2B: Right fundus photograph showing retinal oedema with cherry red spot suggestive of CRAO.
Two days later, she complained of reduced vision in her right eye. Examination also revealed no perception of light in the right eye. The fundus showed a pale, swollen disc suggestive of AION (Figure 2A) with signs of retinal oedema and a cherry red spot (Figure 2B) suggestive of central retinal artery occlusion (CRAO). The left fundus was normal. Urgent blood tests were repeated including ESR and CRP, which was markedly raised. The temporal artery USS did not show any evidence of inflammation. On further questioning, she complained of scalp tenderness as well as pain in the left side of her jaw. A diagnosis of AION with CRAO secondary to GCA was made and the patient was promptly started on IV Methylprednisolone for 3 days followed by oral prednisolone. The MRI scan was performed at this stage but had very similar results to the CT scan showing only small vessel disease and no other abnormalities. The diagnosis was later confirmed with temporal artery biopsy. Two weeks later, her inflammatory markers returned to normal and her complaints of double vision had completely resolved, with resolution of the restricted left upgazed. Six months later there had been no change in her visual acuity in the right eye and left eye vision was also preserved.
Case Report 3
An 82-year-old lady with a background of cerebrovascular accidents (CVA) presented with a history of transient loss of vision in the right eye associated with history of flashes and floaters in both eyes. She reported to be suffering from headache, scalp tenderness and jaw claudication, which had been on-going for 3 weeks. On examination, her unaided vision was 6/12 bilaterally. Her anterior segment was normal. Fundal examination revealed a posterior vitreous detachment (PVD) in the left eye; both the optic discs appeared normal. Superficial temporal artery pulsations were feeble on both sides. No focal neurological deficits were noted. While PVD was felt to explain the symptoms of floaters and flashing lights, there was a high index of suspicion for GCA. Hence the patient was referred to the medical team for further investigation. ESR and CRP were raised at 55 and 19 respectively. Her platelet count was also raised. Temporal artery USS revealed signs of inflammation in both temporal arteries. Due to the strong suspicion of GCA, she was started on 60mg of oral prednisolone. As the patient had history of allergy to aspirin, it was not started.
She presented again 4 days later with a one-day onset of seeing ‘shadows’ in her left central vision, though her headache had now improved. Her unaided vision was 6/12-2 in the left eye, but she had a left relative afferent pupillary defect and reduced colour vision. Fundal examination revealed a left AION with cilioretinal artery occlusion. Her right eye examination was normal. She was treated with intravenous methylprednisolone for 3 days, before being stepped down to oral prednisolone. She also received a course of therapeutic Clexane for 1 week. During the admission, her vision continued to deteriorate despite treatment. Her unaided left visual acuity had decreased to 6/36. It further deteriorated to hand movements despite continued treatment. Fortunately, her vision in the right eye was preserved.
Discussion
Giant cell arteritis is a challenging condition and is considered as a medical emergency. The primary reason for this is the risk of strokes and visual loss. Outcome following visual loss is usually poor with little recovery. In the first 2 cases, delays in recognition of this condition led to profound visual loss in one eye. In the 3rd case, while it was correctly suspected and treated, the patient unfortunately continued to lose vision in the affected eye. It is difficult to determine whether she would have had better visual prognosis if treated with IV Methylprednisolone in the first instance rather than oral prednisolone. Though evidence is limited, literature does however support that IV Methylprednisolone leads to better visual outcomes as opposed to oral steroids alone [8, 9].
Case 1 highlights the importance of keeping a broad differential. The symptoms described by the patient were very much in keeping with B symptoms of lymphoma of which the patient had a known diagnosis of. However, when all the investigations returned as negative, the differential did not appear to have been revisited by the referring team to include GCA. This patient had a very profound ophthalmic involvement with complete Ophthalmoplegia, visual loss (through both anterior ischaemic optic neuropathy and arterial occlusion) and anterior segment ischaemia. A similar presentation was described previously in a series of patients, one of which had GCA, which was subsequently termed orbital infarction due to the apparent ischaemia of all intraorbital and intraocular structures [10]. While this presentation is rare, it highlights the devastating effect GCA can have if left untreated over a lengthy period.
Case 2 is another unusual case presenting with diplopia. Due to this and the relatively borderline inflammatory markers, the initial diagnosis was suspected to be an intracranial lesion. When the patient lost vision 2 days later, it became apparent that the diagnosis was GCA. Diplopia, a rarer presenting symptom of GCA compared to reduced vision, is reported to occur in 2-15% of patients. In addition, patients may also report transient diplopia which poses a further diagnostic difficulty [11-13]. Pathophysiology of diplopia in GCA is multifactorial as well. Causes include ischaemia of the cranial nerves, the extraocular muscles, brainstem ocular motor nuclei or a combination of these [3]. This can make it difficult to determine the underlying aetiology of the diplopia, particularly if patients present solely with this symptom. Those with constant diplopia from GCA however will often have other visual symptoms (58%) and are also more likely to experience systemic symptoms of GCA [14].
This case highlights the importance of including GCA in the differential for an elderly patient with diplopia particularly if their history suggests systemic symptoms. It was also interesting to note that the patient initially had a negative temporal artery USS and diagnosis was later confirmed by TAB. The 2 modalities differ in terms of sensitivity and specificity as described by a recent multinational trial (TABUL) [15]. The study reports that the sensitivity of biopsy for diagnosis of GCA was 39%, however, the specificity was 100%. In contrast, the sensitivity of USS was higher at 54% but the specificity was only 81%. This suggests both modalities have their strengths and weaknesses, however, The TABUL trial also described how a combination strategy is superior, where all patients receive an initial USS scan but only scan negative cases undergo biopsy. In this instance, the reported sensitivity is greater at 65%. This combination also led to an accurate diagnosis in our patient.
Case 3 highlights that even when GCA is correctly identified and treated, patients may continue to lose vision. In this case, it is interesting that the patient did report an episode of transient visual loss several weeks prior to presenting to hospital services. This may have been an indication to consider using intravenous methylprednisolone instead of oral prednisolone from the outset although whether that would have prevented her from developing visual loss is difficult to determine. In these instances, it’s important to keep in mind that while prompt diagnosis and treatment may not always preserve vision in an affected eye, it can prevent the development of visual loss in the other eye, as second eye involvement can occur within 10 days [6, 16].
Conclusion
Giant cell arteritis is a complex medical condition, which can have devastating consequences for patients if there is a delay in diagnosis and appropriate treatment; particularly whilst they are also being investigated for other differentials. Our cases highlight the variable and atypical presentations of this condition as well as the devastating ophthalmic implications that GCA can have. A high index of suspicion should be maintained in elderly patients with atypical presentations as clinicians may miss typical constitutional symptoms of GCA, particularly when patients have history of other co-morbidities such as systemic lymphoma or cancers and their symptoms may be attributed to those conditions. Headache with double vision may also mislead clinicians to investigate for neurological causes. These patients should be directly questioned about ophthalmic symptoms as those with visual symptoms including transient symptoms, are at an increased risk of blindness. Urgent blood tests for inflammatory markers as well as temporal artery USS and biopsy should be considered when the diagnosis is in doubt, but if highly suspicious then treatment should not be delayed in favour of investigations. Visual loss from GCA is often profound with poor prognosis for any recovery, which is why it is so important to ensure that bilateral involvement does not occur.
Acknowledgements
None.
Disclosure
None.
Conflicts of Interest
None.
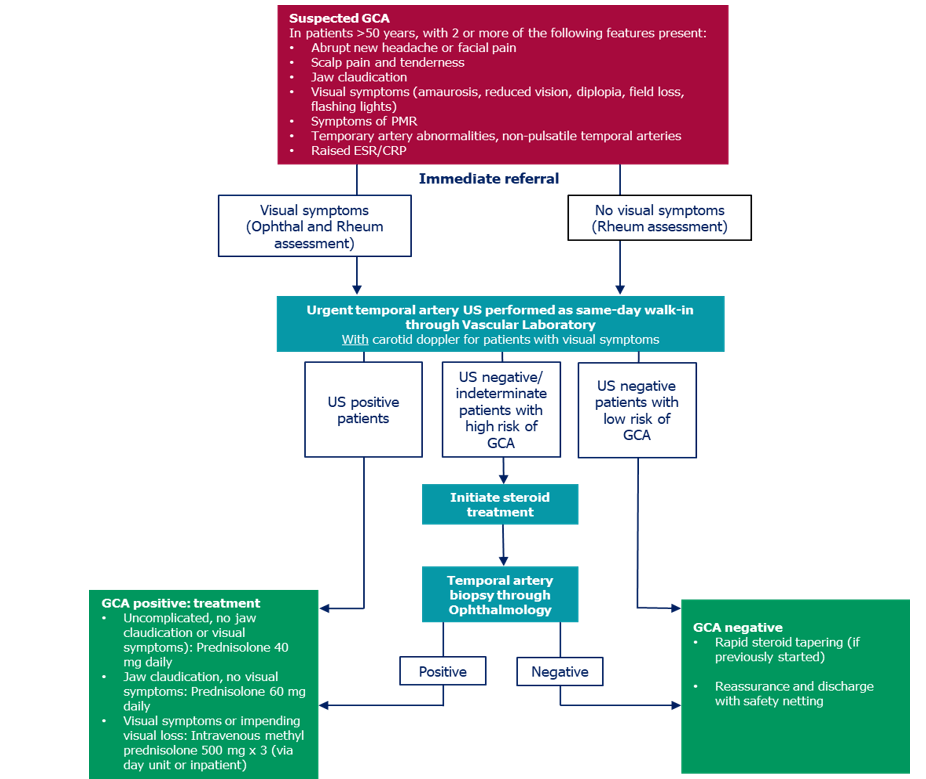
Addendum 1: Coventry Fast-Track Pathway for Giant Cell Arteritis [7].
CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate; Ophthal: Ophthalmology; Rheum: Rheumatology; GCA: Giant Cell Arteritis; PMR: Polymyalgia Rheumatica; US: Ultrasound.
Article Info
Article Type
Case ReportPublication history
Received: Sat 04, Jul 2020Accepted: Mon 20, Jul 2020
Published: Mon 27, Jul 2020
Copyright
© 2023 Purnima Mehta. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.03.06
Author Info
Faaiq Hassan Muhammed Omar Qadir Shirish Dubey Sergio Pagliarini Purnima Mehta
Corresponding Author
Purnima MehtaUniversity Hospital Coventry & Warwickshire, Clifford Bridge Road, Coventry, UK
Figures & Tables


CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate; Ophthal: Ophthalmology; Rheum: Rheumatology; GCA: Giant Cell Arteritis; PMR: Polymyalgia Rheumatica; US: Ultrasound.
References
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B (2016) Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 315: 2442-2458. [Crossref]
- Patil P, Karia N, Jain S, Dasgupta B (2013) Giant cell arteritis: a review. Eye Brain 5: 23-33. [Crossref]
- Kawasaki A, Purvin V (2009) Giant cell arteritis: an updated review. Acta Ophthalmol 87: 13-32. [Crossref]
- Ezeonyeji AN, Borg FA, Dasgupta B (2011) Delays in recognition and management of giant cell arteritis: results from a retrospective audit. Clin Rheumatol 30 : 259-262. [Crossref]
- Soriano A, Muratore F, Pipitone N, Boiardi L, Cimino L et al. (2017) Visual loss and other cranial ischaemic complications in giant cell arteritis. Nat Rev Rheumatol 13: 476-484. [Crossref]
- Yates M, MacGregor AJ, Robson J, Craven A, Merkle PA et al. (2017) The association of vascular risk factors with visual loss in giant cell arteritis. Rheumatology (Oxford) 56: 524-528. [Crossref]
- Dubey S, Pinnell J, Tiivas C, Mehta P (2020) Coventry fast-track pathway for managing giant cell arteritis. Int J Clin Rheumtol 15: 21-25.
- Chan CCK, Paine M, O'Day J (2001) Steroid management in giant cell arteritis. Br J Ophthalmol 85: 1061-1064. [Crossref]
- Dasgupta B, Borg FA, Hassan N, Alexander L, Barraclough K et al. (2010) BSR and BHPR Guidelines for the management of giant cell arteritis. Rheumatology (Oxford) 49: 1594-1597. [Crossref]
- Borruat FX, Bogousslavsky J, Uffer S, Klainguti G, Schatz NJ (1993) Orbital infarction syndrome. Ophthalmology 100: 562-568. [Crossref]
- Hayreh SS, Podhajsky PA, Zimmerman B (1998) Ocular manifestations of giant cell arteritis. Am J Ophthalmol 125: 509-520. [Crossref]
- González Gay MA, Garcia Porrua C, Llorca J, Hajeer AH, Branas F et al. (2000) Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore) 79: 283-292. [Crossref]
- Glutz von Blotzheim S, Borruat FX (1997) Neuro-ophthalmic complications of biopsy-proven giant cell arteritis. Eur J Ophthalmol 7: 375-382. [Crossref]
- Ross AG, Jivraj I, Rodriguez G, Pistilli M, Chen JJ et al. (2019) Retrospective, Multicenter Comparison of the Clinical Presentation of Patients Presenting With Diplopia From Giant Cell Arteritis vs Other Causes. J Neurophthalmol 39: 8-13. [Crossref]
- Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA et al. (2016) The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess 20: 1-238. [Crossref]
- De Smit E, O’Sullivan E, Mackey DA, Hewitt AW (2016) Giant cell arteritis: ophthalmic manifestations of a systemic disease. Graefes Arch Clin Exp Ophthalmol 254: 2291-2306. [Crossref]
