Cervical Thymic Cyst: A Unique Case Report with Related Embryogenesis
A B S T R A C T
Cystic lesions of neck containing thymic tissue are rare and usually difficult to diagnose. Postulated hypothesis for this entity is the persistence and/or degeneration of thymopharyngeal duct derived from pharyngeal pouch endoderm. We report a unique case of persistent thymopharyngeal duct which was diagnosed antenatally at 30 weeks’ gestation, and it preserved its connection with the pharynx. Postnatal radiological studies were non-specific and misleading, and diagnosis was confirmed by histological examination of the excised specimen.
Keywords
Persistence thymopharyngeal duct, thymic cyst, cervical cyst
Introduction
The cervical thymic cyst (CTC) is a rare benign lesion accounting for 0.3% -1% of all congenital neck masses [1]. It locates anywhere along the thymopharyngeal tract extending from hyoid to the anterior mediastinum with close vicinity to the carotid sheath (adjacent or within) [2, 3]. CTCs are not usually found at birth, about two-third of cases presented in the first decade of life, most of the remainder were in the second, with only occasional cases in the third decade [4, 5]. About 50% of CTCs are continuous with mediastinal mass, either by direct extension of the cyst substernally or by a fibrous connection to a vestigial remnant of thymopharyngeal duct [5]. It is more common on the left (LT) side, vary in size and few reports have suggested an increased incidence in males [1, 2, 5]. Majority presented with painless swelling, less common symptoms including dyspnea, hoarseness, dysphagia, and life-threatening respiratory symptoms, especially in infants [1, 6].
To our knowledge; firstly; there are only few reports of antenatally detected intrathoracic thymic cyst, but there is none for CTC [7]. Secondly, there are rare reports of CTCs due to persist patency throughout the entire course of normal thymic descent, but none of them preserved the connection with pharynx. In this report we present a case of symptomatic CTC in a newborn, which was diagnosed antenatally and it maintained a connection to the pharynx. Also, we discuss the relevant embryogenesis.
Case Report
A 37-year-old lady, gravida 3, para 2+0, living 1, with history of preeclampsia in previous pregnancy at 28 weeks of gestation caused intra uterine fetal death. She presented to emergency with symptoms of preeclampsia for two days. Fetal US showed gestational age 29 +6 days, cephalic presentation, mild increase in the amniotic fluid and normal cardiac anatomy. There was an anterior thoracic simple avascular thin-walled cyst that was posterior to the sternum and anterior to the great vessels, measuring 22×15 mm likely representing a congenital thymic cyst which unlikely to cause airway obstruction (Figure 1).
Figure 1: Antenatal US shows cystic lesion posterior to the sternum and anterior to the great vessels measuring 22×15 mm.
Two days later, she developed fetal distress and underwent emergency caesarian section. It yielded a baby boy, weighed 1.57 Kg, with no dysmorphic features. Apgar’s score was 7 at 1 minute and 9 at 5 minutes. As the decision was made for elective intubation after birth, the baby was intubated in delivery room (Ex-utero intrapartum treatment). He required low ventilation setting for first two days of life and weaned from oxygen support at 8 days old.
During the first 5 days of life the baby had recurrent hypoglycemic episodes which were resolved uneventfully. On examination, there was a slight soft rounded bulge on LT anterior neck. Cardiac echo revealed small PDA and head US was normal. MRI neck showed the trachea was shifted rightward and a 2×1.5×4 cm cystic lesion extending from level of the hyoid bone on LT side down to superior mediastinum with a small focal projection seen associated with the LT lateral surface of the esophagus with air/fluid level and two small air loculi, highly suggestive of communication with aero-digestive tract (Figures 2A & 2B). Most likely diagnosis was esophageal duplication cyst.
Figure 2: A & B) MRI neck sagittal and axial views show LT neck longitudinal cystic lesion with air/fluid level and two small air loculi.
At 10 days old oral feeding was started and increased gradually until it reached to full feeding. The baby developed increasing breathing efforts which required oxygen support; therefore, feeding was held. CT-scan neck showed a stable size cystic lesion in the LT upper mediastinum extending to the LT lower neck with thick peripheral enhancement, contained a small air focus at non-dependent portion of the cyst, keeping with diagnosis of esophageal duplication cyst (Figures 3A and 3B).
Figure 3: A & B) CT-scan neck coronal and axial views show cystic lesion in the LT upper mediastinum extending to the LT lower neck with thick peripheral enhancement, arrow indicate air foci in the cyst.
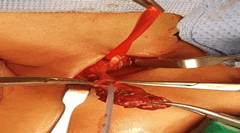
Direct laryngobronchscopy revealed normal larynx and trachea, an orifice with deep hypopharyngeal pouch was identified anterolateral (LT) to the esophagus. On compressing the LT neck, drops of milk came out from the orifice. Due to symptomatic nature of the cyst, surgery was performed at 55-days-old through transverse LT cervical incision. On lateral retraction of sternomastocloid muscle, carotid sheath and dissecting the cyst, the tract was found passing anterior to the carotid sheath reaching up to hypopharynx. To confirm the connection, cyst was opened and feeding tube was inserted (Figure 4). Cyst’s neck was transfixed followed by formal excision of the cyst. No stalk was identified.
Figure 4: Vessels loop around the carotid sheath, feeding tube passing through the cyst into the pharynx.
Post-operatively, boy was extubated on day-1, feeding was started on day-4, and was discharged home on day-6 with a weight of 2.9 kgs. Microscopic examination of the specimen revealed a cyst lined by stratified squamous epithelium (Figure 5A) with cholesterol granulomas in the cyst wall (Figure 5B). It contained lobules of thymic tissue with Hassall’s corpuscles with intervening connective tissue septae (Figure 5C). Hassall’s corpuscle degeneration reveals calcification (Figure 5D) and cellular debris (Figure 5E).
Follow up; the child presented at 15-months-old with surgical site abscess LT neck (Figure 6), underwent drainage of foul smell pus, culture reviled E. coli ESBL (extend spectrum beta-lactamase). It responded to antibiotics course and UGI contrast study was unremarkable. The child is now 3-years-old, thriving well without any feeding difficulties.
Figure 5: A) Microscopic examination; the cyst wall lined by stratified squamous epithelium. B) Cholesterol granulomas in the cyst wall. C) Lobules of thymic tissue with intervening connective tissue septae. D) Hassall’s corpuscle degeneration reveals calcification. E) Hassall’s corpuscle degeneration reveals cellular debris.
Figure 6: LT neck surgical site abscess.
Discussion
To understand the etiology of the CTC, one must first be aware of the thymopharyngeal duct, thymus and branchial apparatus development. During sixth week of fetal life, thymopharyngeal ducts start to form as endodermal invaginations along the ventral aspect of the third pharyngeal pouches (the region destined to form the pyriform sinus). By the seventh week, the thymopharyngeal ducts detach from pharynx and form a pair of hollow structures called thymic primordia which later become paired solid masses by a process of endodermal cell proliferation. These primordia descend into the thorax over a period of 3 weeks by developing elongated stalks which approach each other but never fuse. When the process of thymic descent ends, the proximal portions of the elongated stalks usually atrophy and later disappear. By the tenth week, adjacent mesenchymal cells invade the thymic primordia and destroy the previous solid cord of primitive pharyngeal endoderm. The remaining endodermal epithelium degenerates later into Hassall’s corpuscles. The fourth pouches are thought to contribute a small amount of thymic tissue which becomes incorporated into the main gland primordia [4, 7].
Presence of thymic tissue in the neck has been found in up to 30% of infants studied at autopsy [8]. CTCs are very rarely encountered and less than 7% of patients initially have respiratory symptoms [6]. Macroscopically, they are variable in size, multilocular or unilocular [4, 5]. Microscopically, the cyst wall epithelium may consist of squamous, cuboidal, columnar and/or ciliated columnar cells. This lining is often degenerated and replaced by granulation tissue, inflammatory cellular infiltrate and foamy macrophages. The thymic tissue included the pathognomonic Hassall’s corpuscles are found in intimate relation with the cyst wall. The cyst fluid may be clear or turbid because of cholesterol clefts or hemorrhage [5, 9].
Speer (1938) was the first one who classified the thymic cysts on the basis of pathogenesis, although the exact etiology was not known. He hypothesized five models; i) embryonal remnants of the persistence thymopharyngeal duct, branchial cleft or thymic tubules (congenital), ii) cystic degeneration of Hassall's corpuscles, primitive endodermal cells, lymphocytes, and reticular cells (acquired), iii) sequestration or pathologic involution of the thymus, iv) lymph or blood vessels, v) neoplastic process in lymphoid or connective tissue in lymphoid [9]. Only the first theory has a strong evidence-based documentation, the rest are a theoretical possibility of their existence.
Most of CTCs occur in first decade of life when degenerative changes are unlikely, and cystic degeneration in Hassall’s corpuscles is not a constant finding in thymic cysts (krech et al., 1954). Moreover, the authors who described cystic degeneration in Hassall’s corpuscles, conclude that the cysts are of congenital origin [4].
Few reports mentioned that the unilocular cyst is remnant of thymopharyngeal duct while multilocular cyst is acquired arising from degeneration of thymic Hassall’s corpuscles, but microscopic studies showed multiple minute cysts sometimes detected in the walls of CTC indicate that one cyst has enlarged at the expense of the others (Dyer 1967) [3, 9].
Observations like CTC location, longitudinal shape, frequent finding of the intrathoracic connection, and occasional finding of other endocrine tissue (parathyroid and less common thyroid) within the CTC support the congenital origin [3, 5, 9]. Few case reports presented longitudinal cysts with persistence patency throughout the entire course of the normal thymic descent, but they pinched off from larynx [2]. Literature mentioned that the fistulous tract extending to the pharynx is rare [8]. We think that is because the thymopharyngeal duct separates early from pharynx, sooner after elongation during 7th week of gestation, which make preservation of this connection rare. To our knowledge, our case is the first documentation of the presence of this connection.
The main entity to be differentiated from a CTC is a branchial cyst, which arises from the thymopharyngeal tract of the third pouch, it locates along the line of the thymopharyngeal duct and it has similar epithelial lining to that of the CTC. The branchial cyst presents in the third decade of life, rarely extend to the clavicle, and its tract passes between the internal and external carotid arteries and ends in the superior tonsillar fossa which help in differentiation. According to Fahmy, the thymic cyst should be considered a variant of the branchial cyst affecting a younger age group [4, 5].
In conclusion, the thymopharyngeal duct cyst is an uncommonly encountered lateral neck mass. We reported a unique case of CTC which; diagnosed prenatally, symptomatic required early surgical excision during neonatal period and thymopharyngeal duct preserved a patent connection to the pharynx. Therefore, it is logical to attribute the CTC origin to residues of the thymopharyngeal tract being left behind along the normal embryonic path of descent. Adequate reporting of cases is necessary to improve the CTC awareness.
Funding
None.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Consent
The consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Conflicts of Interest
None.
Acknowledgement
We appreciate all the efforts of Dr. Salman T. AL Malki, Consultant Pathologist Dermatopathology and Soft tissue/Bone Pathology. Who provide us with the full pathology report about our patient.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Sat 10, Dec 2022Accepted: Mon 02, Jan 2023
Published: Wed 11, Jan 2023
Copyright
© 2023 Majd A. Hadad. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSCR.2022.02.05
Author Info
Majd A. Hadad Mohammad S. Mallick Alam A. Shafi Abdullah Badughaish
Corresponding Author
Majd A. HadadPediatric Surgery Department, King Fahad Medical City, Riyadh, Saudi Arabia
Figures & Tables





References
1. Betti M, Hoseini N,
Martin A, Buccoliero A, Messineo A et al. (2015) Cervical Thymic Cyst in
Childhood: A Case Report. Fetal Pediatr Pathol 34: 65-69. [Crossref]
2. Boyd J, Templer J,
Havey A, Walls J, Decker J (1993) Persistent thymopharyngeal duct cyst. Otolaryngol
Head Neck Surg 109: 135-139. [Crossref]
3. Niranjan J, Santosh
K, Prabhakar G (2011) Multiloculated cervical thymic cyst. J Indian Assoc
Pedatr Surg 16: 24-25. [Crossref]
4. Fahmy S (1974)
Cervical thymic cysts: their pathogenesis and relationship to branchial cysts. J
Laryngol Otol 88: 47-60. [Crossref]
5. Barat M, Sciubba
JJ, Abramson AL (1985) Cervical thymic cyst: case report and review of
literature. Laryngoscope 95: 89-91. [Crossref]
6. Raines JM, Rowe LD
(1981) Progressive neonatal airway obstruction secondary to cervical thymic
cyst. Otolaryngol Head Neck Surg 89: 723-725. [Crossref]
7. Luthra M, Kumar C, Ahlawat K (2019) Congenital
Thymic Cyst: Antenatal Diagnosis and Postnatal Management. J Indian Assoc
Pediatr Surg 24: 206-208. [Crossref]
8. Kaufman RM, Smith S, Rothschild AM, Som P (2001) Thymopharyngeal duct cyst: an unusual variant of cervical thymic anomalies. Arch Otolaryngol Head Neck Surg 127: 1357-1360. [Crossref]
9. Leong AS (1990) Thymic Cysts. Surgery of the Thymus. Springer-Verlag Berlin, Heidelberg.
