Journals
Cervical biopsy after chemoradiation for locally advanced cervical cancer to identify residual disease: a retrospective cohort study
A B S T R A C T
Objective
To evaluate the value of cervical biopsies 12 to 16 weeks post chemoradiation (CRT) in patients with locally advanced cervical cancer (LACC), to identify patients eligible for salvage surgery.
Methods
We retrospectively collected data of 154 patients with LACC, who received chemoradiation as primary treatment between 1998 and 2015 at the Radboud university medical center in Nijmegen (Radboud). In patients eligible for salvage surgery, without suspicious lymph nodes on imaging, a cervical biopsy was taken post CRT under general anesthesia to evaluate whether complete clinical remission was obtained. We analyzed data from 78 patients with cervical biopsy post CRT and 60 patients without cervical biopsy. Follow-up, biopsy results, patient characteristics and subsequent treatment modalities were reviewed.
Results
In total, 154 patients with LACC received CRT. The median follow up time was 30.5 months. Sixteen patients (10.4%) were lost to follow up. Of the remaining 138 women, 78 patients underwent a cervical biopsy post CRT. Eight out of the 78 patients (10.3%) had residual disease after CRT, six of these patients underwent salvage surgery, with long-term complete remission in 4 of these patients (66.7%).
Conclusion
Post CRT cervical biopsy in locally advanced cervical cancer patients was beneficial in 4 out of 78 patients (5%). As 1 in 20 patients will achieve long-term survival by salvage hysterectomy, taking a cervical biopsy 16 weeks post radiation seems thus advisable.
Keywords
Cervical cancer, Chemoradiation, cervical biopsy, salvage surgery
I N T R O D U C T I O N
Chemoradiation (CRT) is the standard treatment in locally advanced stage cervical cancer (LACC), i.e. FIGO stage 1B2-4A (FIGO: The International Federation of Gynecology and Obstetrics) [1-3]. Treatment failure is associated with a very poor prognosis, with a 5-year survival of just 10% [4]. Currently, there are no validated screening methods to detect residual disease pre-symptomatic. If the residual tumor isconfined to the cervix, patients could still be salvaged with surgery. The success of salvage surgery is mainly determined by the localization of the residual tumor [4]. Cervical biopsy post CRT could detect residual disease at an early stage when it might still be localized in the cervix only. To determine whether patients primary treated with CRT in LACC will benefit from post treatment cervical biopsies, we conducted a retrospective study among all patients treated with CRT for cervical cancer stage 1B2-4A at our academic hospital. Twelve to sixteen weeks after CRT, patients underwent an examination under general anesthesia (EUA) including a cervical biopsy.
Methods and Materials
Patients
We conducted a retrospective cohort study of all patients with LACC treated with CRT at the Radboud university medical centre between 1998 and 2015. In total, 154 patients met the criteria but follow up was incomplete in 16 patients. The resulting 138 women were included in this study. Staging classification of patients was performed according to FIGO guidelines. Prior to treatment, all patients underwent an EUA with additional imaging (MRI and /or PET-CT scan) for local extension and to detect metastases.
Treatment
All patients were treated with both external beam radiotherapy (EBRT) and brachytherapy (BT). Target definition for EBRT was based on a planning-CT obtained in treatment position. External pelvic irradiation consisted of a total dose of 45 Gy in 25 daily fractions of 1.8 Gy, 5 times a week. Since 1999, this was combined with weekly Cisplatin (40mg/m²) for 5 weeks [1]. Before July 2009 a four-field box technique was used. Since July 2009, radiotherapy was administered by Intensity-Modulated Radiation Therapy (IMRT). Since 2009, 3D MR-based image guided adaptive BT was given, according to the GEC-ESTRO guidelines [5]. In the end or after EBRT, patients received 4 times 7 Gy high dose rate brachytherapy to the D90 of the high-risk clinical target volume (HR-CTV) with the Utrecht interstitial CT/MR applicator (Elekta, Veenendaal, The Netherlands).
Follow up after initial given treatment
In patients with no suspicion of lymph node metastases prior to treatment, EUA including cervical biopsy was performed 12 to 16 weeks after CRT to detect residual disease. All biopsy specimens were assessed by an experienced gynecologic oncologic pathologist. Patients with residual disease in cervical biopsy were additionally assessed with CT-scan or MRI, combined with a PET-CT-scan (Positron Emission Tomography scan in combination with a CT-scan). If distant metastases were present, patients received palliative chemotherapy. Patients with residual disease and no distant metastases on imaging, who were medically fit enough were offered salvage surgery. Type of salvage surgery was tailored to the patient and varied from type I, type II, or type III hysterectomy, or partial/complete exenteration.
Statistical analyses
Total follow up time of each patient was calculated using a subtraction of the date of diagnosis and the last time the patient was seen alive for a follow up consultation. When the exact date of diagnosis was unknown, the date of physical exam under general anesthesia prior to treatment was used.
In case a patient succumbed to disease, the date of death was used as the last follow up date. Patients with palliative treatment and lost to follow up (due to migration or treatment by their general practitioner) were scored as alive when no message of their passing had been received.
Statistical analyses were performed using SPSS software, version 22 [6]. A Fishers exact test was used to compare baseline characteristics between patients with and without post CRT biopsy. The sensitivity and specificity of the cervical biopsy were calculated, the Clopper-Pearson was used to determine the 95% confidence intervals [7].
We used a Log-Rank test to detect possible differences in five-year survival and created a survival curve with Kaplan-Meier analysis. A p-value of <0.05 was considered significant.
Table 1
|
All patients |
Patients with biopsy after CRT N=78 |
Patients without biopsy after CRT N=60 |
|||||
|
Mean age diagnose (min-max) |
49.82 (24 - 83) |
47.49 (24-83) |
52.88 (26-79) |
||||
|
FIGO-stadium |
n |
% |
n |
% |
n |
% |
|
|
IB1 |
8 |
5.8 |
3 |
3.8 |
5 |
8.2 |
|
|
IB2 |
22 |
15.8 |
12 |
15.4 |
10 |
16.4 |
|
|
IIA |
10 |
7.2 |
7 |
9.0 |
3 |
4.9 |
|
|
IIB |
77 |
55.4 |
46 |
59.0 |
31 |
50.8 |
|
|
IIIA |
4 |
2.9 |
3 |
3.8 |
1 |
1.6 |
|
|
IIIB |
17 |
12.2 |
7 |
9.0 |
10 |
16.4 |
|
|
Histology |
|||||||
|
Squamous |
112 |
81.2 |
60 |
76.9 |
52 |
86.7 |
|
|
Adeno |
20 |
14.5 |
14 |
18.0 |
6 |
10.0 |
|
|
Unknown |
6 |
4.3 |
4 |
5.1 |
2 |
3.3 |
|
|
Biopsy taken |
|||||||
|
Yes |
78 |
56.5 |
78 |
100 |
0 |
0.0 |
|
|
No |
60 |
43.5 |
0 |
0.0 |
60 |
100 |
|
Results
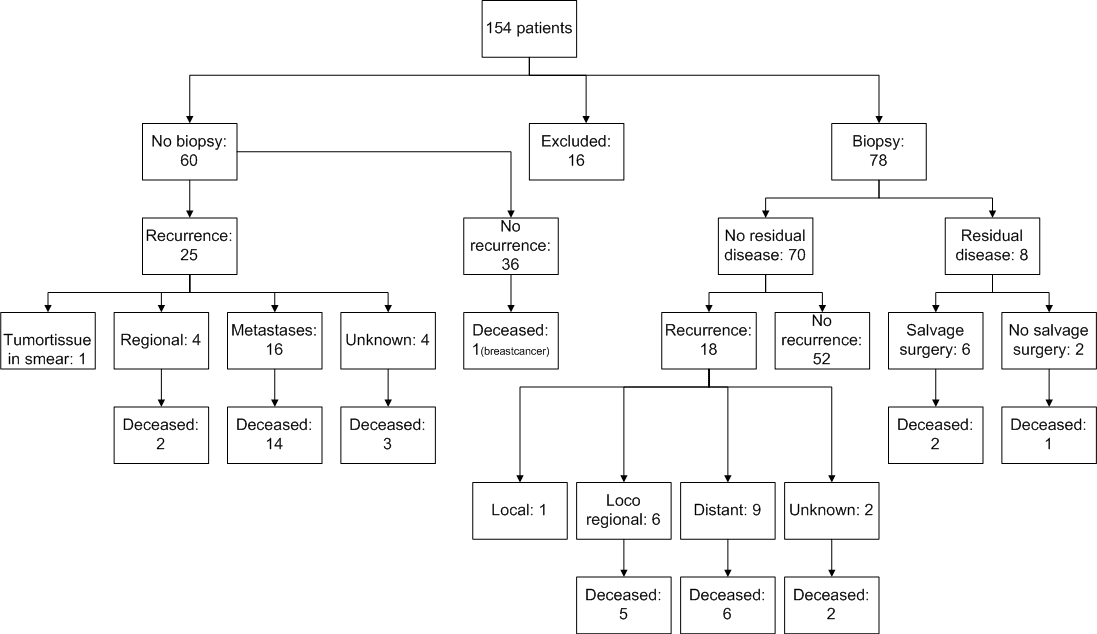
The study cohort consisted of 154 patients with cervical cancer FIGO stages IB2 to IVA, treated with chemoradiation. Patient characteristics are displayed in table 1. A total of 16 out of the 154 patients were excluded from this study because of loss to follow up. Eight patients were lost to follow up immediately after diagnosis, 2 patients lacked a date of diagnosis, and of 6 patients no follow up data, nor a date of diagnosis was available. Of the remaining 138 patients, 60 had lymph node metastases on MRI or PET/CT prior to CRT treatment and were excluded from the post-treatment biopsy group. The remaining78 patients (56.5%) without suspicious lymph nodes, underwent an additional EUA and cervical biopsy post CRT (Figure 1). The median time between last brachytherapy and biopsy under EUA was 12.8 weeks. Median follow up time of all patients was 30.5 months [interquartile range (IQ) 17.8 – 55.3]. There was no significant difference between patients with and without biopsy in terms of median follow up time, FIGO stage or histology of the tumor (Table 1). Seventy out of 78 patients (90%) had no residual disease in biopsy. Of these 70 patients, 18 (25.7%) developed recurrent disease; 6 patients developed a regional recurrence, 9 had distant recurrences. Only one patient had a local cervical recurrence which was presumably missed by the biopsy. In two patients the recurrence site was unknown. Of the patients who had a recurrence, 13 (72.2%) died of cervical cancer. The other 52 patients were cured and showed no recurrence during follow up.
Eight (10%) patients showed residual disease following their post treatment biopsy specimen. FIGO stages of these patients were; 2 IB2 (25,0%), 1 IIA (12,5%), 3 IIB (37,5%), 1 IIIA (12,5%) and 1 IIIB (12,5%). The 8 patients with residual disease following biopsy underwent a PET/CT scan to detect possible metastasis. Four of the patients (50%) did not have clinical symptoms or any indication of residual disease during physical examination. In two patients no residual disease was visible at PET/CT, although residual disease was present, proven by the cervical biopsy. Two patients showed metastatic disease on PET/CT and where considered not eligible for salvage surgery, they received palliative chemotherapy (Figure 2). Six out of 8 patients received salvage surgery tailored to the extensiveness of the residual disease (Figure 2). In concurrence with the biopsy, all patients with salvage surgery had residual tumor in the hysterectomy-specimen. The test characteristics of the biopsy post chemoradiation were as follows: The sensitivity of biopsy to detect residual disease was 88.9% (8/9; 95% CI 57%-100%) the specificity was 100% (69/69; 95% CI 63%-100%).
Of the 6 patients who underwent extensive salvage surgery, three patients suffered from complications. One patient developed an ileus and multiple fistulas, which required multiple re-interventions. The second patient suffered from extensive hemorrhage after surgery, requiring packed cells. The third patient had a gastrointestinal infection caused by Clostridium difficile (Figure 2).
Four patients (66.7%) treated with salvage surgery survived and were considered cured of disease after 5 years of follow up. Unfortunately, 2 patients were diagnosed with recurrent disease after salvage surgery: both succumbed to disease. In 60 patients, no biopsy was taken after CRT, because before start of initial treatment the disease was considered metastasized to pelvic lymph nodes. Twenty-five (41.7%) developed a recurrence: 4 regional recurrences, 16 distant recurrences, and in 4 patients the place of recurrence was unknown. In one case tumor tissue was seen in the vagina during the first physical assessment. A smear showed atypical cells suspect for residual disease. Nineteen (76.0%) patients who had a recurrence died of cervical cancer. There was no significant difference in overall survival between patients with residual disease and patients without residual disease in the cervical biopsy group.
Figure 1:
Figure 2:
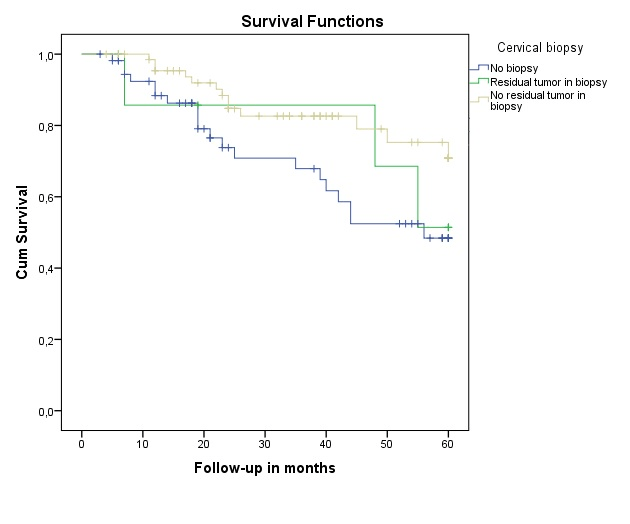
Survival analysis
Figure 3 illustrates the cumulative survival in patients with residual disease in cervical biopsy, patients without residual disease in biopsy and patients with no biopsy at all. There was no significant difference between the three groups (p=0.065)
Figure 3: Time dependent increase in comet tail moment.
Treatment with 4NQO induces DNA damage, as revealed by formation of comet. Tail moment, as estimated using COMET software has been found to increase with time. Simultaneous intervention with black tea reduces comet tail moment, indicating its inhibitory effect on DNA damage.
Discussion
Currently there are no validated screening methods to detect residual disease pre-symptomatic. Treatment failure is associated with a very poor prognosis, published literature reports a 5-year survival of just 10% [4]. Other studies show that up to 50% of the patients with a recurrent cervix carcinoma were asymptomatic at diagnosis [8].
With cervical biopsy post-treatment in a selected group of patients, we singled out those patients who have no other curative treatment options and might benefit from salvage surgery. Eight out of 78 patients undergoing biopsy showed residual disease, with 6 patients eligible for salvage surgery, resulting in long-term survival of 4 of these women.
The sensitivity of the biopsy is 88.9% (95% CI 57%-100%) with a specificity of 100% (95% CI 63%-100%). When compared to surveillance with PET-CT in non-symptomatic patients, (sensitivity 92%, specificity 75%), surveillance with biopsy is favorable [9]. Other screening tests (SSC antigen, cervix smear) showed lower sensitivity and specificity [10-12]. In our patient cohort four (50%) of the patients with biopsy proven residual disease showed no clinical symptoms or any indication of residual disease during physical examination, pre-biopsy. Additionally, in two patients no residual disease was visible at PET/CT, although residual disease was present as proven by the cervical biopsy.
In patients with no biopsy, treatment effect was determined by MRI imaging, followed by clinical follow up. Only when symptoms of recurrent disease occurred new imaging was performed. This could lead to a diagnostic delay of recurrence, eventually limiting salvage treatment options for patients treated with curative intent. Timing of biopsy is crucial, as biopsies taken too early could detect residual disease still going into regression due to the radiation effect. Studies on cervical biopsies taken in the first 6 weeks after radiation therapy reported false-positive biopsy rates up to 65% [4, 13]. Furthermore, a study which assed clinical response after chemoradiation in squamous cell carcinoma of the anus, presumably partially comparable to our cases, showed an overtreatment percentage of 29% when biopsies were taken too early [14]. To prevent unnecessary surgery in our patient cohort, salvage treatment is reserved for patients with residual disease proven by histology. Since 2015, cervical biopsies are taken at 16 weeks after last brachytherapy instead of 12 weeks postradiotherapy.
In 2006, a study presented by Nijhuis et al. reported that 38% of the patients identified with residual disease through cervical biopsy were cured of disease after salvage surgery. Their advice was to prolong post treatment biopsies [15]. A follow-up study of Boers et al. showed that about half of the patients with residual disease were salvaged with surgery. However, a contradictive advice was given: They suggested to stop taking routine biopsies after CRT for LACC. Radical surgery in patients with residual disease did not result in lower locoregional recurrences, nor in a better disease specific survival, while increasing morbidity [16]. In our study we only performed post radiation biopsies in patients without suspicious lymph nodes in order to select patients with the best chance of salvage. Furthermore, type of salvage surgery was tailored to the extensiveness of the residual disease.
Limitations of the present study are the retrospective design and the small cohort of patients. Therefore, the sample size was too small to conduct multivariate statistical analysis. Although there was no significant difference, there is a clear trend towards better overall survival in patients treated for proven residual disease versus patients with no biopsy. In our cohort one out of every 20 patients with biopsy proven residual disease is salvaged with surgery. As cervical cancer affects most patients at a young age, an increased morbidity rate might be more acceptable to salvage one patient. Furthermore, in our study, only one patient suffered from long-term complications. Another limitation is timing of biopsy, between 1998 and 2015, timing of biopsy shifted from 12 weeks, to 16 weeks post CRT. Though in our study there were no false-positives, in all patients with biopsy proven residual disease extensive disease was present in the hysterectomy specimen after salvage surgery. Therefore, in our opinion, salvage surgery in these patients was justifiable. Considering their young age and the fear of potential metastasis and consequently no curative options left when patients were ‘treated’ with a watchful waiting approach.
In our study, patients with pelvic lymph node metastases at first diagnosis were excluded from the post treatment biopsy group. Even though these lymph nodes are boosted with RT after EBRT and therefore treated with curative intent. It is known that nodal involvement at diagnosis is a strong prognostic factor for recurrence, explaining the difference between our biopsy and no biopsy group [17]. Still, this patient group might benefit from salvage surgery if residual disease in biopsy after CRT is found as well.
Further research is necessary to prove if women with LACC and positive pelvic lymph nodes, boosted with RT may benefit from post CRT cervical biopsy. As we expect an even higher number to treat and increasing morbidity due to cervical biopsy, we do not advise a standard biopsy after completing treatment until there are more studies available showing any beneficial effect of this strategy.
Conclusion
Post chemoradiation biopsies, 12 to 16 weeks after completion of cervical cancer treatment, in medically fit patients without signs of metastases may benefit 1 out of every 20 patients and seems an appropriate screening test to select patients eligible for salvage surgery. In patients with positive lymph nodes prior to CRT treatment, selection with post CRT biopsy remains a point of discussion. As there is no evidence of a better overall survival at the moment, we do not recommend biopsies for these cervical cancer patients.
Acknowledgements
Authors report no acknowledgements.
Details of ethical approval
As this was an anonymous retrospective database study it was exempted from Ethical approval at our hospital.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Conflict of interest
The authors report no conflict of interest.
Disclosure
The authors have nothing to disclose.
Author’s roles
YMH, PLMZ and RLMB have been involved in the study design. All authors collected data for this study. YMH,PLMZ and RLMB analyzed and interpreted the data. All authors were equally involved in writing, reviewing and editing the manuscript.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 20, Dec 2018Accepted: Mon 21, Jan 2019
Published: Fri 22, Mar 2019
Copyright
© 2023 Yvonne Marije Hoeijmakers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2019.01.001
Author Info
Yvonne Marije Hoeijmakers A. Snyers M.A.P.C. v. Ham P.L.M. Zusterzeel R.L.M. Bekkers
Corresponding Author
Yvonne Marije HoeijmakersDepartment of Obstetrics and Gynecology, Radboud University Nijmegen Medical Center, the Netherlands
Figures & Tables

Treatment with 4NQO induces DNA damage, as revealed by formation of comet. Tail moment, as estimated using COMET software has been found to increase with time. Simultaneous intervention with black tea reduces comet tail moment, indicating its inhibitory effect on DNA damage.
Table 1
|
All patients |
Patients with biopsy after CRT N=78 |
Patients without biopsy after CRT N=60 |
|||||
|
Mean age diagnose (min-max) |
49.82 (24 - 83) |
47.49 (24-83) |
52.88 (26-79) |
||||
|
FIGO-stadium |
n |
% |
n |
% |
n |
% |
|
|
IB1 |
8 |
5.8 |
3 |
3.8 |
5 |
8.2 |
|
|
IB2 |
22 |
15.8 |
12 |
15.4 |
10 |
16.4 |
|
|
IIA |
10 |
7.2 |
7 |
9.0 |
3 |
4.9 |
|
|
IIB |
77 |
55.4 |
46 |
59.0 |
31 |
50.8 |
|
|
IIIA |
4 |
2.9 |
3 |
3.8 |
1 |
1.6 |
|
|
IIIB |
17 |
12.2 |
7 |
9.0 |
10 |
16.4 |
|
|
Histology |
|||||||
|
Squamous |
112 |
81.2 |
60 |
76.9 |
52 |
86.7 |
|
|
Adeno |
20 |
14.5 |
14 |
18.0 |
6 |
10.0 |
|
|
Unknown |
6 |
4.3 |
4 |
5.1 |
2 |
3.3 |
|
|
Biopsy taken |
|||||||
|
Yes |
78 |
56.5 |
78 |
100 |
0 |
0.0 |
|
|
No |
60 |
43.5 |
0 |
0.0 |
60 |
100 |
|
References
- Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, et al. (1999) Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 340: 1144-1153. [Crossref]
- Waggoner SE (2003) Cervical cancer. Lancet 361: 2217-2225. [Crossref]
- Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, et al. (2001) Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 358: 781-786. [Crossref]
- Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, et al. (2004) Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys 60: 249-257. [Crossref]
- Touboul C, Uzan C, Mauguen A, Gouy S, Rey A, et al. (2010) Prognostic factors and morbidities after completion surgery in patients undergoing initial chemoradiation therapy for locally advanced cervical cancer. Oncologist 15: 405-415. [Crossref]
- IBM. Corp. IBM SPSS Statistics for Windows VA, NY: IBM Corp.; Released 2013.
- Brown L C, TT and DasGupta (2001) A Interval Estimation for a proportion. Statistical Science 16: 101-133.
- Zola P, Fuso L, Mazzola S, Piovano E, Perotto S, et al. (2007) Could follow-up different modalities play a role in asymptomatic cervical cancer relapses diagnosis? An Italian multicenter retrospective analysis. Gynecol Oncol 107: 150-154. [Crossref]
- Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER (2005) FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol 97: 183-191. [Crossref]
- Yoon SM, Shin KH, Kim JY, Seo SS, Park SY, et al. (2010) Use of serum squamous cell carcinoma antigen for follow-up monitoring of cervical cancer patients who were treated by concurrent chemoradiotherapy. Radiat Oncol 5: 78. [Crossref]
- Chan YM, Ng TY, Ngan HY, Wong LC (2002) Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol 84: 7-11. [Crossref]
- Chien CR, Ting LL, Hsieh CY, Lai MS (2005) Post-radiation Pap smear for Chinese patients with cervical cancer: a ten-year follow-up. Eur J Gynaecol Oncol 26: 619-622. [Crossref]
- Keys HM, Bundy BN, Stehman FB, Okagaki T, Gallup DG, et al. (2003) Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol 89: 343-353. [Crossref]
- Glynne-Jones RJ, R Meadows H; Begum R; Cunningham D; Northover J; et al. (2012) Optimum time to assess complete clinical response (CR) following chemoradiation (CRT) using mitomycin (MMC) or cisplatin (CisP), with or without maintenance CisP/5FU in squamous cell carcinoma of the anus: Results of ACT II 2012.
- Nijhuis ER, van der Zee AG, in 't Hout BA, Boomgaard JJ, de Hullu JA, et al. (2006) Gynecologic examination and cervical biopsies after (chemo) radiation for cervical cancer to identify patients eligible for salvage surgery. Int J Radiat Oncol Biol Phys 66: 699-705. [Crossref]
- Boers A, Arts HJ, Klip H, Nijhuis ER, Pras E, et al. (2014) Radical surgery in patients with residual disease after (chemo)radiation for cervical cancer. Int J Gynecol Cancer 24: 1276-1285. [Crossref]
- Suprasert P, Charoenkwan K, Siriaree S, Cheewakriangkrai C, Saeteng J, et al. (2013) Outcome of cervical cancer patients with single-node compared with no nodal involvement treated with radical hysterectomy and pelvic lymphadenectomy. Int J Gynaecol Obstet 121: 45-48. [Crossref]
