Case Report of Cerebral Venous Sinus Thrombosis Complication after Microvascular Decompression Surgery in the Inactive Phase of Ulcerative Colitis
A B S T R A C T
Background: The risk of thrombosis is high in patients with Ulcerative Colitis. The main location of thrombosis is in the extremity and pulmonary vein. Cerebral venous thrombosis is a rare location for thrombosis with high mortality. One of the risk factors for developing thrombosis is in association with surgery, especially during an active phase of Ulcerative Colitis (UC), and very few studies report cases occurring during the inactive phase. Hence, it is important for the surgeon to understand the risk of thrombosis, especially of cerebral sinus thrombosis in the Ulcerative Colitis patient.
Case: We are presenting a fatal complication in a male patient of 62, who had sagittal sinus thrombosis and multiple intra-cranial hemorrhages in both frontal lobes 3 weeks after microvascular decompression surgery for hemifacial spasm.
Conclusion: The risk of cerebral venous sinus thrombosis is higher in Ulcerative Colitis patients, especially in those who undergo surgery regardless of its type. Careful peri-operative assessment and treatment related to thromboembolism prevention are important to reduce complications in such cases.
Keywords
Venous Sinus thrombosis, microvascular decompression, ulcerative colitis, inflammatory bowel disease
Introduction
Venous Thromboembolism (VTE) is one of the complications of Inflammatory Bowel Disease (IBD) which includes Ulcerative Colitis (UC) and Crohn’s Disease (CD). The risk of thrombosis is estimated at a three-fold increase compared to controls, with an overall incidence estimated between 1%-8% [1, 2]. VTE occurs mainly during the active phase, but a study indicates that one-third of thromboembolic complications occurred during inactive phase [3].
Factors that affect the homeostasis system in IBD include prolonged immobilization, surgical procedures, central venous catheters, glucocorticoid therapy, cigarette smoking, hyperhomocysteinemia, vitamin deficiency, dehydration, and damage of the vascular endothelium [4]. Changes in the coagulation and fibrinolytic mechanisms in patients with IBD have been proposed as the pathophysiology of VTE. Higher levels of coagulation factors, lower levels of fibrinolytic factors and plasma coagulation inhibitors have been also considered. Other factors can be endothelial damage in hyperhomocysteinemia, higher platelet activation, and a higher level of autoantibodies [3, 4].
Most VTE events occur in the lower extremities or appear as pulmonary embolisms, but they can also occur in other locations such as hepatic, portal, cerebral, and mesenteric veins [2]. Cerebral venous sinus thrombosis (CVST) has a reported incidence between 0.5% - 6.7% and is associated with a high mortality rate [5].
The clinical presentation of CVST includes headache, vomiting, seizure, focal neurological deficit, speech impairment, blurred vision, and drowsiness. One study reported that the most common location for CVST is the transverse sinus followed by sagittal, however other papers reported that the most common location for CVST is superior sagittal sinuses [5-7].
Most of the publications that report CVST in IBD are single case reports, however, CVST in IBD after craniotomy has not yet been reported. Cerebral venous thrombosis is of significant clinical importance due to its association with a high mortality rate. Surgeons’ awareness has to be increased regarding this kind of complication. Here, we present a patient with a fatal outcome from CVST after microvascular decompression surgery for hemifacial spasm, on the background of ulcerative colitis in the inactive phase.
Case Report
A male patient of 62 years complained of frequently recurring left-side facial twitching and was diagnosed with hemifacial spasm 5 years before admission. He had previously received carbamazepine drug therapy and botulinum neurotoxin injection treatment, but it didn’t relieve the symptoms well. The twitching frequency gradually deteriorated and became almost impossible to open his left eye. MRI showed that the facial nerve root exit area from the medulla was compressed by a large vertebral artery.
The patient decided to undergo surgery, and a microvascular decompression surgery was adopted. The MRI showed left vertebral artery compression to the facial nerve in the root exit zone (Figure 1). The CT angiography showed good visualization of the superior sagittal sinus and transverse-sigmoid sinuses bilaterally (Figure 1).
Figure 1: Pre-Operative radiological image.
A) Left vertebral artery (red arrow) compression facial nerve (Green arrow) at root exit zone. B) Post-operative MRI showed the left vertebral artery already separated from the root exit zone (red arrow). C) The normal superior sagittal sinus can be seen in 3DCTA (green arrow). D) 3DCTA image shows the sigmoid-transverse sinus is normal and there is no stenosis pre-operatively (green arrow).
The patient had previously been diagnosed with ulcerative colitis (UC), Psoriasis Vulgaris, and hypertension. Pre-operative evaluations were performed also by a gastroenterologist and dermatologist. Both concluded that the ulcerative colitis condition was currently inactive and stable, and surgery could be performed. The patient also had treatment with cyclosporin, corticosteroids, and mesalazine suppositories. All the medication was already stopped because of the remission.
The surgery was performed via lateral suboccipital approach successfully without any complications. A three-centimeter small craniotomy was done without any damage to the sigmoid sinus and transverse sinus. Transposition of the vertebral artery was done using Teflon sling stitching to the dura mater, and hemifacial spasm symptoms disappeared immediately after the operation. Post-operative MRI showed that adequate transposition of the left vertebral artery was achieved (Figure 1). Post-operatively the patient complained of constipation that was resolved and was discharged 1 week after surgery. He had also some generalized fatigue and appetite loss, but he could do his daily activities independently. Before he was discharged from the hospital, his general condition improved, and he did not have any bowel symptoms.
One week after discharge, swelling of the face and both upper limbs were detected. The patient was consulted by a dermatologist, and as an allergic reaction was suspected, he received a single dose of oral steroid. That improved the swelling and the appetite of the patient.
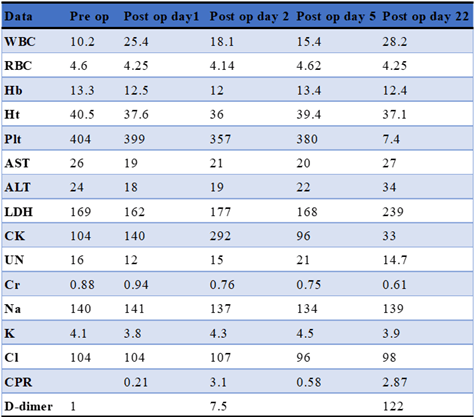
However, one week later or 3 weeks after the surgery, the patient was admitted to the hospital emergency department due to impaired consciousness. A CT scan showed multiple frontal hemorrhages, and the following angiography showed complete occlusion of the superior sagittal sinus (SSS). An endovascular thrombectomy was performed in emergency, and the SSS was recanalized (Figure 2). However, peripheral veins were still occluded. During this hospitalization, Laboratory examination showed an elevated white blood cell count, low platelet count, and high levels of D-dimer (Table 1). The patient expired 3 days after admission.
Figure 2: Radiological images when the patient developed a decrease in consciousness.
A & B) Head CT scan shows bilateral frontal multiple hemorrhages. C) One-third anterior and middle part of the superior sagittal sinus was not visualized (red arrow), D) Middle part of the superior sagittal sinus was visualized after the thrombectomy was performed.
Table 1: Laboratory examination.
Discussion
The risk of CVST is high after surgery in IBD patients [6]. Until now, there has been no case report with such observation after craniotomy surgery, more in microvascular decompression surgery. CVST in IBD incidence was reported in the range of 0.5 – 6.7%. We analysed the risk factors that possibly lead to CVST in this patient. Aishah Ibrahim Albakr and Noor Almohish analysed risk factors for CVST in patients with IBD and pointed at corticosteroid therapy, anemia, previous thrombotic events, inherited thrombophilia, high homocysteine level, hormonal therapy, and post-operative condition [6]. Another study added D-dimer level as a risk factor [5]. Owczarek et al. mention acquired prothrombotic factors in Inflammatory Bowel disease dehydration, glucocorticosteroid therapy, prolonged immobilization, central venous catheters, surgical procedures, oral contraceptives/hormonal replacement therapy, smoking and hyperhomocysteinemia [4]. Ulcerative Colitis was more common than Chron’s Disease to lead to CVST [6].
It is more often for thrombosis to occur in active IBD conditions, but occasionally that can be seen during remission [5]. Control of disease activity is an important factor in reducing the risk of venous and arterial thrombotic events in patients with IBD [8]. Clinical and endoscopic remission should be confirmed given that persistent subclinical inflammation can also increase the risk of a thrombotic event [8]. In the present case, mesalazine suppositories were used occasionally, indicating that the patient might have been occasionally symptomatic. The surgical procedure was an additional factor that elevated CVST risk.
Treatment with cyclosporin and corticoids increased the risk of venous thromboembolism, although not specifically for CVST [9-11]. The mechanism of cyclosporin thrombogenicity is still controversial. It was proposed that cyclosporin enhances relies from endothelial cells of von Willebrand factor (VWF), a classic platelet agonist, and platelet aggregation was increased due to a higher level of VWF in the circulation. Cyclosporin also induces endothelial cell dysfunction by suppressing nitric oxide production and initiating the coagulation pathway. Increased D-dimer and fibrinogen level was also associated with cyclosporin [11]. The time range in which cyclosporin could induce CVST is between 9 days to 11 months, and a specific time for this induction remains unclear [10]. However, in our case cyclosporin was already stopped before surgery and the risk of CVST from cyclosporin intake for this patient was ruled out.
White blood cell (WBC) level was higher than normal patient post-operative condition compared with the CRP level. It might be this WBC reaction is also one of the risk signs. The IBD patient tends to have an easy inflammatory response. Possible effects of psoriasis, history of taking immunosuppressive drugs such as Cyclosporine, and other immunosuppressive drugs, and complications such as IBD and autoimmune diseases, as well as interactions, are also possible.
Mesalazine is the only drug that was still administered occasionally. A single study showed evidence for the possibility of mesalazine increasing the risk of thromboembolism [12]. However, data from RCTs investigating the use of mesalazine in patients with ulcerative colitis have not shown any safety signals toward an increased risk of venous thromboembolism [8, 13].
The risk of thrombotic events in IBD patients increased also with steroid consumption. Several studies and a meta-analysis have shown corticosteroids in IBD treatment increased the rate of VTE [14-16]. Steroid was taken only once after the patient complained of the face and upper extremity swelling which the suspected cause was an allergic reaction. Steroids are needed for IBD in the active phase, and that also can increase the risk of thrombosis.
Thromboprophylaxis administration is still debatable. Prophylaxis is recommended to be given to IBD patients who will be treated by major surgery [6, 17]. However, there is also a report that CVST occurs even when thromboprophylaxis is given [18]. There are no recommendations yet for thromboprophylaxis in CVST prevention for craniotomy surgery in IBD patients. Recommendations for prophylaxis were given to prevent the risk of systemic thromboembolism. Anti-coagulant prophylaxis administration is recommended for IBD patients who must undertake major abdominal–pelvic or general surgery [17]. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease recommends that patients with IBD should receive pharmacological thromboprophylaxis during the in-patient period regardless of the cause of hospitalization. IBD patients have a higher risk of VTE than those without IBD, even during periods of remission [8]. Low molecular weight heparin or fondaparinux is recommended over low-dose unfractionated heparin. Prophylaxis should be maintained during the whole in-patient period [8]. In the future, the risk, and benefits of giving thromboprophylaxis in the inactive phase of IBD before surgery should be considered.
Cerebral venous sinus thrombosis in remission UC is rare but is related to high morbidity and mortality. After the patient’s consciousness level deteriorated was found thrombosis of the superior sagittal sinus. Venous congestion caused multiple intracranial hemorrhages in the frontal lobe. Within the previously reported 10 cases of cerebral hemorrhage associated with ulcerative colitis, there was 50% mortality, 30% partial resolution of symptoms, and 20% recovery [19]. Paper by Aishah Ibrahim Albakr and Noor Almohish which collected the published case report showed that most of the patients have a good outcome with complete recovery after treatment. This happens because of improvements in diagnostic and treatment for CVST patients. If we can find in the early stage of the condition, there is a higher possibility of getting a better outcome. Headache was the most common symptom, followed by seizures, focal neurologic disorder, changes in mental status, and symptoms of increased intracranial pressure, such as nausea, vomiting, and papilledema [6].
Peri-operative assessment of VTE can be measured by D-dimer examination [20, 21]. Pre-operative D-dimer level of this patient had been checked and showed a normal level. However, during the CVST event, D-dimer became markedly elevated. There was a possibility that thrombosis had occurred also in other locations. The greatest risk of post-operative VTE is within 10 days after hospital discharge [22]. Benlice and colleagues found that 61% of VTE events occurred within the first 2 weeks after hospital discharge [23]. In this case, the patient developed CVST 3 weeks after the first hospital discharge, so it was possible that the CVST was progressing for 3 weeks. In patients of high risk for developing VTE, as when being performed on surgery, D-dimer examination peri-operative for VTE screening in 1-2 weeks period can be a possible future policy, even if the patient has not developed any symptoms of VTE or CVST yet. If an elevated level is found, additional tests have to be performed. Further treatment can be done in the early stages too.
The mortality outcome was not directly related to the surgery itself, but surgery was one of the risk factors for developing CVST in this patient. There should be awareness of the risks of cerebral venous sinus thrombosis and all thromboembolism in IBD patients, even when the disease is in an inactive phase (remission).
Peri-operative precautions for IBD patients:
i. Avoid dehydration for at least 3 weeks during the peri-operative period; D-dimer is an indicator of thrombosis, so regular D-dimer measurements should be performed according to the patient's physical condition during the peri-operative period.
ii. Care must be taken for the worsening of IBD symptoms due to surgical invasion.
iii. If there is a possibility of thrombotic symptoms or an elevation of D-dimer, confirm the absence of bleeding complications and then aggressively administer anticoagulants to ensure a decrease in D-dimer.
iv. Early aggressive treatment, including endovascular therapy, is recommended if an extensive intravenous sinus thrombus is identified.
Conclusion
The risk of venous sinus embolization in surgery for patients with IBD is very high, and great care must be taken, especially during surgery in the active phase of IBD symptoms. At the same time, surgery during the inactive phase can also lead to thrombotic tendencies as well. Whether in the active or inactive phase, one should be aware of systemic thrombotic complications of the cephalic sinus, including the SSS, as well as the deep veins.
Disclosure
The authors have no personal financial interest in any of the drugs, materials and devices described in this article.
Abbreviation
CD: Crohn’s Disease
CVST: Cerebral Venous Sinus Thrombosis
IBD: Inflammatory Bowel Disease
SSS: Superior Sagittal Sinus
VTE: Venous Thromboembolism
VWF: Von Willebrand factor
BC: White Blood Cell
UC: Ulcerative Colitis
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Thu 22, Dec 2022Accepted: Sat 07, Jan 2023
Published: Thu 19, Jan 2023
Copyright
© 2023 Kentaro Watanabe. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2022.04.06
Author Info
Kentaro Watanabe Ande Fachniadine Fumiaki Maruyama Karagiozov Kostadin Yuichi Murayama
Corresponding Author
Kentaro WatanabeDepartment of Neurosurgery, Tokyo Jikei University school of Medicine, Tokyo, Japan
Figures & Tables
Table 1: Laboratory examination.

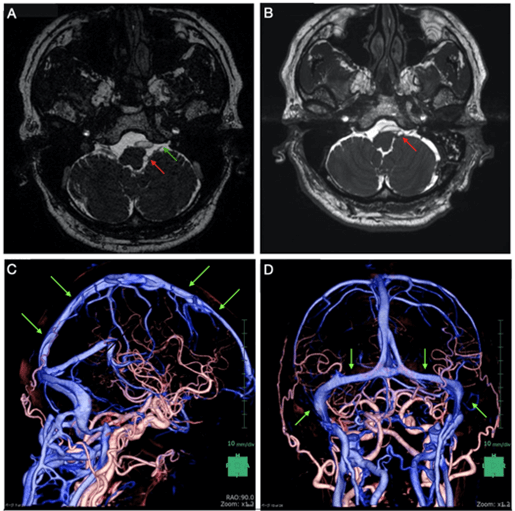
A) Left vertebral artery (red arrow) compression facial nerve (Green arrow) at root exit zone. B) Post-operative MRI showed the left vertebral artery already separated from the root exit zone (red arrow). C) The normal superior sagittal sinus can be seen in 3DCTA (green arrow). D) 3DCTA image shows the sigmoid-transverse sinus is normal and there is no stenosis pre-operatively (green arrow).
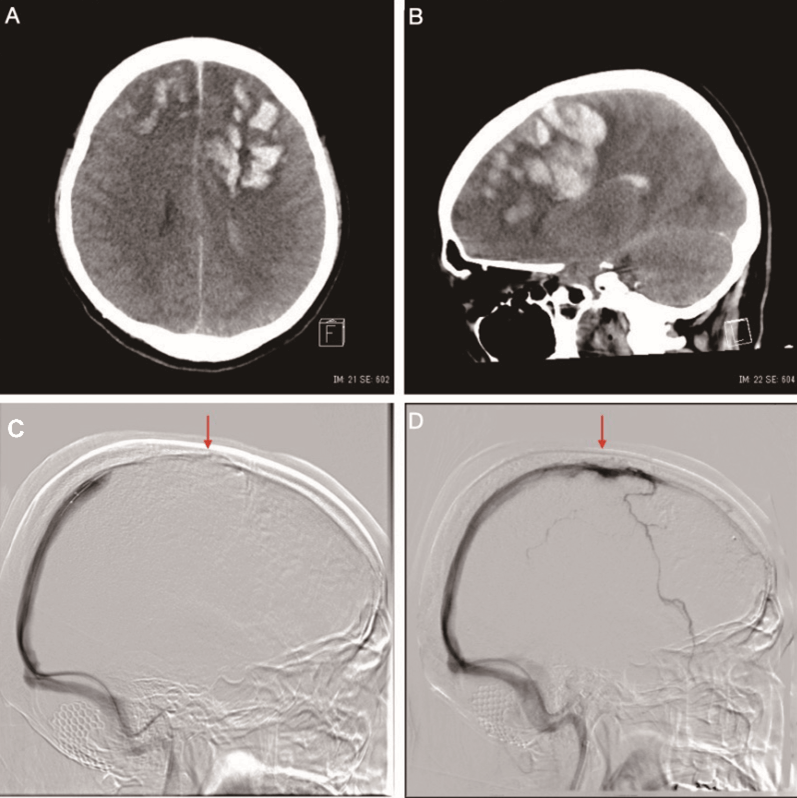
A & B) Head CT scan shows bilateral frontal multiple hemorrhages. C) One-third anterior and middle part of the superior sagittal sinus was not visualized (red arrow), D) Middle part of the superior sagittal sinus was visualized after the thrombectomy was performed.
References
1.
Meštrović
A, Žaja I, Ardalić Z, Cindro PV, Šustić I et al. (2018) A Patient with
Ulcerative Colitis Complicated by Systemic Vein Thrombosis. Case Rep
Gastroenterol 12: 322-326. [Crossref]
2.
Giannotta
M, Tapete G, Emmi G, Silvestri E, Milla M (2015) Thrombosis in inflammatory
bowel diseases: what’s the link? Thromb J 13: 14. [Crossref]
3.
Scaldaferri
F, Lancellotti S, Pizzoferrato M, Cristofaro RD (2011) Haemostatic system in
inflammatory bowel diseases: New players in gut inflammation. World J
Gastroenterol 17: 594-608. [Crossref]
4.
Owczarek
D, Cibor D, Głowacki MK, Rodacki T, Mach T (2014) Inflammatory bowel disease:
Epidemiology, pathology and risk factors for hypercoagulability. World J
Gastroenterol 20: 53-63. [Crossref]
5.
Shujun
W, Huijie Z, Xia B, Hongjian W (2021) Cerebral venous sinus thrombosis in
patients with inflammatory bowel disease: a retrospective study. Sci Rep
11: 17004. [Crossref]
6.
Albakr
AI, AlMohish N (2021) Cerebral Venous Sinus Thrombosis in Inflammatory Bowel
Disease: A Review of Published Case Reports. Perm J 25: 21.031. [Crossref]
7.
Houissa
F, Salem M, Bouzaidi S, Rejeb MB, Mekki H et al. (2011) Cerebral thrombosis in
inflammatory bowel disease: A report of four cases. J Crohn’s Colitis 5:
249-252. [Crossref]
8.
Olivera
PA, Zuily S, Kotze PG, Regnault V, Al Awadhi S et al. (2021) International
consensus on the prevention of venous and arterial thrombotic events in
patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol
18: 857-873. [Crossref]
9.
Al
Shekhlee A, Oghlakian G, Katirji B (2005) A case of cyclosporine‐induced
dural sinus thrombosis. J Thromb Haemost 3: 1327-1328. [Crossref]
10. Gao F, Zhang J,
Wang F, Xin X, Sha D (2018) Cyclosporin A-related cerebral venous sinus
thrombosis. Medicine 97: e11642. [Crossref]
11. Song S, Wang Z,
Ding Y, Ji X, Meng R (2021) Cyclosporine-A-Induced Intracranial Thrombotic
Complications: Systematic Review and Cases Report. Front Neurol 11:
563037. [Crossref]
12. Carty E, Macey M,
Rampton DS (2000) Inhibition of platelet activation by 5‐aminosalicylic
acid in inflammatory bowel disease. Aliment Pharmacol Ther 14:
1169-1179. [Crossref]
13. Sehgal P, Colombel
JF, Aboubakr A, Narula N (2018) Systematic review: safety of mesalazine in
ulcerative colitis. Aliment Pharmacol Ther 47: 1597-1609. [Crossref]
14. Sarlos P, Szemes K,
Hegyi P, Garami A, Szabo I et al. (2017) Steroid but not Biological Therapy
Elevates the risk of Venous Thromboembolic Events in Inflammatory Bowel
Disease: A Meta-Analysis. J Crohns Colitis 12: 489-498. [Crossref]
15. Waljee AK, Wiitala
WL, Govani S, Stidham R, Saini S et al. (2016) Corticosteroid Use and
Complications in a US Inflammatory Bowel Disease Cohort. PloS One 11:
e0158017. [Crossref]
16. Higgins PDR, Skup
M, Mulani PM, Lin J, Chao J (2015) Increased Risk of Venous Thromboembolic
Events With Corticosteroid vs Biologic Therapy for Inflammatory Bowel Disease. Clin
Gastroenterol Hepatol 13: 316-321. [Crossref]
17. Nguyen GC et al.
(2014) Consensus Statements on the Risk, Prevention, and Treatment of Venous
Thromboembolism in Inflammatory Bowel Disease: Canadian Association of
Gastroenterology. Gastroenterology 146: 835.e6-848.e6. [Crossref]
18. Kosmidou M, Vagias
I, Katsanos KH, Tsianos EV (2013) Agitation as the only symptom of cerebral
venous sinus thrombosis in a patient with Crohn’s disease. Ann Gastroenterol
26: 373. [Crossref]
19. Yanagisawa T,
Mizukami H, Akiyama H, Hasegawa Y (2020) Recurrent Cerebral Hemorrhage
Associated with Ulcerative Colitis. J St Marianna Univ 10: 115-121.
20. Ando K, Fujiya M,
Nomura Y, Inaba Y, Sugiyama Y et al. (2018) The incidence and risk factors of
venous thromboembolism in Japanese inpatients with inflammatory bowel disease:
a retrospective cohort study. Intest Res 16: 416-425. [Crossref]
21. Ohya H, Kimura H,
Watanabe J, Nakagawa K, Suwa Y et al. (2022) Preoperative prevalence and risk
factors of deep-vein thrombosis in Japanese surgical patients with ulcerative
colitis: a retrospective investigational study. Surg Today 52: 251-259. [Crossref]
22. Faye AS, Wen T, Ananthakrishnan AN, Lichtiger S, Kaplan GG et al. (2020) Acute Venous Thromboembolism Risk Highest Within 60 Days After Discharge From the Hospital in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 18: 1133.e3-1141.e3. [Crossref]
23. Benlice C, Holubar SD, Gorgun E, Stocchi L, Lipman JM et al. (2018) Extended Venous Thromboembolism Prophylaxis After Elective Surgery for IBD Patients: Nomogram-Based Risk Assessment and Prediction from Nationwide Cohort. Dis Colon Rectum 61: 1170-1179. [Crossref]
