Cardioprotective Effect of Zinc Oxide Nanoparticles/Green Tea Extract Complex on Monosodium Glutamate Toxicity
A B S T R A C T
Monosodium glutamate (MSG) is used worldwide as a food additive, the survey has disclosed some of MSG's deleterious effects on various organs and tissues of rats. This research was achieved to determine the impact of MSG on the albino rats' antioxidant and histology of cardiac tissue. 48 male rats were divided into six groups of eight rats each. Group one used for monitoring and normal saline, whereas rats of group two were given the lower dosage of MSG 6 mg/Kg, rats in group three received 17.5 m/kg body weight of MSG, while rats in group four were given 10 mg/kg body weight of zinc oxide nanoparticles /green tea extract (ZnO NPs/GTE) complex, the fifth and sixth groups were treated with the lower and the higher doses of MSG with ZnO NP/GTE complex for 30 days. Each animal was sacrificed at the end of the treatment period and the heart was thoroughly separated, determining the antioxidant parameter and the histopathological changes. MSG administration to the rats has shown a substantial rise in peroxidation of cardiac heart tissue, decline in the activities of catalase, superoxide dismutase, and glutathione peroxidase beside the decrease in the glutathione level as compared to those in the control animals. The treatment the induced-rats with MSG using complex showed some degree of recovery by a reduction in LPO of the heart and enhancement of antioxidant enzymes. The findings suggested that the intrinsic antioxidant of the heart tissue can significantly be alternated with the use of MSG in a dose-dependent manner and these changes could be improved by using the green ZnO NPs complex. This complex acts as a factor to decrease the histological damage that could be happening by the induction of MSG.
Keywords
ZnO NPs / Green tea complex, heart, antioxidants, histology
Introduction
Monosodium Glutamate (MSG) is recognized as a sodium salt discovered as a non-essential amino acid; found to improve the flavor in food. The survey has shown that MSG can trigger adverse effects on various organs and tissues with signs as inflammation in liver, kidney alteration as well as in testis, pancreas, and brain (unpublished data) [1-3]. Increasing the dose of MSG consumption by adult rats, raise the oxidative stress in many tissues.
The basic feature of the heart is the close-inexhaustible cardiac muscle, the myocardium, which mainly consists of a set of specific skeletal muscle cells called myocytes. In many pathological conditions, cardiac hypertrophy preceded the start of heart failure; the myocardium's compensatory reaction enhanced mechanical work. In the excessive hemodynamic stress or disturbance in myocardial contractility, the cardiac system retains arterial pressure and perfusion of essential organs by a variety of processes. Moreover, Scientific literature assessment represents the review that oxidative stress is probable to be engaged in the pathogenesis of different illnesses such as atherosclerosis due to the creation of free radical [4-6]. In our previous studies, we reported that MSG was used as a flavor enhancer in a variety of products cooked at restaurants, home, and industry to increase the oxidative stress in different tissues [1-3]. Also, it has now been discovered that the current younger generation is more willing toward Chinese's and Japanese's foods. This phenomenon is concomitant with increasing the prevalence of CHD and atherosclerosis in developing nations that also increased dramatically the past century [7]. There are many kinds of literature claims that atherosclerosis has been occurred in very young people (25-30 years) in the current times [4-7].
ZnO NPs/GTE complex act as partial hepato-protective against MSG through its hyperlipidemia reducing effect and potent antioxidant properties [1, 2]. Also, ZnO NPs/GTE reduced the effects of MSG significantly by reduction of the level peroxidation and enhancement intracellular antioxidant in the kidneys of rats. These biochemical findings were supported by histopathology evaluation, which showed minor morphological changes [3]. Since MSG is considered to be one of the triggers of tissue harm, the objective of this study was to investigate the long-term effect of MSG on oxidative stress markers histology of the heart. The lipid peroxidation as an oxidative stress marker, as well as the superoxide dismutase, catalase, glutathione as free radical scavenging parameters glutathione peroxidase as a metabolizing enzyme were evaluated. Moreover, the effectiveness of ZnO NPs/GTE complex to decrease the damage induced by MSG in the cardiac tissue of the rats at oxidative stress and histological levels.
Materials and Methods
I Animals and protocol of the experiment
In this study, forty-eight mature rats weighing 200-250 g were used. They were acquired from the Faculty of Pharmacy, Zagazig University, Egypt. The rats were left to acclimatize with nutrition and water for two weeks under normal circumstances. The animals were then split into six groups of eight rats each. The animals were given orally the following treatment regime for 30 consecutive days:
• Group-I (Control): 0 mg MSG/g body weight (b.w).
• Group-II: 6 mg MSG/g b.w.
• Group-III: 1.7 mg MSG/g b.w.
• Group-IV: 10 mg/Kg ZnO NPs/GTE complex that prepared and its characters has been published before [2-3].
• Group-V: 6 mg MSG/g b.w. + 10 mg/Kg ZnO NPs/GTE complex.
• Group-VI: 1.7 mg MSG/g b.w. + 10 mg/Kg ZnO NPs/GTE complex.
Based on the smaller number of animals, the sample size of rats was selected for real outcomes. This study was conducted with confirmation from the Biological Department, University of Taif's Local Ethics Committee on Use and Care for Animal Experiments (license number: 39-31-0034).
II Biochemical assays
Lipid peroxidation (LPO) was has been tested by evaluating the purple chromophore created by malondialdehyde (MDA) response of thiobarbituric acid [8]. The activity of superoxide dismutase (SOD) was measured according to its capacity to inhibit pyrogallol autooxidation at 440 nm for 3 min [9]. The catalase (CAT) activity was estimated in which decomposition of H2O2 catalyzed by this enzyme was measured by a decrease in absorbance at 240 nm, taking 0.0394 mM-1 cm-1 extinction coefficient and enzyme activity was expressed as nmol of H2O2degraded/min/ g tissue [10]. The glutathione peroxidase (GPx) activity of heart tissue was assessed using H2O2 as a substrate by applying the method of Hafeman et al. [11]. Reduced glutathione (GSH) was evaluated by the method of Beutler et al. using 5-5’ dithiobis2-nitrobenzoic acid [12].
III Histological evaluation
After 30 days of treatment, specimens were gathered from the heart and maintained for histopathological examination in a neutral buffer formalin solution (10%) [13]. The fixed heart specimens were regularly dehydrated by graded alcohol sequence, cleared in xylol and lastly embedded in paraffin. serial sectioned were performed at 4 µm thickness and stained with hematoxylin and eosin stains (H&E). The sections were subjected to histopathological examination using the electric light microscope Olympus BH2 (Tokyo, Japan).
IV Statistical Analysis
Results have been depicted as means ± SE. Results were analyzed using analysis of variance (ANOVA), followed by post hoc with SPSS for Windows version 20.0. The significance level was regarded to be at the P-value below 0.05.
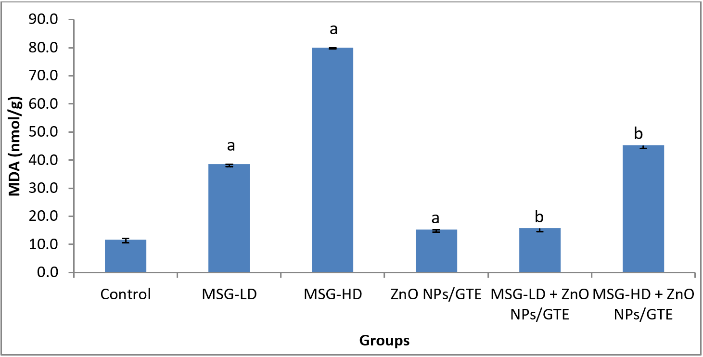
Figure 1: Changes in lipid peroxidation levels in cardiac tissue of different groups. Values are expressed as mean ± S.E (n=8). aSignificant difference as compared to control and b significant difference as compared to the corresponding group treated with MSG alone. GTE; green tea extract, ZnO NPs; zinc oxide nanoparticles, MSG-LD; the lower dosage of monosodium glutamate, MSG-HD; the higher dosage of monosodium glutamate.
Results
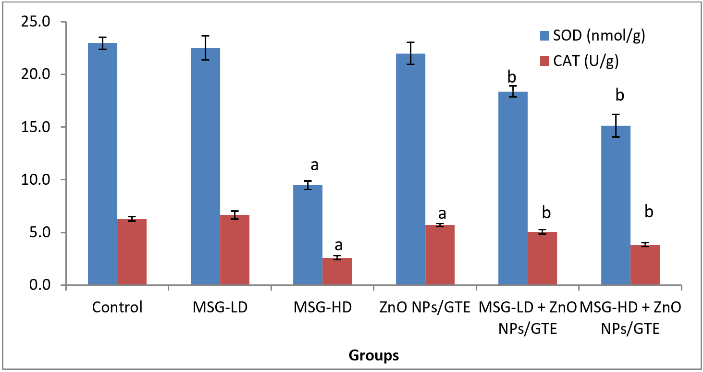
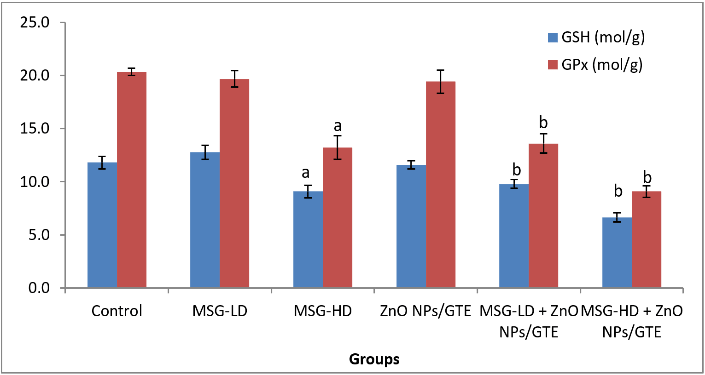
A significant rise in concentrations of LPO in all the MSG-treated groups (Figure 1) was observed in the present study by 3.3- and 6.8- fold as compared to control animals. Treatment the MSG-induced rats with ZnO NPs/GTE complex decreased the LPO by 59% and 43% as compared to its relative group of MSG alone. The activity of SOD was decreased significantly by 58.6% in group MSG-HD as compared to control animals (Figure 2). ZnO NPs/GTE increased the SOD activity in rats treated with MSG-HD by 1.6-fold as compared with MSG-HD alone. The activity of CAT was also significantly decreased in cardiac tissue 58.6% in group-MSG-HD as compared to the control animals (Figure 2). When the animals treated with a ZnO NPs/GTE + MSG-HD, the activity of CAT was increased by 1.5-fold as compared to the control group. A significant decline in the levels of GSH could be accompanied by a significant increase in the levels of LPO. The level of GSH significantly decreased by 23.1% in 17.5 mg MSG/g body weight group as compared with normal adult animals (Figure 3). Also, a significant decrease was observed by 23.4% and 26.9% (P < 0.01) in ZnO NPs/GTE + MSG-LD and ZnO NPs/GTE + MSG-HD as compared their relative group of MSG, respectively. The activity of GPx was found to be decreased by 35.1% in MSG-HD group (Figure 3). There was also a significant inhibition of GPx activity by 30.9% in ZnO NPs/GTE + MSG-LD, and 31.4% in MSG-LD and ZnO NPs/GTE as compared to MSG-LD and MSG-HD, respectively. not receiving MSG (Figure 3).
Figure 2: Changes in superoxide dismutase (SOD) and catalase (CAT) activities in cardiac tissue of different groups. Values are expressed as mean ± S.E (n=8). aSignificant difference as compared to control and b significant difference as compared to the corresponding group treated with MSG alone. GTE; green tea extract, ZnO NPs; zinc oxide nanoparticles, MSG-LD; the lower dosage of monosodium glutamate, MSG-HD; the higher dosage of monosodium glutamate.
Figure 3: Changes in glutathione peroxidase (GPx) activity and glutathione (GSH) level in cardiac tissue of different groups. Values are expressed as mean ± S.E (n=8). aSignificant difference as compared to control and b significant difference as compared to the corresponding group treated with MSG alone. GTE; green tea extract, ZnO NPs; zinc oxide nanoparticles, MSG-LD; the lower dosage of monosodium glutamate, MSG-HD; the higher dosage of monosodium glutamate.
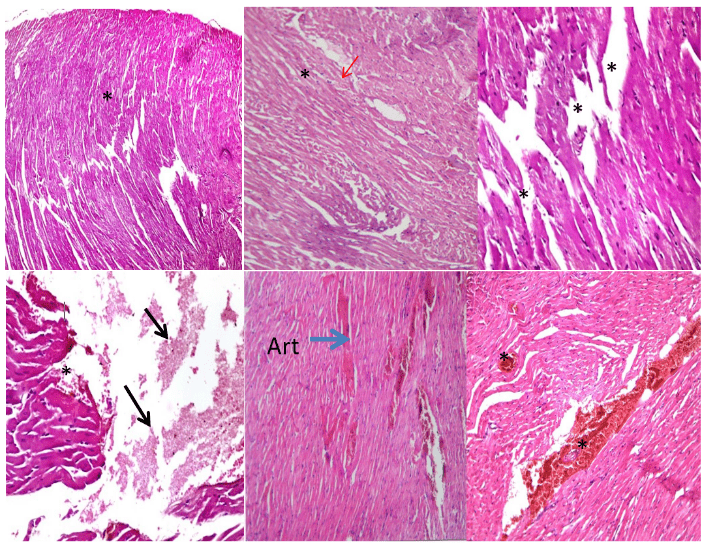
The control group of rat cardiac muscles was normal appearance with intercommunicating muscle fibers in different directions bundles of cardiomyocytes (Figure 4A). Figure 4B illustrated the cross-section of rat cardiac myocytes treated with ZnO NPs/GTE complex that shows normal cardiac muscle with striations and branched appearance as well as normal-sized nucleus. MSG-LD treated group (6 mg/kg) effect on cardiac muscles, and focal necrosis of myocytes with ruptured muscle fibers and eosinophilia in cytoplasm were observed (Figure 4C). The necrotic and atrophic muscle fibers as well as areas of intramuscular hemorrhage with deformation of muscle fibers were noticed in MSG-HD treated animals (17.5 mg/kg) showing (Figure 4D). The lower dosage of MSG and ZnO NPs/GTE complex showing partial restoration of cardiac muscles with mild congested area of muscle fibers and exhibiting relatively mycocardial cells (Figure 4E). The higher dosage of MSG and ZnONPs/GTE complex treated-animals showing restoration of cardiac muscles with moderate congested area and degeneration of some fibril cells (Figure 4F).
Figure 4: Histopathological slides of the cardiac myocytes in the basal level of rat left ventricle with hematoxylin and eosin. (A) control group of rat showing normal appearance of cardiac muscle (**) formed of intercommunicating muscle fibers in different directions bundles of cardiomyocytes (200X); (B) cross-section of rat cardiac myocytes treated with ZnO NPs/GTE complex (10 mg/Kg) showing normal cardiac muscle with striations and branched appearance (*) as well as normal-sized nucleus (Red arrow) (400X); (C) MSG-LD treated group (6 mg/kg) showing cardiac muscles, and focal necrosis of myocytes with ruptured muscle fibers and eosinophilia in cytoplasm (**) (400X); (D) MSG-HD treated animals (17.5 mg/kg) showing necrotic and atrophic muscle fibers (Black arrow) and areas of intramuscular hemorrhage with deformation of muscle fibers (*) (400X); (E) The lower dosage of MSG and ZnO NPs/GTE complex showing partial restoration of cardiac muscles with mild congested area of muscle fibers and exhibiting relatively mycocardial cells (*) (400X); (F) The higher dosage of MSG and ZnONPs/GTE complex treated-animals showing restoration of cardiac muscles with moderate congested area and degeneration of some fibril cells (**) (400X).
Discussion
MSG is widely used as food enhancer but its outstanding bleaching characteristics may adversely affect the body's tissues and organs [1-3,14]. There is insufficient evidence to determine whether or not long-term consumption of MSG caused damage to the cardiac tissue. Thus, the first objective of the present investigation was to examine the impact of low and high doses of MSG on cardiac tissues. Moreover, the Ameliorative efficacy of ZnO NPs/GTE complex against the MSG toxicity was evaluated on antioxidants parameters and histological changes in cardiac tissue of rats.
Increasing the LPO could help increase the susceptibility of the membrane and ultimately cause damage to the cardiac tissue. This indicates that MSG-induced cell permeability disruption at the sub-chronic and two dosages in a dose-dependent way. In the current research, the elevation of LPO reduced antioxidant status, and associated cardiotoxicity may be correlated with inadequate antioxidant capacity. Due to the capacity of antioxidants as prophylactic and therapeutic agents in many illnesses, antioxidant therapy has become very important in the latest year. Antioxidant consumption enhances biological mechanisms and prevents illnesses and organ toxicity associated with oxidative stress. SOD is regarded to be one of the first lines of defense enzymes against the hurtful impact of oxygen radicals in the cells and scavenges ROS by catalyzing the O2.- radical to H2O2 and O2 [15]. The existence of SOD in different compartments in our body allows O2. radicals to disputed. So, the cells are protected against oxidative damage. Significant inhibition of SOD activity in cardiac tissue as the impact of 17.5 mg MSG/g body weight may result in an elevated flux of O2.- radicals and cause Tissue injuries as shown in our research.
Also, the considerable reduction of rats that treated with MSG-HD in the activity of CAT, another powerful antioxidant enzyme, particularly against the O2.- radicals and singlet oxygen. CAT prevents cells from H2O2 accumulation by either disputing it into H2O and O2 or by using it as an oxidant in which it functions as a peroxidase. The reduction in CAT activity found in the current work may be due to lower accessibility of NADH as MSG promotes lipogenesis [16]. Therefore, MSG had no positive impact on CAT activity to decrease lipogenesis or oxidative stress. Glutathione (GSH), is effective on GPx and glutathione reductase system to maintain a reduced state in the body. GSH is a powerful endogenous antioxidant capable of protecting the body cells against a variety of noxious stimuli, including ROS [17, 18].
GSH endorses an elevation of sensitivity to oxidative damage that is an agreement exists between LPO and GSH status with a study that found an inverse relationship. GSH depletion of 20% to 30% may influence the body defense against xenobiotics and can contribute to cell injury [19]. Moreover, GPx uses GSH as a substrate for H2O2 and thus saves mammalian cells from oxidative stress [20]. Low activity of GPx may make the tissue more vulnerable to LPO harm. Correspondingly, after an increase in LPO concentration, we observed a significant reduction in the GPx activity. This finding is consistent with the assumption that LPO and GPx may be involved in tissue harm [21-23].
These results agree with AL-Salmi et al. who stated that animals treated with MSG orally at a dose of 17.5 mg/g for 30 days induced hepatic damage through oxidative stress, which caused LPO, diminished levels of antioxidant enzymes; SOD, CAT, and GPx with diminished GSH [2]. In the same context, Valko et al. indicated that oxidative stress could be caused and presented in the cardiac tissue at a dosage of 4.8 mg/g body weight with changes in CAT and SOD activities after administration of MSG. The present study has shown that the cardiac tissues of rats treated with MSG exhibited remarkable histopathological derangements [24]. Comparable findings have been mentioned in other studies [25, 26]. They discovered that left ventricle hypertrophy happens prevalent causes as well as substance abuse in which MSG can be defined as one in response to elevated stress on the heart [27].
These histopathological changes are in the same line with previous researchers [4, 25] who reported that the heart showed an increase in its weight. The information might be clarified by the glutamate's excitotoxic function. In order to study the ameliorative effect of ZnO NPs/GTE complex, rats received two dosages of MSG and a dose of ZnO NPs/GTE (10 mg/kg) by oral gavages daily for 30 days. The dose of ZnO NPs/GTE used in this study was not toxic as evidenced by normal levels of antioxidant parameters and histology of cardiac tissue. In the current research, rats were subjected to MSG along with ZnO NPs/GTE indicated the protective role of complex in reducing the LPO markers in cardiac tissue. Significant increase in the level of endogenous antioxidants such as SOD, CAT, GSH, and GPx in cardiac tissue indicates the protection against oxidative stress. Cardiac tissue showed normal architecture. Administration of ZnO NPs/GTE protects the cardiac function from MSG intoxication as indicated by the significant restoration of biochemical and functional parameters. Additionally, it has ameliorated cardiac histopathological changes. This finding affirmed by other authors who mentioned that the histopathological changes in the diseased organs as the MSG effect are relieved with ZnO NPs/GTE treatment approximately to control groups [1-3].
The green ZnO NPs/GTE complex antioxidant property can be attributed to its ingredients from phenol compounds in GTE, which give hydrogen ions to free radicals preventing oxidation reactions in the cells. It had the ability to get rid of free radicals, which are the fundamental cause of LPO and disturbance in antioxidant as well as cardiac tissue damage [28]. In conclusion, the above observations proposed that MSG increased oxidative stress by altering the levels of oxidative stress indicators such as LPO, SOD, CAT, GSH, and GPx in adult male rats cardiac tissue at a dose level of 17.5 mg/g body weight. Hence, MSG might lead to cardiac dysfunction. Therefore, all results indicated that ongoing and enhanced use of MSG could considerably boost health danger and could result from hypertrophy and oxidative stress of cardiac muscle. Treatment of ZnO NPs/GTE along with MSG ameliorated LPO, increased antioxidant activities and prevents oxidative damages. The processes that contribute to its efficacy include the capacity of the green complex (ZnO NPs/GTE) to scavenge free radicals and improve the architecture of cardiac myofibers.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 14, Aug 2019Accepted: Wed 04, Sep 2019
Published: Tue 05, Nov 2019
Copyright
© 2023 Nahla S El-Shenawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JBEM.2019.01.03
Author Info
Reham Z. Hamza Fawziah A. Al-Salmi Nahla S El-Shenawy
Corresponding Author
Nahla S El-ShenawyZoology Department, Faculty of Science, Suez Canal University, Ismailia, 41522, Egypt
Figures & Tables
References
- Hamza RZ, Al-Salmi FA, El-Shenawy NS (2018) Nanoparticles effects on zinc oxide/green tea complex on the lipid profile and liver functions of rats after monosodium glutamate treatment. J Appl Sci 18: 65-70.
- Al-Salmi FA, Hamza RZ, El-Shenawy NS (2019) The interaction of zinc oxide/green tea extract complex nanoparticles and its effect on monosodium glutamate toxicity in liver of rats. Curr Pharm Biotechnol 20: 465-475. [Crossref]
- El-Shenawy NS, Hamza RZ, Al-Salmi FA, Al-Eisa RA (2019) Evaluation of the effect of nanoparticles zinc oxide/Camellia sinensis complex on the kidney of rats treated with monosodium glutamate: Antioxidant and histological approaches. Curr Pharm Biotechnol 21. [Crossref]
- Chaudhary P, Malik VB, Puri S, Ahluwalia P (1996) Studies on the effect of monosodium glutamate (MSG) on hepatic microsomal lipid peroxidation, calcium, ascorbic acid and glutathione and its dependent enzymes in adult male mice. Toxicol Lett 9: 71-76. [Crossref]
- Kuldip S, Ahluwalia P (2003) Studies on the effect of MSG administration on some antioxidant enzymes in arterial tissue of adult male mice. J Nutr Sci Vitaminol (Tokyo) 49: 145-148. [Crossref]
- Kuldip S, Ahluwalia P (2005) Alteration in some antioxidant enzymes in cardiac tissue upon MSG administration to adult male mice. Indian J Clin Biochem 20: 43-46. [Crossref]
- Singh K, Ahluwalia P (2012) Effect of monosodium glutamate on lipid peroxidation and certain antioxidant enzymes in cardiac tissue of alcoholic adult male mice. J Cardiovasc Dis Res 3: 12-18. [Crossref]
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358. [Crossref]
- Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469-474. [Crossref]
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105: 121-126. [Crossref]
- Hafeman DG, Sunde RA, Hoekstra WG (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104: 580-587. [Crossref]
- Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882-888. [Crossref]
- Banchroft DJ, Cook CH, Stirling RW, Turner DR (1996) Manual of histopathological techniques and their diagnostic application. Churchill Livingston Edinburg.
- Inuwa HM, Aina VO, Gabi B, Ola IA, Jaafaru L (2011) Determination of nephrotoxicity and hepatoxicity of monosodium glutamate (MSG) consumption. Br J Pharmacol Toxicol 2: 148-153.
- Marklund SL (1984) Properties of extracellular superoxide dismutase from human lung. Biochem J 220: 269-272. [Crossref]
- Malik VB, Ahluwalia P (1994) Studies on effect of MSG on various fractions of lipids and certain carbohydrate metabolizing enzymes in liver and blood of adult male mice. Toxicol Lett 74: 69-77. [Crossref]
- Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP et al. (2006) Increased oxidation and degradation of cytosolic proteins in alcohol exposed mouse liver and hepatoma cells. Proteomics 6: 1250-1260. [Crossref]
- Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO (2006) Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys 43: 20-24. [Crossref]
- Padmini E, Sundari BT (2008) Erythrocyte Glutathione depletion impairs resistance to hemolysis in women consuming alcohol. J Clin Biochem Nutr 42: 14-20. [Crossref]
- Nakazawa H, Genka C, Fujishima M (1996) Pathological aspects of active oxygen/ free radicals. Jpn J Physiol 46: 15-32. [Crossref]
- Blum J, Fridovich I (1985) Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys 240: 500-508. [Crossref]
- Raes M, Mihiels MC, Remacle J (1987) Comparative study of the enzymatic defence systems against oxygen derived free radicals the key role of glutathione peroxidase. Free Radic Biol Med 3: 3-20. [Crossref]
- Lapennna D, Gioia S, Ciotani G, Mezzetti TA, Ucchino S et al. (1998) Glutathione related antioxidants defense in human atherosclerotic plaques. Circulation 97: 1930-1934. [Crossref]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M et al. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44-84. [Crossref]
- Ortiz GG, Bitzer-Quintero OK, Zárate CB, Rodríguez-Reynoso S, Larios-Arceo F et al. (2006) Monosodium glutamate-induced damage in liver and kidney: a morphological and biochemical approach. Biomed Pharmacother 60: 86-91. [Crossref]
- Kingsley OA, Jacks PTW, Amaza DS, Peters TM, Otong ES (2013) The effect of monosodium glutamate (MSG) on the gross weight of the heart of Albino rats. Scholar J Appl Med Sci 1: 44-47.
- Kang PM, Izumo S (2000) Apoptosis and heart failure: A critical review of the literature. Circ Res 86: 1107-1113. [Crossref]
- Srivastava RC, Husain MM, Hasan SK, Athar M (2000) Green tea polyphenols and tannic acid act as potent inhibitors of phorbol ester-induced nitric oxide generation in rat hepatocytes independent of their antioxidant properties. Cancer Lett 153: 1-5. [Crossref]
