Blastic Plasmacytoid Dendritic Cell Neoplasm: A Case Report of a Rare Leukemic Variant
A B S T R A C T
Blastic plasmacytoid dendritic cell neoplasm, originally described in 1995, is a very rare hematological malignancy with an aggressive clinical course characterized by a rapid progression to systemic disease via hematogenous dissemination. It represents around 0.5% of all hematologic cancers and typically affects older males. Early recognition remains a challenge as it largely overlaps with other hematological malignancies.
Keywords
blastic plasmacytoid dendritic cell, neoplasm, dentritic cells, CD123, hematological malignancy, hematologic cancer, leukemic variant, cutaneous lesion, tagraxofusp-erzs, elzonris
Introduction
Originally described in 1995, blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a very rare hematological malignancy with an aggressive clinical course [1, 2]. The overall incidence rate is 0.04 cases per 100,000 people, representing around 0.5% of all hematologic cancers [3, 4]. It typically affects older males with a median age of 43-68 years with a 2.0-3.3:1 male to female ratio [5, 6]. Despite an increasing number of reports and biologic insights about BPDCN, early recognition of the disease still remains a challenge because its phenotype largely overlaps with that of other hematological malignancies; in fact, it was in 2008 that the World Health Organization (WHO) established the term BPDCN and only later in 2016, classified it as a distinct entity [7].
While most BPCDN cases present with cutaneous lesions and multiple erythematous papules, the disease actually manifests as two clinically and pathologically distinct variants: the first and more common one presents with distinctive skin lesions, and with proliferating cells deriving from plasmacytoid dendritic cells precursors. The second variant is represented by nodular aggregates of clonally expanded plasmacytoid dendritic cells (DC) in lymph nodes, skin and bone marrow and a leukemic dissemination and presentation [4, 5, 8, 9]. The latter is rare and affects predominantly males [9]. Clinically, the course of BPDCN is characterized by a rapid progression to systemic disease via hematogenous dissemination, reflecting in an aggressive disease with poor outcome and a median overall survival ranging from 12 to 14 months [4, 9]. Herein, we report a case of a patient with BPDCN with a rare leukemic variant of the disease at presentation.
Case Description
A 71-year old man with a past medical history significant for coronary artery disease, hypertension and dyslipidemia presented to the emergency department complaining of shortness of breath and dyspnea upon exertion. A review of systems was otherwise negative. Physical exam was also negative, namely for any cervical, axillary or inguinal palpable lymphadenopathy, but was relevant for a diffuse rash, and petechiae (Figure 1) that had started three months earlier. Work up for the chief complaint was negative for a coronary event, congestive heart failure or pulmonary embolism. Laboratory work included a complete blood count (CBC), which showed a white blood cell count (WBC) of 6,100 cell/mm3 with the differential reported as 83% lymphocytes and 5% atypical lymphocytes, hemoglobin of 11.8 g/dl with a mean corpuscular volume of 97.4 fl, and a platelet count of 39,000 cell/mm3. Computer-Tomography (CT) further revealed splenomegaly.
Figure 1: Cutaneous manifestation of blastic plasmacytoid dendritic cell neoplasm demonstrated by bruising and disseminated petechiae.
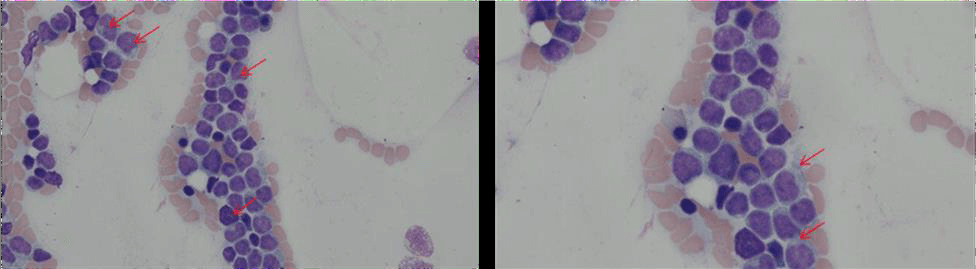
Figure 2: Morphology of blastic plasmacytoid dendritic cell neoplasm (arrows) demonstrated in patient’s bone marrow aspirate (A: 400x magnification and B: 600 magnification). Characteristic features include irregularly shaped nuclei with fine chromatin with one or more nucleolus. Cytoplasmic vacuoles localized along the cell membrane in a pearl necklace pattern are also seen (A top right corner).
The patient was referred for bone marrow biopsy which revealed blast cells of variable sizes, agranular/finely regular cytoplasm and irregular nuclei, concerning for leukemia (Figure 2). Peripheral blood flow cytometry revealed abnormal CD123+ dendritic cell population compatible with a leukemic phase of a blastic plasmacytoid dendritic cell neoplasm and comprising about 34% of the leukocytes. Consistently, review of the peripheral smear and CBC examination revealed a WBC of 7,100 cell/mm3 with 34% blasts, as well as macrocytic anemia and thrombocytopenia without schistocytosis. Based on these results, a diagnosis of BPDCN with rare leukemic variant was made and discussed with the patient. A therapy plan consisting of Tagraxofusp-erzs (ELZONRIS®) was further established.
Discussion
BPDCN is considered to arise from precursors of the type 2 or plasmacytoid dendritic cells and is characterized by the proliferation of intermediate-sized, monotonous immature-appearing cells with finely dispersed chromatin and small nucleoli [10]. Typically, cells are positive for CD123, CD4, BDCA2 and TCL1 and show aberrant expression of CD56 while being negative for CD34 and other lineage-defining markers such as CD19, CD20 and myeloperoxidase [11, 12].
Although BPDCN is categorized into two main clinical and pathological variants (cutaneous vs. leukemic dissemination), a wide spectrum of presentations has been reported: most cases of BPDCN manifest as cutaneous lesions with or without bone marrow involvement and leukemic dissemination [13]. Fewer cases present with leukemia with no skin involvement [14]. The skin lesions widely vary and can be brown to violaceous bruise-like lesions, plaques, or tumors, and may be solitary or widespread [6]. Cytopenia, lymphadenopathy, and/or splenomegaly are present in a considerable number of cases [5, 13]. Liver involvement has also been reported and appears to be more associated in cases with extensive bone marrow involvement [5]. Tonsillar, paranasal cavities, lungs, eyes, central nervous system (CNS), and paravertebral involvement have also been reported [5, 13].
In our case, the patient uniquely presented with the diffuse rash and the symptoms of dyspnea likely related to anemia. None of the other typical physical examination findings commonly seen in BPDCN and described above were appreciated at presentation. Such patients with only skin involvement constitute a minority of reported cases [15]. Further characterization by BM aspirate and peripheral smear examination and flow cytometry characterization was crucial in directing our diagnosis towards the rare leukemic variant. Unlike the common dermatologic variant which consists of proliferating cells deriving from plasmacytoid dendritic cells precursors, the leukemic variant is characterized by nodular aggregates of clonally expanded plasmacytoid DCs in lymph nodes, skin and bone marrow and a leukemic dissemination and presentation.
An accurate diagnosis of BPDCN is essential in order to provide treatment promptly, especially considering that the initial clinical presentation is often indolent. BPDCN may be suspected from a set of converging features from the clinical presentation and histological findings but overlaps with other hematologic neoplasms are considerable and the final diagnosis relies on a compatible immunophenotype. The triple positive CD4+CD56+CD123+ phenotype associated with negativity for lineage-specific markers is a minimum requirement for defining BPDCN. In addition, the highly specific marker BDCA2/CD303, as well as other plasmacytoid dendritic cell-associated antigens (e.g. TCL1 and CD2AP), might be of great support for establishing the diagnosis and for excluding potential mimickers of BPDCN (acute myeloid and monocytic leukemias, precursor lymphoblastic T-cell leukemia/lymphomas and T- and NK/T cell lymphomas) [5].
This case highlights the importance of a carefully selected flow cytometry panel that will identify BPDCN in leukemic phase, or with bone marrow involvement, regardless of skin involvement pattern. Tagraxofusp is used to induce remission. However, long-term outcomes are not well defined. It is administered at 12 mcg/kg intravenously over 15 minutes daily on days 1 to 5 of a 21-day cycle; the optimal number of cycles is not currently defined. Patients should be premedicated with antihistamines and steroids as capillary leak syndrome is a potential life-threatening complication [16].
In 44 patients treated with the higher dose of tagraxofusp (12 μg per kilogram), capillary leak syndrome was reported in 18 percent of patients, including 1 death [3]. The median survival of BDPCN is less than 2 years [17]. Our patient was transferred to a tertiary referral center to receive Tagraxofusp but, unfortunately, passed away few weeks later and this highlights the poor prognosis of the disease especially when it comes to elderly patients with a leukemic variant of the disease as younger age and early hematopoietic stem cell transplantation are predictive of better outcomes[13, 17] and some other small studies have also reported somewhat better complete remission rates with the cutaneous-only form of the disease[4, 16, 18].
TdT expression has been shown to correlate with longer survival, this suggests that differentiation state may correlate with sensitivity to therapy. Moreover, biallelic loss of the 9p21.3 locus and NR3C1 haploinsufficiency was associated with poor outcome, as were mutations involving the DNA methylation machinery. However, due to the rarity of the disease and the lack of a characteristic clinical and molecular profiles, prognostication using clinical and molecular data in BPDCN remains a challenge [4].
Article Info
Article Type
Case ReportPublication history
Received: Tue 17, Mar 2020Accepted: Tue 31, Mar 2020
Published: Mon 06, Apr 2020
Copyright
© 2023 Ammar Al-Obaidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2020.01.03
Author Info
Ammar Al-Obaidi Chloe McBride Christopher Dakhil Khalil Choucair William Palko
Corresponding Author
Ammar Al-ObaidiUniversity of Kansas School of Medicine - Wichita Campus, Department of Internal Medicine, Wichita, Kansas, USA
Figures & Tables

References
- Brody JP, Allen S, Schulman P, Sun T, Chan WC et al. (1995) Acute agranular CD4‐positive natural killer cell leukemia. Comprehensive clinicopathologic studies including virologic and in vitro culture with inducing agents. Cancer 75: 2474-2483. [Crossref]
- Kameoka J, Ichinohasama R, Tanaka M, Miura I, Tomiya Yet al. (1998) A cutaneous agranular CD2- CD4+ CD56+ “lymphoma”: report of two cases and review of the literature. Am J Clin Pathol 110: 478-488. [Crossref]
- Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S et al. (2019) Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N Engl J Med 380: 1628-1637. [Crossref]
- Venugopal S, Zhou S, El Jamal SM, Lane AA, Mascarenhas J (2019) Blastic Plasmacytoid Dendritic Cell Neoplasm–Current Insights. Clin Lymphoma Myeloma Leuk 19: 545-554. [Crossref]
- Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P et al. (2013) Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica 98: 239-246. [Crossref]
- Julia F, Dalle S, Duru G, Balme B, Vergier B et al. (2014) Blastic plasmacytoid dendritic cell neoplasms: clinico-immunohistochemical correlations in a series of 91 patients. Am J Surg Pathol 38: 673-680. [Crossref]
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ et al. (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127: 2391-2405. [Crossref]
- Pennisi M, Cesana C, Cittone MG, Bandiera L, Scarpati B et al. (2017) A Case of Blastic Plasmacytoid Dendritic Cell Neoplasm Extensively Studied by Flow Cytometry and Immunohistochemistry. Case Rep Hematol 2017: 4984951. [Crossref]
- Viviano M, Cocca S, Miracco C, Parrini S (2018) Blastic plasmacytoid dendritic cell neoplasm: a rare case of gingival lesion with leukaemic presentation. BMJ Case Rep 2018: 224623. [Crossref]
- Facchetti F, Pileri SA, Agostinelli C, Martelli MP, Paulli M et al. (2009) Cytoplasmic nucleophosmin is not detected in blastic plasmacytoid dendritic cell neoplasm. Haematologica 94: 285-288. [Crossref]
- Pagano L, Valentini CG, Grammatico S, Pulsoni A (2016) Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol 174: 188-202. [Crossref]
- Garnache Ottou F, Feuillard J, Ferrand C, Biichle S, Trimoreau F et al. (2009) Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol 145: 624-636. [Crossref]
- Feuillard J, Jacob MC, Valensi F, Maynadié M, Gressin R et al. (2002) Clinical and biologic features of CD4(+) CD56(+) malignancies. Blood 99: 1556-1563. [Crossref]
- Rauh MJ, Rahman F, Good D, Silverman J, Brennan MK et al. (2012) Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation, lacking cutaneous involvement: Case series and literature review. Leuk Res 36: 81-86. [Crossref]
- Chang HJ, Lee MD, Yi HG, Lim JH, Lee MH et al. (2010) A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat 42: 239-243. [Crossref]
- Hashikawa K, Niino D, Yasumoto S, Nakama T, Kiyasu J et al. (2012) Clinicopathological features and prognostic significance of CXCL12 in blastic plasmacytoid dendritic cell neoplasm. J Am Acad Dermatol 66: 278-291. [Crossref]
- Alsidawi S (2016) Blastic Plasmacytoid Dendritic Cell Neoplasm. a Population-Based Analysis from the SEER and NCDB Databases. Blood 128: 4789-4789.
- Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G et al. (2010) Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res 34: 438-446. [Crossref]
