Journals
Bacterial Aerosols Released During Dental Ultrasonic Scaling in Dogs
A B S T R A C T
Periodontal disease is a high prevalent and multi-factorial oral disease in dogs and ultrasonic scaling is used to remove dental plaque and calculus, releasing contaminated aerosols, which may represent a hazard to animal and human health. This study aimed to identify the microorganisms present in aerosols produced during dental scaling of canine patients. A random sample of 15 dogs with periodontal disease was included, and aerosol samples were collected, incubated, and isolated bacteria were identified. Dogs without previous antibiotic treatment (n=4) and dogs that received systemic antibiotics up to two weeks before to dental scaling and polishing, either amoxicillin and clavulanic acid (n=3), or a combination of metronidazole and spiramycin (n=8), were included in the study. The highest percentage of pathogenic bacteria present corresponded to Pseudomonas spp. (20%), followed by Staphylococcus spp. (13%) and Escherichia coli (10%). The most prevalent bacteria identified in the non-treated group was Pseudomonas spp. Within the group subject to prior antibiotherapy, the predominant bacterial species was also Pseudomonas spp. Followed by Staphylococcus spp. In spite of previous antibiotherapy, strong bacterial contamination was still present, suggesting that this is not a warranty of less contamination of the released aerosols. The bacteria identified in this study represent a serious hazard to public and animal health, so strict hygiene and prevention measures during ultrasonic scaling in dogs are mandatory.
Keywords
Canine periodontal disease, dental scaling, aerosols, bacterial contamination, hygiene and prevention
Summary
Periodontal disease is a high prevalent and multi-factorial oral disease in dogs. Ultrasonic scaling is used to remove dental plaque and calculus. In our study we found a high number of dangerous pathogenic bacteria in aerosol samples. Contaminated aerosols represent a serious a hazard to animal and human health. Strict hygiene measures during ultrasonic scaling in dogs are mandatory.
Introduction
Periodontal disease is a multi-factorial inflammatory disease both in humans and dogs, affecting tooth supporting tissues. The prevalence of gingivitis and periodontitis in dogs is approximately 95 to 100% and 50 to 70%, respectively [1]. Bacteria present in periodontal dental plaque are known to be an important factor in the course of the disease, especially anaerobic Gram-negative bacteria when compared to aerobic Gram-positive bacteria [2].
A wide range of bacteria has already been associated with periodontal disease in dogs. The first studies on dental plaque, based on traditional cultured methods, identified mainly the presence of: Porphyromonas spp. Actinomyces spp., Neisseria, Fusobacterium spp., Bacteroides spp., Pasteurella spp., Prevotella and Escherichia coli. [3, 4]. More recently, molecular methods have been used to detect oral bacteria present in the clinical specimens collected from dogs with periodontal disease [5]. Dental scaling and polishing are part of the periodontal therapy. Sonic and ultrasonic scalers are widely used in veterinary medicine, and these high-speed machines lead to the emission of highly concentrated aerosols [6]. Aerosols are less than 100ųm in size and can be inhaled into lungs or get in contact with skin and mucous membranes. They can transport microorganisms from the patient’s saliva, dental plaque, calculus, blood, the machine water supply, or the clinical environment [7].
Bacterial contamination from dental aerosols has been a growing concern in human dentistry, due to the infectious risk they pose to patients and to medical professionals. Measures for personal protection, equipment handling, and cleaning and space layout have been published [8]. This study aimed to identify potentially dangerous aerosolized microorganisms released during dental scaling in dogs.
Materials and Methods
I Subject Population
This prospective clinical study comprised fifteen client-owned dogs, from the Teaching Hospital of the Veterinary Medicine Faculty of Lisbon University that presented for treatment of periodontal disease. The experimental protocol was approved by the Ethics Committee of the Veterinary Medicine Faculty of Lisbon University. Owners gave written consent for inclusion of their animals in the study. Inclusion criteria for enrolment in the study included being a canine patient, having periodontal disease but no other concomitant diseases that could increase an aesthetic risks or promote immunosuppression or bacteria overgrowth, or interfere with microbiological results plus dogs were not under systemic medication other than antibiotic treatment related to the periodontal disease.
The staging of periodontal disease of the patients was done according to Sowkup J.W. (2010) [9]. According to the veterinary index of periodontal disease, staging should be performed for each tooth individually, based on the gingival recession, gingival sulcus, dental mobility and periodontal ligament loss Clinically patients were subdivided into: healthy gum (stage 0), gingivitis (stage 1), mild periodontitis (stage 2), moderate periodontitis (stage 3), and severe periodontitis (stage 4). Of the 15 dogs, group 1 did not receive previous antibiotic treatment (n=4) and group 2 was started on systemic antibiotics 2 weeks before dental scaling and polishing was performed (n=11). A total of 3 received amoxicillin and clavulanic acid and a total of 8 dogs received a combination of metronidazole and spiramycin.
II Sample Collection
Dogs were catheterized in the cephalic vein and administered propofol 1% (10 mg/1 ml (Fresenius Kabi Pharma Portugal, Carnaxide, Portugal) at a dosage of 5 mg/Kg for anesthetic induction. Following endotracheal intubation, anesthesia was maintained with isoflurane (IsoFlo® 100% p/p, Abbot Laboratories, North Chicago, USA), and the procedure of dental scaling and polishing was performed.
The ultrasonic scaling procedure involved the use of an ultrasonic piezoelectric scaler (Model KRUUSE-SP2, Jorgen Kruuse®, Langeskov, Denmark). Sterile water was used in the ultrasonic piezoelectric scaler. After collection of the aerosol samples, all the patients underwent dental polishing. Aerosols sample collection occurred at two different time points of the dental procedure in both groups. The first time point was during the dental scaling procedure, using two different culture plates, located at a distance of approximately 10 cm from the tip of the scaler, for 15 min. The plates used to collect the aerosol samples contained Columbia 5% sheep blood agar (COS) (bioMereux®, Marcy l’Etoile, France) and Schaedler 5% sheep blood agar (SCS) (bioMereux®, Marcy l’Etoile, France) medium.
The second time point of aerosol sample collection was at the end of the scaling procedure, using a sterile swab with activated charcoal (Normax, ref. 6091501). This sample was obtained directly by gently stroking with a swab the surface of the superior canine and premolar teeth of each patient. The technicians involved wore protective equipment during dental scaling procedures, namely safety googles, mask, cap, gown and gloves.
III Microbiological Analysis
The blood agar plates were immediately transported to the Bacteriology Laboratory. The COS agar plate was incubated aerobically at 37°C for 24 hours, while the SCS agar plate was incubated anaerobically at 37°C for 48 hours. The activated charcoal swabs were inoculated onto SCS agar plates which were also incubated anaerobically for 48 hours at 37°C.
The aerobic plates were evaluated and the colonies with similar macroscopic morphology and showing more than 5 well-isolated colony forming units were sub- cultured to a COS plate for isolation. These plates were incubated aerobically for 24 hours at 37°C. Aerobic bacteria were identified by Gram staining and by catalase and oxidase biochemical tests. The bacteria identified were classified until the species level using the manual test kits: API® Staph for Gram positive cocci /Catalase positive, API® 20 Strep for Gram positive cocci/Catalase negative, API® 20E for Gram negative bacillus/Oxidase negative, API® 20NE for Gram negative bacillus/Oxidase positive and API® Coryne for Gram positive bacillus; and BBL® GP for Gram positive Ccocci/Gram positive Bacillus and BBL E/NF® for Gram negative bacillus (BioMerieux®, Marcy l’Etoile, France). The results were interpreted using the database provided by the APIWEB™ software service.
The anaerobic plates were also evaluated after the incubation period and following macroscopic evaluation, the colonies were sub cultured onto a new SCS plate and incubated anaerobically for 48 hours at 37°C. Subsequent identification of the bacterial colonies was performed using Gram’s staining and the biochemical test API 20 A® NF (bioMerieux®, Marcy l’Etoile, France). Results are presented using descriptive statistical methods.
Results
There were nine females (60%) and six male dogs (40%) included in the study. The average age corresponded to 9.8 years, varying between 2 and 15 years. The majority of dogs were crossbreeds. Other breeds included Cocker spaniel, Toy poodle, Yorkshire terrier and German spitz. Concerning staging of periodontal disease, 2 dogs presented with stage two, 7 dogs with stage three and six dogs with stage four. Regarding the distribution of animals depending on prior antibiotic therapy, 27% were not doing antibiotherapy while 73% were on a systemic antibiotic course. Within these, 53% of the dogs were on a combination of spiramycin and metronidazole, and 20% were on a combination of amoxycillin plus clavulanic acid.
Microbiological Results
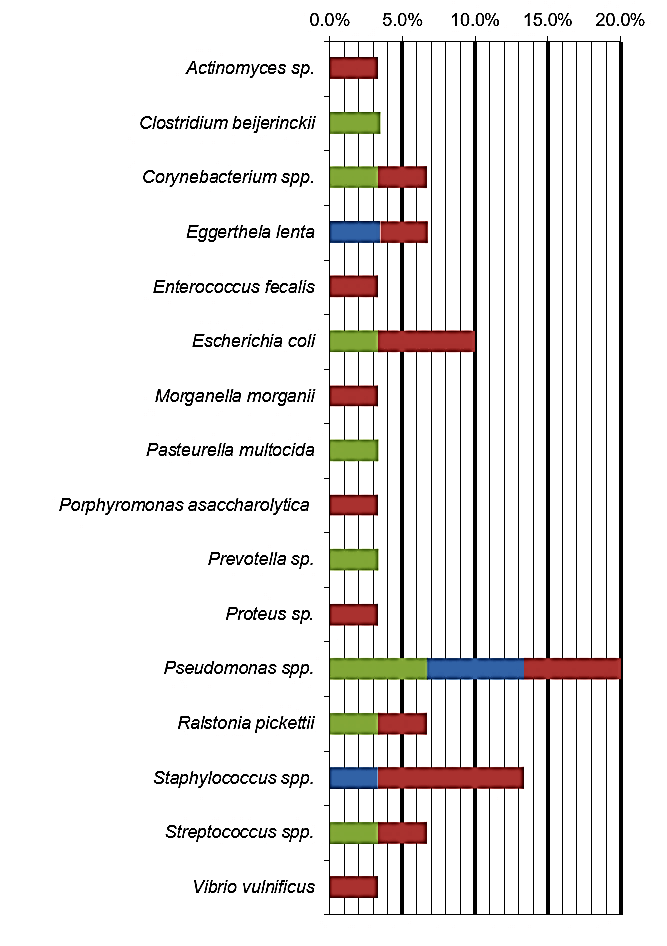
The bacterial species identified from dental aerosols collected during the ultrasonic scaling procedure are presented in (Table 1). The highest percentage of pathogenic bacteria present in the aerosol samples collected from our studied population corresponded to Pseudomonas spp. (20%), followed by Staphylococcus spp. (13%) and Escherichia coli (10%). The most prevalent bacteria identified in the G1 was Pseudomonas spp. In spite of the previous antibiotherapy in the majority of animals (n=11), there was a large number of bacterial species identified in group 2. Within G2, the predominant bacterial species were Pseudomonas spp. and Staphylococcus spp. (Table 2).
Table 1: Bacterial species identified from dental aerosols collected during the ultrasonic scaling procedure in each case (n=15).
|
Case number |
Microorganisms isolated |
|
|
||
|
Group 1 |
Case 1 |
Clostridium beijerinki Pasteurella muktocida Prevotella sp. Ralstonia pickettii |
|||
|
Case 2 |
Corynebacterium glucoronolyticum Streptococcus constellatus |
||||
|
Case 3 |
Echerichia coli Pseudomonas aeruginosa |
||||
|
Case 4 |
Pseudomonas aeruginosa |
||||
|
Group 2 |
Case 5 |
Staphylococcus aureus |
|||
|
Case 6 |
Eggertella lenta |
||||
|
Case 7 |
Pseudomonas aeruginosa Pseudomonas sp. |
||||
|
Case 8 |
Morganella morgani Pseudomonas aeruginosa Staphylococcus chromogenes |
||||
|
Case 9 |
No bacterial growth |
||||
|
Case10 |
Vibrio vulnificus Actinomyces sp. |
||||
|
Case 11 |
Corinebacterium sp. Enterococcus fecalis Escherichia coli |
||||
|
Case 12 |
Escherichia coli Staphylococcus aureus |
||||
|
Case 13 |
No bacterial growth |
||||
|
Case 14 |
Pseudomonas aeruginosa |
||||
|
Case 15 |
Proteus sp. Streptococcus sp. Ralstonia pickettii Staphylococcus aureus Porphyromonas asaccharolytica Eggerthela lenta |
||||
Discussion
Ultrasonic scaling procedures release potentially contaminated aerosols to the environment. In dogs the prevalence and severity degree of periodontal disease are high. The aerosolized bacteria may represent a serious hazard to both public and animal health. The concentration of microorganisms present on the aerosols is higher closer to the tip of the hand piece of the ultrasonic scaling machine [10]. Studies performed in human medicine environments showed that the area becoming contaminated by the aerosols extends to more than 1,5m from the patient mouth and that high concentrations of bacteria can last for 30 minutes in the environment [11].
When evaluating aerosol sampling methods, the studies in human medicine have a broad variety technique regarding time and sampling distance [11, 12]. The distance and duration of aerosols sampling in our study were within the same range. In addition to the agar plates positioned at 10 cm from the ultrasonic scaling tip, at the second time point we used an activated charcoal sterile swab for collection of aerosols from the tooth surface, gingival sulcus area, and/or periodontal pockets. This method of collection was also supported by previous studies that analyzed the composition of the dental plaque in dogs with periodontal disease [13]. Likewise, the culture methods used were similar to the ones selected in human medicine [11, 12].
From the total isolated microorganisms, Pseudomonas spp. were the most prevalent bacteria (20%). Pseudmonas spp. have been isolated from the oral cavity of healthy dogs and dogs with periodontal disease [5]. In human medicine studies, these bacteria has also been identified both before and after dental scaling procedures [8, 11]. Additionally, Pseudomonas has also been subject of study as a waterborne bacterium, since it has the ability to form part of dental unit waterline biofilms [14]. Its pathogenic effects are well known, and the release of this bacteria to the environment represents a serious hazard to both animal and human health. Staphylococcus spp. was identified as the second most prevalent microorganism (13%). In several aerosol studies from human dentistry, Staphylococcus was also one of the most common bacteria found [8, 11]. Staphylococcus is most commonly isolated from the upper respiratory airways, eyes, skin, urinary and reproductive tract of dogs and humans [15]. Studies in the composition of the canine dental plaque have also identified Staphylococcus both in both in healthy and in dogs with periodontal disease [16, 17].
In third place came Escherichia coli, which represents 10% of the isolated microorganisms. This bacterial species has been isolated in several studies that focus on the microorganisms that are present in the mouth of dogs with periodontal disease [16, 17]. However, E. coli has not been reported to be present in isolates of dental aerosols in human dentistry.
Presenting with a lower prevalence, Corynebacterium spp., Eggerthela lenta, Ralstonia picketii and Streptococcus spp. were also present, representing 7% of the total isolated bacteria. Corynebacterium and Streptococci have both been isolated before from dogs with periodontal disease [5, 16]. Streptococcus was also found in some of the studies analysing human dental aerosols [8, 12]. E. lenta has only been found in humans as being part of the intestinal microbiome, and it has also been found as one of the microorganisms forming part of the subgingival flora in patients with periodontal disease [18]. R. pickettii has been identified in cases of human medicine infections due to contaminated solutions, as water for injections, saline solutions, and sterile drug solutions [19]. Szymanska (2007) conducted several studies in the microflora of dental unit water lines, and 94% of the bacteria identified were R. picketti [20].
The less prevalent bacteria were Actinomyces sp., Clostridium beijerinckii, Enterococcus fecalis, Morganella morganii, Pasteurella multocida, Porphyromonas asccharolytica, Prevotella sp., Proteus sp. and Vibrio vulnificus, representing 3-4% of the isolates each. In the evaluation of the bacteria in dogs with periodontal disease, Actinomyces sp., Clostridium sp., Pasteurella sp., Porphyromonas sp., Prevotella and Proteus have been widely isolated [5, 16]. Regarding Clostridium beijerinckii, Enterococcus fecalis and Morganella morganii, these have all been identified as commensal microorganisms of the gastrointestinal tract in dogs [21]. Vibrio vulnificus is reported in the literature as being present in sea and estuarine waters [22]. For future identification of specific bacterial species, as periodontal pathogens and waterborne bacteria, specific molecular methods would be necessary [5].
Antibiotic therapy is commonly used as part of the treatment for canine periodontal disease. According to previous studies, preoperative treatment with clindamycin prior to removal of calculus using an ultrasonic scaler can reduce aerosolized bacteria [23] . In our study, in spite of the previous antibiotherapy in some patients, strong bacterial contamination of the released aerosols was still present, suggesting that antibiotherapy per se is not a warranty of less contaminated aerosols. Antibiotherapy should not be part of the management of periodontal disease in the vast majority of dogs to avoid bacterial resistance. Dogs from the present study suffered from chronic periodontal disease for months or even years. Mechanical removal of plaque and calculus from supra- and subgingival spaces as well as extraction of moderately to severely affected teeth are the advised treatment for periodontal disease, followed by strict home oral hygiene. In subsequent studies it would be of interest to analyze a larger sample to draw meaningful conclusions on this parameter.
Infection control and protection measures should be implemented. Preventive mouth rinse with chlorhexidine solution of patients undergoing this procedure should be performed. The use of personal protective barriers by the operator such as gown, gloves, face mask, surgical cap and safety googles should be mandatory. Regular monitoring of the ultrasonic scaler, machine cleaning and water supply renewal, disinfection and ventilation of the working space should be part of the measures undertaken in every veterinary clinical facility [24, 25].
The results of this clinical study show that a large number of microorganisms may be present in the aerosols released during dental scaling and polishing in dogs with periodontal disease, which can contaminate patients, operators and clinical environment. These bacteria can contaminate patients, operators, and clinical environments. Strict infection control and protection measures should be implemented. We hope to contribute to increase awareness of the risk of bacterial contamination of patients and veterinary practitioners when exposed to aerosolized microbial pathogens during dental scaling procedures in dogs.
Conflicts of Interest
None.
Funding
This work was supported by the Project UID/CVT/276/2019 (CIISA).
Article Info
Article Type
Research ArticlePublication history
Received: Thu 07, May 2020Accepted: Sun 24, May 2020
Published: Tue 30, Jun 2020
Copyright
© 2023 Esmeralda Delgado. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2020.03.02
Author Info
Corresponding Author
Esmeralda DelgadoCIISA, Centre for Interdisciplinary Research in Animal Health, Faculty of Veterinary Medicine, University of Lisbon, Portugal
Figures & Tables
Table 1: Bacterial species identified from dental aerosols collected during the ultrasonic scaling procedure in each case (n=15).
|
Case number |
Microorganisms isolated |
|
|
||
|
Group 1 |
Case 1 |
Clostridium beijerinki Pasteurella muktocida Prevotella sp. Ralstonia pickettii |
|||
|
Case 2 |
Corynebacterium glucoronolyticum Streptococcus constellatus |
||||
|
Case 3 |
Echerichia coli Pseudomonas aeruginosa |
||||
|
Case 4 |
Pseudomonas aeruginosa |
||||
|
Group 2 |
Case 5 |
Staphylococcus aureus |
|||
|
Case 6 |
Eggertella lenta |
||||
|
Case 7 |
Pseudomonas aeruginosa Pseudomonas sp. |
||||
|
Case 8 |
Morganella morgani Pseudomonas aeruginosa Staphylococcus chromogenes |
||||
|
Case 9 |
No bacterial growth |
||||
|
Case10 |
Vibrio vulnificus Actinomyces sp. |
||||
|
Case 11 |
Corinebacterium sp. Enterococcus fecalis Escherichia coli |
||||
|
Case 12 |
Escherichia coli Staphylococcus aureus |
||||
|
Case 13 |
No bacterial growth |
||||
|
Case 14 |
Pseudomonas aeruginosa |
||||
|
Case 15 |
Proteus sp. Streptococcus sp. Ralstonia pickettii Staphylococcus aureus Porphyromonas asaccharolytica Eggerthela lenta |
||||

References
- Harvey CE, Shofer FS, Laster L (1996) Correlation of Diet, Other Chewing Activities and Periodontal Disease in North American Client-Owned Dogs. J Vet Denti 13: 101-105. [Crossref]
- Wallis C, Marshall M, Colyer A, O’Flynn C, Deusch O et al. (2015) A Longitudinal Assessment of Changes in Bacterial Community Composition Associated with the Development of Periodontal Disease in Dogs. Vet Microbiol 181: 271-282. [Crossref]
- Domingues LM, Alessi AC, Schoken Iturrinho RP, Dutra LS (1999) Microbiota saprófita associada à doença periodontal em cães. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 51: 1-6.
- Syed S, Svanberg M, Svanberg G (1980) The Predominant Cultivable Dental Plaque Flora of Beagle Dogs with Gingivitis. J Periodontal Res 15: 123-136. [Crossref]
- Riggio MP, Lennon A, Taylor DJ, Bennett D (2011) Molecular identification of bacteria associated with canine periodontal disease. Vet Microbiol 150: 394-400. [Crossref]
- Bennett AM, Fulford MR, Walker JT, Bradshaw DJ, Martin MV et al. (2000) Microbial Aerosols in General Dental Practice. Br Dent J 189: 664-667. [Crossref]
- Leggat PA, Kedjarune U (2001) Bacterial Aerosols in the Dental Clinic: A Review. Int Dent J 51: 39-44. [Crossref]
- Kobza J, Pastuszka JS, Brągoszewska E (2018) Do exposures to aerosols pose a risk to dental professionals? Occup Med (Lond) 68: 454-458. [Crossref]
- Sowkup JW (2010) Periodontitis. In Ettinger SJ &. Feldman EC (Eds.), Textbook of Veterinary Internal Medicine. (7th ed.) Missouri: Saunders Elsevier 174-185.
- Prospero E, Savini S, Anmino I (2003) Microbial Aerosol Contamination of Dental Healthcare Workers' Faces and Other Surfaces in Dental Practice. Infect Control Hosp Epidemiol 24: 139-141. [Crossref]
- Al Maghlouth A, Al Yousef Y, Al Bagieh N (2004) Qualitative and Quantitative Analysis of Bacterial Aerosols. J Contemp Dent Pract 5: 91-100. [Crossref]
- Sawhney A, Venugopal S, Babu GRJ, Garg A, Mathew M et al. (2015) Aerosols how dangerous they are in clinical practice. J Clin Diagn Res 9: ZC52-ZC57. [Crossref]
- Augusto J, Discacciati C, Sander HH, Silva L, Castilho D et al. (1998) Verificação da dispersão de respingos durante o trabalho do cirurgião-dentista. Pan Am J Public Health 3: 84-87.
- Pasquarella C, Veronesi L, Castiglia P, Liguori G, Montagna MT et al. (2010 Italian Multicentre Study on Microbial Environmental Contamination in Dental Clinics: A Pilot Study. Sci Total Environ 408: 4045-4051. [Crossref]
- Hoekstra K, Paulton RJL (2002) Clinical Prevalence and Antimicrobial Susceptibility of Staphylococcus Aureus and Staph. Intermedius in Dogs. J Appl Microbiol 93: 406-413. [Crossref]
- Bota A, Muste A, Beteg F, Scurtu L, Krupaci A (2010) Microbiological investigations in dogs with periodontal disease. Cluj Veterinary J 17: 59-63.
- Braga C, Resende C, Pestana A, Carmo L, Costa J et al. (2005) Isolamento e identificação da microbiota periodontal de cães da raça Pastor Alemão. Ciência Rural 35: 385-390.
- Nakazawa F, Miyakawa H, Fujita M, Kamaguchi A (2011) Significance of Asaccharolytic Eubacterium and Closely Related Bacterial Species in the Human Oral Cavity. J Exp Clin Med 3: 17-21.
- Moreira BM, Leobons MBGP, Pellegrino FLPC, Santos M, Teixeira LM et al. (2005) Ralstonia Pickettii and Burkholderia Cepacia Complex Bloodstream Infections Related to Infusion of Contaminated Water for Injection. J Hosp Infect 60: 51-55. [Crossref]
- Szymańska J (2007) Dental Bioaerosol as an Occupational Hazard in a Dentist's Workplace. Ann Agric Environ Med 14: 203-207. [Crossref]
- Harwood VJ, Delahoya NC, Ulrich RM, Kramer MF, Whitlock JE et al. (2004) Molecular Confirmation of Enterococcus Faecalis and E. Faecium From Clinical, Faecal and Environmental Sources. Lett Appl Microbiol 38: 476-482. [Crossref]
- Strom MS, Paranjpye RN (2000) Epidemiology and Pathogenesis of Vibrio Vulnificus. Microbes Infect 2: 177-188. [Crossref]
- Zetner K, Thiemann G (1993) The Antimicrobial Effectiveness of Clindamycin in Diseases of the Oral Cavity. J Vet Dent 10: 6-9. [Crossref]
- Bowersock TL, Wu CC, Inskeep GA, Chester ST (2000) Prevention of Bacteremia in Dogs Undergoing Dental Scaling by Prior Administration of Oral Clindamycin or Chlorhexidine Oral Rinse. J Vet Dent 17: 11-16. [Crossref]
- Kohn WG, Harte JA, Malvitz DM, Collins AS, Cleveland JL et al. (2004) Guidelines for Infection Control in Dental Health Care settings--2003. J Am Dent Assoc 135: 33-47. [Crossref]
