Augmented Pain and Inflammation with Obesity: A Role for the Pro-Inflammatory Cytokine Visfatin
A B S T R A C T
Obesity is associated with several co-morbidities including chronic pain. Systemic low-grade chronic inflammation and dysregulation of pro-inflammatory cytokines have been proposed to underlie these phenomena. This study characterized pain and inflammation, and levels of the pro-inflammatory cytokine visfatin, in a rodent model of obesity, and investigated whether treatment with the visfatin inhibitor, FK866, has anti-inflammatory and/or analgesic effects in normal and obese rats. The effects of pre-administration of FK866 (3, 10 mg/kg; i.p.) on carrageenan (3%; i.d. into the left paw)-induced thermal and mechanical hypersensitivity and paw oedema was measured in adult male Wistar rats fed a normal diet (ND) or high fat diet (HFD) for 12 weeks. HFD-fed rats displayed an increased sensitivity to acute mechanical nociceptive stimulation, and potentiated mechanical hyperalgesia and peripheral inflammation to carrageenan. Levels of circulating visfatin were increased in HFD-fed rats. Treatment with FK866, a visfatin inhibitor, was effective in reducing carrageenan-induced hyperalgesia and paw oedema in both ND-fed and HFD-fed rats. These data show that FK866 has anti-inflammatory and analgesic properties. The potentiated response to pain and inflammation, and elevated visfatin levels in HFD-fed rats supports the hypothesis that obesity is a chronic low-grade inflammatory disorder. Reversal of this co-morbidity by blocking visfatin may be a novel therapeutic strategy for managing pain with obesity.
Keywords
Visfatin, obesity, pain, inflammation, cytokines, high fat diet
Introduction
Obesity is an energy-rich condition associated with over-nutrition, which impairs systemic metabolic homeostasis and physical mobility [1, 2]. Increased body weight in humans is directly linked to development of chronic pain conditions including osteoarthritis, lower back pain, migraine, hyperuricemia, gouty arthritis and musculoskeletal pain [3-5]. Expansion of adipose tissue with obesity is associated with altered inflammatory cytokine or ‘adipokine’ secretion, which is now known to directly contribute to inflammation and associated pain [6-8].
Visfatin, also known as a nicotinamide phosphorybosyltransferase (NAMPT), the limiting enzyme in nicotinamide adenine dinucleotide (NAD) biosynthesis, and identical to previously identified pre-B cell colony-enhancing factor (PBEF), is a well characterized 52 kDa adipokine with insulin mimetic properties [9, 10]. Visfatin is expressed in lymphocytes, hepatocytes, muscle and in white adipose tissue macrophages and is up-regulated in cancer, inflammation and obesity [11-15]. In brain, visfatin is emerging as a neuroprotective factor for ischaemic brain injury [16, 17]. A recent study reported that direct intracerebroventricular infusion of recombinant NAMPT reduced infarct volume in a mouse stroke model through an anti-inflammatory mechanism [18]. In other pathways, visfatin/NAMPT has been shown to have a pro-inflammatory function, inducing microglial activation in the hypothalamus, promoting inflammatory arthritis, and expression of inflammatory cytokines in atherosclerosis [19-21]. In addition, the specific non-competitive anti-visfatin inhibitor FK866 has been shown to reduce inflammation in several animal models including spinal cord injury, acute lung injury, and brain injury, by reducing activation of astrocytes and Iba1-positive macrophages/microglia and inhibition of pro-inflammatory cytokines [16, 22-24]. This suggests that blocking visfatin may be a promising therapeutic target for treatment of pain by attenuating the inflammatory cytokine cascade associated with inflammatory conditions, including obesity.
The aim of this study was to investigate the effects of inhibition of visfatin after treatment with FK866, on development of pain and inflammation in two models of inflammation; an acute experimentally-induced pain model (carrageenan) and a chronic low-grade inflammation model induced by feeding a high fat diet (HFD) for 3 months (model of dietary-induced obesity). The expression of visfatin was also characterized in peripheral and central tissues in both models.
Materials and Methods
I Animals
Adult male Wistar rats (n = 78; 260 - 440 g) were obtained from the Bioscience unit at Glasgow Caledonian University. Animals were housed in plastic cages (3 rats per cage) under a 12h light/12h dark schedule in a humidity-controlled room. Animals had access to food and water ad libitum and monitored daily. All studies were approved by the Institute’s Ethics and Welfare Committee and all procedures were in accordance with the UK Animals Scientific Procedures Act (1986). Animals were treated in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals as issued by the International Association for the Study of Pain.
II Characterization of a Model of Pre-Diabetic Obesity
Adult male Wistar rats (n = 12; 267 - 390 g) were fed either a normal diet (ND; RM1; 2.9% of fat; Special Diet Services, UK; n = 6), or high fat diet (HFD; 22%; #821424; Special Diet Services, UK; n = 6) for 12 weeks. Body weight and blood glucose levels from non-fasting rat tail vein was measured weekly from week 0 to week 12 using an electrical scale and Accu-Chek Aviva Glucometer, respectively. Thermal and mechanical nociceptive responses to hind paw stimulation were recorded every second week for the duration of the study. At the end of the study (week 12) rats were fasted for 8 hours before being terminally anaesthetized by i.p. injection of pentobarbital sodium (5 mg/100 g; pharmasol, JM Loveridge PLC, Southampton, UK) and abdominal white adipose tissue and spinal cord tissues were collected and stored at -80°C. Blood (5 ml collected by cardiac puncture) was also collected, and serum separated by centrifugation and stored and stored at -80°C.
III Measurements
For the nociception recordings, animals were placed in the recording apparatus (thermal and mechanical nociception) for 20 minutes for 5 consecutive days to acclimatize the rat to the apparatus. Behavioural testing for rats was performed between the hours of 0900 and 1200. Pre-readings (baseline readings) were taken 24 hours prior to start of the study (time 0). The Hargreaves test was used to measure thermal response latency (in seconds) (Harvard Apparatus, UK). Mechanical response thresholds (in grams/Force) were measured using the Ugo Basile dynamic plantar aesthesiometer (Harvard Apparatus, UK). Three or four readings were taken from each hind paw, and the average of these readings taken. Paw volume (cm3) of both hind paws was measured at 0, 6 and 24 h post-carrageenan using an Ugo Basile plethysmometer (Linton Instrumentation, UK). Paw oedema is represented as mean percentage difference in volume between ipsilateral (left) and contralateral (right) paw.
IV Metabolic Measurements
Total fasting-plasma triglyceride levels were measured using a Triglyceride (Trigs) determination kit (Randox, UK), cholesterol measured using a Cholesterol Quantitation Kit (Sigma-Aldrich, UK), and insulin levels assayed using an Insulin Rat Insulin ELISA Kit (Thermo Scientific, UK), all following the manufacturer’s protocol.
V Characterisation of Visfatin Expression in Serum, White Adipose and Spinal Cord Tissue
Levels of visfatin mRNA in spinal cord and white adipose tissue were measured using TaqMan semi-quantitative real-time PCR, on the CFX96 Real-Time PCR detection System (Bio-Rad, UK). Each cDNA sample was tested in duplicate and all PCR reactions contained 10 µl Master Mix (Primer design, UK), 6.5 µl molecular biology grade water (Fisher Scientific, UK), 0.75 µl all of each of forward (10 pmol/μl) and reverse primer (10 pmol/μl) and 1 μl of FAM/TAMRA dual labelled probe (5 pmol/μl) (Primer design, UK). For relative quantification of mRNA, the comparative ΔΔCt method was used (Applied Biosystems; User Bulletin 2). The visfatin Enzyme Immunoassay (EIA) Kit (Sigma Aldrich, UK) was used to measure visfatin peptide levels in serum, following the manufacturer’s protocol.
VI The Effect of FK866 on Carrageenan-Induced Inflammation in Normal Rats
Adult male Wistar rats (n = 22; 260 - 313 g) fed a normal diet (ND; RM1; 2.9% of fat; Special Diet Services, UK) were injected intraperitoneally (i.p.) with either vehicle (0.5 ml/kg; control group, n = 8), 3 mg/kg FK866 (n = 6) or 10 mg/kg FK866 (n = 8) 15 minutes before carrageenan injection (3 mg/ml; i.d.) into the left hind paw. A separate group of rats were terminally anaesthetized 6 hours post carrageenan or saline (n = 6/group; i.d.) and abdominal white adipose tissue and spinal cord tissues were processed for expression of visfatin mRNA using Taqman semi-quantitative real-time PCR, as above.
VII The Effect of FK866 on Carrageenan-Induced Inflammation in Obese Rats
Adult male Wistar rats (n = 18; 313 - 440 g) were fed either ND (control group; n = 6), or high fat diet (HFD; n = 6/group) for 12 weeks. The effects of carrageenan on ND fed rats injected with vehicle (0.5 ml/kg; i.p.), and HFD rats injected with either vehicle (0.5 ml/kg; i.p.) or FK866 (10 mg/kg; i.p.) 15 minutes before carrageenan injection (3 mg/ml; i.d.) into the left hind paw. Body weight was measured every four weeks from week 0 to week 12 using an electrical scale. Blood glucose levels from non-fasting rat tail vein were also measured on week 0 then 12 weeks using the Accu-Chek Aviva Glucometer.
VIII Drugs
FK866 (Tocris, UK) was dissolved in 2 ml of 10% dimethyl sulfoxide solvent (DMSO). Carrageenan (λ-carrageenan type IV; Sigma, UK) was dissolved in phosphate-buffered saline (Thermo Fisher Scientific, UK) to make a 3% solution 24 h before the start of each study and stored at 4ºC. Rats received a 50 µl intradermal injection of carrageenan or saline into the plantar surface of the left hind paw and responses to thermal and mechanical hind paw stimulation were measured before (time 0) and 2, 4, 6 and 24 h post-carrageenan.
IX Statistical Analysis
Statistical analyses were performed using GraphPad Prism™ (v6.05; UK). Data were analysed using a repeated measures ANOVA test to compare control and experimental groups over time. Body weight and blood glucose were analysed using a t-test to compare control and obese groups. One-way ANOVA test was used to measure carrageenan hyperalgesia in control rats over 24 hours. The maximum effect (Emax) was also calculated for each animal as the maximum change in response or paw volume (after treatment) from baseline. These data were analysed using the one-way ANOVA with post-hoc Tukey’s tests. A value of p < 0.05 was considered significant.
Results
I Characterization of a Model of Pre-Diabetic Obesity
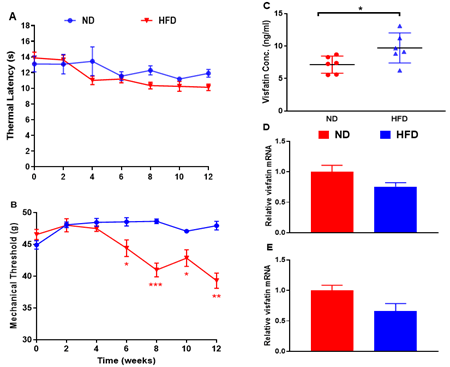
Metabolic Measurements: Rats fed a HFD gained significantly more body weight by 12 weeks (P < 0.01 vs normal diet (ND)-fed rats) but remained normoglycaemic (Table 1). Levels of circulating insulin and cholesterol at 12 weeks were significantly increased in HFD-fed rats (P < 0.01 vs ND-fed rats), while triglycerides remained unchanged (Table 1). Acute Nociception: Rats fed a HFD showed a significant reduction in mechanical withdrawal thresholds at 6 weeks, which persisted until the end of study (all P < 0.05 vs. ND-fed rats; Figure 1B). There was no change in thermal responses (Figure 1A). Visfatin Expression: Levels of circulating visfatin measured by ELISA were significantly increased at 12 weeks in HFD-fed rats (P < 0.05; Figure 1C). Visaftin mRNA was detected in white adipose tissue (Figure 1D) and spinal cord (Figure 1E) but no difference in expression was observed between ND and HFD-fed rats.
Figure 1: Characterisation of a model of pre-diabetic obesity. A) Thermal response latencies (in seconds) and B) mechanical thresholds (in grams) measured in the hind paws of normal diet (ND)- and high fat diet (HFD)-fed rats over 12 weeks. C) Visfatin levels in circulation, D) mRNA in white adipose tissue and E) spinal cord were measured in ND- and HFD-fed rats at 12 weeks. Expression of mRNA levels is expressed relative to the housekeeping gene cyclophilin. All data are mean ± SEM for n = 6 per group: * P < 0.05; **P < 0.01; *** P < 0.001 vs. ND.
Table
1: End
point total weight gain (g) and percentage weight gain (%), serum glucose,
cholesterol, triglyceride (TAG) (mmol/l), and insulin (ng/ml) levels measured
in rats fed a normal Diet (ND) or high fat diet (HFD) for 12 weeks (n = 6 per
group). Significant difference (t-test): ** P < 0.01; *** P < 0.001 vs.
ND rats.
|
Title 1 |
ND-fed rats |
HFD-fed rats |
|
141 ±17.07 |
168 ± 22.6 |
|
|
Body
weight gain (%) |
39.7
± 3.2% |
54.1
± 1.6%** |
|
Glucose
(mmol/L) |
7.1
± 0.2 |
7.5
± 0.2 |
|
Insulin
(ng/ml) |
0.14
± 0.01 |
1.56
± 0.16*** |
|
Cholesterol
(mmol/L) |
4.2
± 0.4 |
6.4
± 0.3** |
|
TAG
(mmol/L) |
0.97
± 0.16 |
1.32
± 0.16 |
II The Effect of FK866 on Carrageenan-Induced Inflammation in Normal Rats
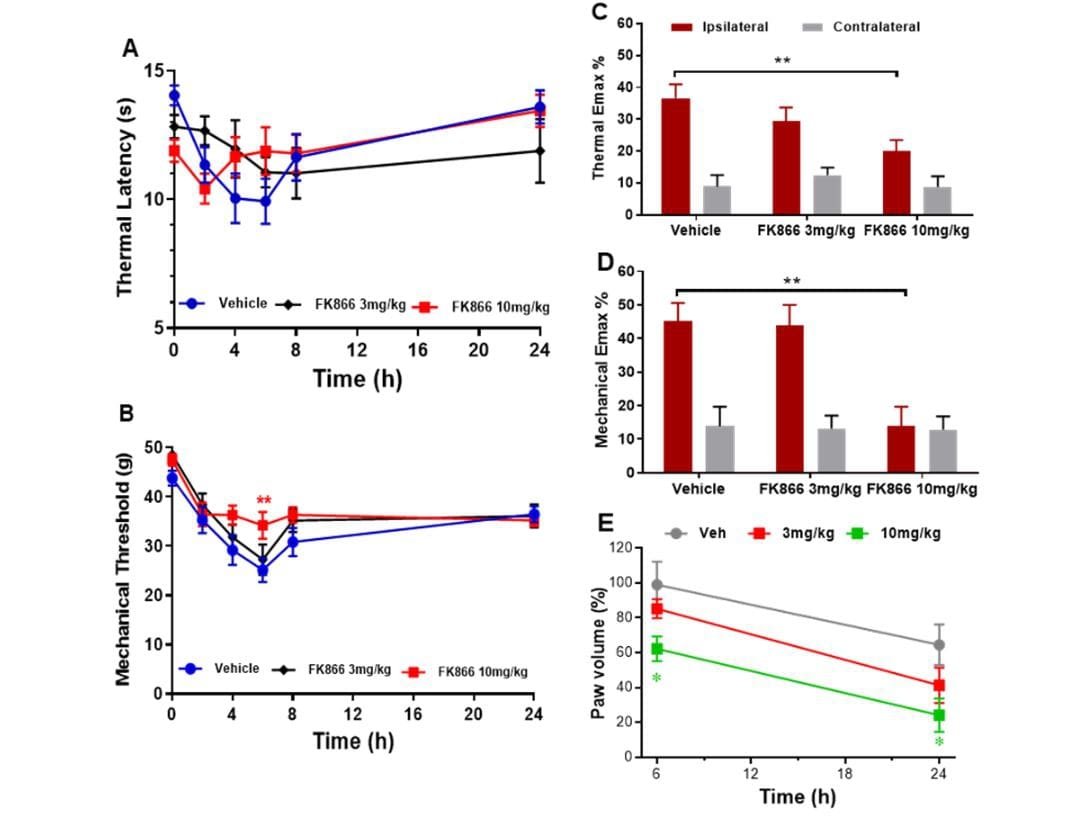
Intraplantar injection of carrageenan into the hind paw induced significant thermal and mechanical hyperalgesia at 2, 4, 6, and 8 h post-carrageenan in vehicle treated animals (One-way ANOVA: P < 0.01 vs. baseline; Figure 2A); maximum hyperalgesia was observed 6 h post-carrageenan (P < 0.001 vs. baseline). Repeated measures ANOVA showed pre-administration of 10 mg/kg FK866 (but not 3 mg/kg) significantly attenuated mechanical hyperalgesia at 6 h (P < 0.01 vs. vehicle; Figure 2B). An analysis of Emax revealed a significant attenuation of carrageenan-induced thermal and mechanical hyperalgesia by FK866 (both P < 0.01 for 10 mg/kg vs. vehicle; Figure 2C & 2D). Paw oedema was also observed 6 and 24 h post-carrageenan in vehicle treated animals (increase of 99 ± 13.2% and 64.5 ± 11.7%, respectively; P < 0.02, P < 0.01 vs. contralateral paw, respectively; Figure 2E). Pre-administration 10 mg/kg FK866 (but not 3 mg/kg) significantly reduced paw oedema to 62.3 ± 7.1% at 6 hours (P < 0.05 vs. vehicle) and 24.1 ± 9.6 % at 24 hours (P < 0.01 vs. vehicle; Figure 2E).
Figure 2: The effect of intraperitoneal administration of FK866 (3 and 10 mg/kg) or drug vehicle (DMSO), 15 minutes pre-carrageenan on A & C) thermal and B & C) mechanical hyperalgesia and D) paw oedema in normal Wistar rats. Mean thermal latency (in seconds; A) and mechanical response threshold (in grams; B) in the ipsilateral hind paw at 0, 2-, 4-, 6-, 8- and 24-hours post-carrageenan. Maximum change in thermal and mechanical responses (C) in the ipsilateral and contralateral paw post-carrageenan are represented as mean % change (Emax %) from baseline responses. Significant attenuation of hyperalgesia by 10 mg/kg FK866: ** P < 0.01 vs. vehicle. E) Paw oedema is represented as mean % change in paw volume from the contralateral paw at 6 and 24 hours. Significant attenuation of paw oedema by 10 mg/kg FK866: * P < 0.05 vs. vehicle.
III The Effect of FK866 on Carrageenan-Induced Inflammation in Obese Rats
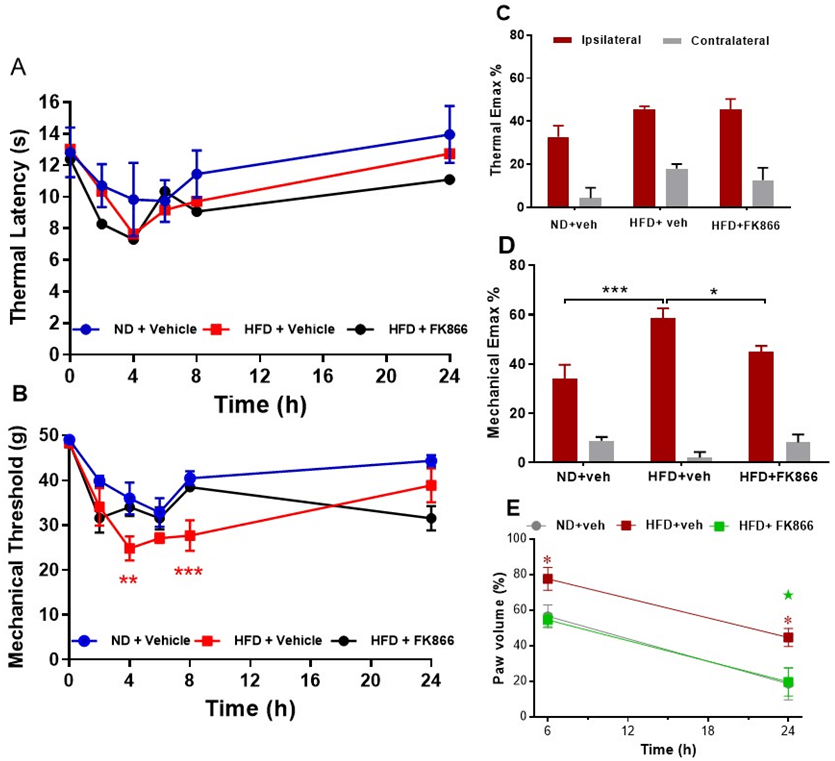
Rats fed a HFD for 12 weeks remained normoglycaemic but gained significantly more weight than ND-fed rats, in line with the previous study (both groups P < 0.001; Table 2). Carrageenan induced significant thermal and mechanical hyperalgesia in ND-fed rats, as expected, but induced a potentiated mechanical hyperalgesia in HFD-fed rats at 4 h (P < 0.01 vs. ND-fed rats) and 8 h (P < 0.001 vs. ND fed rats) post-carrageenan (Figure 3B). An analysis of Emax confirmed augmented carrageenan-induced mechanical hyperalgesia (P < 0.001 vs. ND-fed rats; Figure 3D). There was no change in thermal hyperalgesia in HFD-fed rats compared to ND-fed rats (Figures 3A & 3C). Carrageenan induced paw oedema was also significantly increased in HFD-fed rats at 6 and 24 h post-carrageenan (both P < 0.05 vs. ND-fed rats; Figure 3E). Treatment with FK866 attenuated potentiated mechanical hyperalgesia (P < 0.05 vs. HFD + vehicle; Figure 3D) and paw swelling in HFD-fed rats at 24 h (P < 0.05 vs. HFD + vehicle; Figure 3E).
Figure 3: The effect of intraperitoneal administration of FK866 (10 mg/kg) or drug vehicle (DMSO) 15 minutes pre-carrageenan on B & C) thermal and mechanical hyperalgesia and D) paw oedema in normal diet (ND) and high fat diet (HFD) fed rats. A) Mean thermal latency (in seconds); and mechanical response threshold (in grams); B) measured in the ipsilateral hind paw at 0, 2-, 4-, 6-, 8- and 24-hours post-carrageenan. Maximum change in thermal and mechanical responses (C) in the ipsilateral and contralateral paw post-carrageenan are represented as mean % change (Emax %) from baseline responses. Significant difference between groups: *P < 0.05; ** P < 0.01; ***P < 0.001 vs. vehicle. E) Paw oedema is represented as mean % change in paw volume from the contralateral paw at 6 and 24 hours. Significant increase in paw oedema in HFD rats: *P < 0.05 vs. ND + vehicle. Significant attenuation of paw oedema by FK866: ★ P < 0.05 vs. HFD + vehicle.
Table 2: End point
total weight gain (g) and serum glucose levels (mmol/l) measured in rats (n = 6
per group) fed a normal diet (ND) for 12 weeks and injected with vehicle, and
rats fed a high fat diet (HFD) for 12 weeks and injected with vehicle or FK866
(10 mg/kg). Significant difference (one-way ANOVA): *** P < 0.001 vs. ND
rats.
|
|
ND + Vehicle |
HFD + Vehicle |
HFD + FK866 |
|
Body weight gain
(g) |
62 ± 6.5 |
183 ± 19*** |
195 ± 15*** |
|
Body weight gain
(%) |
17 ± 4.8% |
50 ± 15%*** |
49± 8.7%*** |
|
Glucose (mmol/L) |
3.9 ± 0.2 |
4.1 ± 0.2 |
4.3 ± 0.15 |
IV Expression of Visfatin mRNA in Acute Inflammation
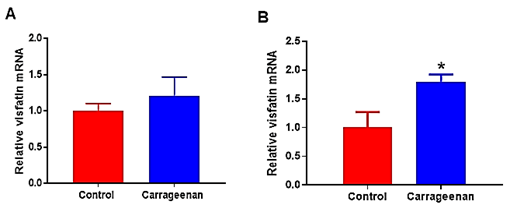
Analysis of levels of visfatin mRNA in spinal cord from ND-fed rats 6 hours post-carrageenan or saline injection revealed a significant up-regulation of visfatin mRNA in spinal cord (1.8-fold increase; P < 0.02 vs. saline-control). There was no change in levels of visfatin mRNA in white adipose tissue between groups (Figure 4).
Figure 4: Visfatin mRNA expression in A) white adipose tissue and in B) spinal cord tissues of normal rats (Control; n = 6) injected intraperitoneally with vehicle or carrageenan (carrageenan; n = 6). Expression of mRNA levels are expressed relative to the housekeeping gene, cyclophilin. Data are presented as mean ± sem. Significant difference between groups: * P < 0.05 vs. control rats.
Discussion
In this study, feeding rats a high fat diet for 12 weeks induced a pre-diabetic phenotype, characterised by increased body weight, hyperinsulinaemia and hypercholesterolaemia but normoglycaemia and normotriglyceridaemia. These rats displayed an increased sensitivity to acute mechanical stimulation, and potentiated mechanical hyperalgesia and peripheral inflammatory response to carrageenan. Levels of circulating visfatin were also increased in these rats, indicative of a pro-inflammatory state and indicating that this mediator may be linked to altered pain processing observed in these rats. Further investigations into the functional role of visfatin in this model, revealed that treatment with FK866, a visfatin inhibitor, was effective in reducing carrageenan-induced hyperalgesia in both normal and high-fat diet fed rats.
It is now well established that pain and obesity are closely related, and that the incidence of pain conditions is higher in obese individuals [5, 25]. Obese animals also display increased pain sensitivity to acute nociceptive stimuli, although responses are variable with some studies reporting no difference in sensitivity to noxious stimulation or reduced sensitivity in obese animals [26-29]. In the present study, rats fed a high fat diet displayed increased mechanical sensitivity by 6 weeks, before significant weight gain, suggesting that changes in diet even in the short-term may have an impact on nociceptive behaviour. Perhaps more striking is the evidence that inflammatory pain and peripheral inflammation are potentiated in obese animals. In the current study, rats fed a high fat diet displayed enhanced paw swelling and mechanical hyperalgesia in response to carrageenan compared to normal diet-fed rats, which is similar to the finding reported previously in this laboratory utilizing obese Zucker rats [28]. Similarly, feeding rats a high-fat diet has been reported to induce visceral hypersensitivity, increased post-operative pain, and increased paw oedema in diet-induced obese rats after intraplantar injection of complete Freund’s adjuvant, a model of arthritis [27, 30, 31]. These studies confirm that exposure to high-fat diet and resulting obesity induces alterations in both central and peripheral pain processing and enhanced inflammatory pain similar to that often reported in patients with obesity.
A number of factors, including increased weight bearing, altered body mechanics, lifestyle factors, disturbed sleep and depression have been linked to co-morbid pain with obesity [5]. Underlying these phenomena appears to be a dysregulation in pro-inflammatory mediators and associated activation of the immune system. The increased level of circulating visfatin observed in high fat-diet fed rats in the current study adds to the growing list of inflammatory biomarkers reported to be increased with obesity, and similarly seen in a range of painful conditions [32-34]. Previous studies have identified visfatin as a marker of inflammation; a meta-analysis carried out by Chang et al. showed that serum visfatin levels were increased in individuals with obesity, type 2 diabetes mellitus, metabolic syndrome and cardiovascular diseases [10, 35, 36]. This study also identified a positive correlation between visfatin levels and insulin resistance in line with the current findings in high fat diet-fed rats. Visfatin promotes inflammation directly through activation of the STAT3/NFκB pathway, and production of pro-inflammatory cytokines, and it’s this mechanism that likely contributes to increased pain observed in high fat diet-fed rats [37, 38]. Interestingly, serum levels of visfatin and nociception were both reduced in an experimental peripheral neuropathic pain model in mice after treatment with pregabalin [39].
Alterations in inflammatory cytokine production are not restricted to the periphery and resulting systemic inflammation. Growing evidence supports alterations in central immune signaling in pain with obesity. For instance, a recent study reported elevated macrophage levels in lumbar dorsal root ganglion in high fat diet-fed rats with enhanced post-operative pain, while another study reported activation of microglia in brain areas related to increased visceral pain in diet-induced obesity [30, 31]. Indeed, visfatin was reported to activate hypothalamic microglial cells, known to be involved in modulation of nociceptive transmission under pathological conditions and to release of neurotoxic factors [19, 40, 41]. In the present study, visfatin mRNA was detected in spinal cord tissue, a key relay station for pain signaling, and although there was no change in expression with obesity, we did see an increase in expression in spinal cord at 6 hours post-carrageenan, presumably as a consequence of the afferent barrage from the inflamed paw. It is interesting to speculate on a function role for visfatin in spinal cord tissue, and the down-stream effects on cytokine signaling; further studies localizing visfatin to specific cell types in this region are warranted.
Visfatin blockers have shown promise as therapeutic agents in a number of conditions. FK866 competitively inhibits NAD enzymatic activity by binding to the active site formed by the dimer [42]. Functional inhibition of visfatin by FK866 in the current study produced anti-inflammatory and anti-hyperalgesic effects. FK866 significantly attenuated carrageenan-induced thermal and mechanical hyperalgesia and reduced paw oedema in both normal and high-fat diet fed rats. This result supports a previous finding, which demonstrated that FK866 injection after spinal cord injury is effective in reducing secondary inflammatory injury and partly reducing permanent damage in the spinal cord [22]. Blocking visfatin has been reported to inhibit production of inflammatory cytokines by human monocytes and reduce inflammation in collagen-induced arthritis [43, 44]. Another recent study reported that FK866 blocks the increase of pro-inflammatory cytokines induced by LPS by reversing activation of the STAT3/NFκB pathway [45]. These results indicate a key role for visfatin in modulating inflammatory pain. Furthermore, the reduction in potentiated hyperalgesia and paw swelling in high-fat diet fed rats, with elevated circulating visfatin levels, supports a role for visfatin in enhanced pain with obesity. Further studies will confirm if anti-visfatin therapies can alleviate inflammatory pain and the increased incidence of painful conditions with obesity.
Acknowledgment
This paper has been taken out from the Ph.D. degree thesis and we thank second supervisor Professor Annette Graham.
Funding
This research was funded by Saudi Arabian Cultural Bureau in London.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 08, Jul 2021Accepted: Sat 24, Jul 2021
Published: Mon 09, Aug 2021
Copyright
© 2023 Sharron Dolan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2021.03.01
Author Info
Corresponding Author
Sharron DolanDepartment of Biological and Biomedical Sciences, Glasgow Caledonian University, Glasgow, UK
Figures & Tables
Table
1: End
point total weight gain (g) and percentage weight gain (%), serum glucose,
cholesterol, triglyceride (TAG) (mmol/l), and insulin (ng/ml) levels measured
in rats fed a normal Diet (ND) or high fat diet (HFD) for 12 weeks (n = 6 per
group). Significant difference (t-test): ** P < 0.01; *** P < 0.001 vs.
ND rats.
|
Title 1 |
ND-fed rats |
HFD-fed rats |
|
141 ±17.07 |
168 ± 22.6 |
|
|
Body
weight gain (%) |
39.7
± 3.2% |
54.1
± 1.6%** |
|
Glucose
(mmol/L) |
7.1
± 0.2 |
7.5
± 0.2 |
|
Insulin
(ng/ml) |
0.14
± 0.01 |
1.56
± 0.16*** |
|
Cholesterol
(mmol/L) |
4.2
± 0.4 |
6.4
± 0.3** |
|
TAG
(mmol/L) |
0.97
± 0.16 |
1.32
± 0.16 |
Table 2: End point
total weight gain (g) and serum glucose levels (mmol/l) measured in rats (n = 6
per group) fed a normal diet (ND) for 12 weeks and injected with vehicle, and
rats fed a high fat diet (HFD) for 12 weeks and injected with vehicle or FK866
(10 mg/kg). Significant difference (one-way ANOVA): *** P < 0.001 vs. ND
rats.
|
|
ND + Vehicle |
HFD + Vehicle |
HFD + FK866 |
|
Body weight gain
(g) |
62 ± 6.5 |
183 ± 19*** |
195 ± 15*** |
|
Body weight gain
(%) |
17 ± 4.8% |
50 ± 15%*** |
49± 8.7%*** |
|
Glucose (mmol/L) |
3.9 ± 0.2 |
4.1 ± 0.2 |
4.3 ± 0.15 |
References
1. Bray GA, Bellanger
T (2006) Epidemiology, trends, and morbidities of obesity and the metabolic
syndrome. Endocrine 29: 109-117. [Crossref]
2. Fujioka K (2015)
Current and emerging medications for overweight or obesity in people with
comorbidities. Diabetes Obes Metab 17: 1021-1032. [Crossref]
3. Hitt HC, McMillen
RC, Thornton Neaves T, Koch K, Cosby AG (2007) Comorbidity of obesity and pain
in a general population: results from the Southern Pain Prevalence Study. J
Pain 8: 430-436. [Crossref]
4. Thijssen E, Van
Caam A, van der Kraan PM (2015) Obesity and osteoarthritis, more than just wear
and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in
obesity-induced osteoarthritis. Rheumatology (Oxford) 54: 588-600. [Crossref]
5. Okifuji A, Hare BD
(2015) The association between chronic pain and obesity. J Pain Res 8:
399-408. [Crossref]
6. Balistreri CR,
Caruso C, Candore G (2010) The role of adipose tissue and adipokines in
obesity-related inflammatory diseases. Mediators Inflamm 2010: 802078. [Crossref]
7. Han JM, Levings MK
(2013) Immune regulation in obesity-associated adipose inflammation. J
Immunol 191: 527-532. [Crossref]
8. Louati K, Berenbaum
F (2015) Fatigue in chronic inflammation - a link to pain pathways. Arthritis
Res Ther 17: 254. [Crossref]
9. Jacques C, Holzenberger M, Mladenovic Z, Salvat C, Pecchi E et al. (2012)
Proinflammatory actions of visfatin/nicotinamide phosphoribosyltransferase
(Nampt) involve regulation of insulin signaling pathway and Nampt enzymatic
activity. J Biol Chem 287: 15100-15108. [Crossref]
10. Kabir F, Jahan FA,
Khan I, Faruque MO, Hassan Z et al. (2015) Increased concentration of
circulating visfatin associates with post-challenged hyperglycaemia and insulin
resistance in IGT subjects. J Taibah Univ Med Sci 10: 481-487.
11. Fukuhara A, Matsuda
M, Nishizawa M, Segawa K, Tanaka M et al. (2005) Visfatin: a protein secreted
by visceral fat that mimics the effects of insulin. Science 307:
426-430. [Crossref]
12. Nakajima TE, Yamada
Y, Hamano T, Furuta K, Gotoda T et al. (2009) Adipocytokine levels in gastric
cancer patients: resistin and visfatin as biomarkers of gastric cancer. J
Gastroenterol 44: 685-690. [Crossref]
13. Oki K, Yamane K,
Kamei N, Nojima H, Kohno N (2007) Circulating visfatin level is correlated with
inflammation, but not with insulin resistance. Clin Endocrinol (Oxf) 5:
796-800. [Crossref]
14. Kocot J, Dziemidok
P, Kiełczykowska M, Hordyjewska A, Szcześniak G et al. (2017) Adipokine Profile
in Patients with Type 2 Diabetes Depends on Degree of Obesity. Med Sci Monit
23: 4995-5004. [Crossref]
15. Kabir F, Haque SA,
Haque K (2018) Serum Visfatin Levels Estimated in Overweight Individuals. Anwer
Khan Mod Med Coll J 9: 91-95.
16. Chen CX, Huang J,
Tu GQ, Lu JT, Xie X et al. (2017) NAMPT inhibitor protects ischemic neuronal
injury in rat brain via anti-neuroinflammation. Neuroscience 356:
193-206. [Crossref]
17. Wang P, Xu TY, Guan
YF, Tian WW, Viollet B et al. (2011) Nicotinamide phosphoribosyltransferase
protects against ischemic stroke through SIRT1-dependent adenosine
monophosphate-activated kinase pathway. Ann Neurol 69: 360-374. [Crossref]
18. Chen F, Weng Z, Xia
Q, Cao C, Leak RK et al. (2019) Intracerebroventricular Delivery of Recombinant
NAMPT Deters Inflammation and Protects Against Cerebral Ischemia. Transl
Stroke Res 10: 719-728. [Crossref]
19. Tu TH, Nam Goong
IS, Lee J, Yang S, Kim JG (2017) Visfatin Triggers Anorexia and Body Weight
Loss through Regulating the Inflammatory Response in the Hypothalamic
Microglia. Mediators Inflamm 2017: 1958947. [Crossref]
20. Présumey J,
Courties G, Louis Plence P, Escriou V, Scherman D et al. (2013) Nicotinamide
phosphoribosyltransferase/visfatin expression by inflammatory monocytes
mediates arthritis pathogenesis. Ann Rheum Dis 72: 1717-1724. [Crossref]
21. Halvorsen B,
Espeland MZ, Andersen GØ, Yndestad A, Sagen EL et al. (2015) Increased
expression of NAMPT in PBMC from patients with acute coronary syndrome and in
inflammatory M1 macrophages. Atherosclerosis 243: 204-210. [Crossref]
22. Esposito E, Impellizzeri D, Mazzon E, Fakhfouri G, Rahimian R et al. (2012) The NAMPT
inhibitor FK866 reverts the damage in spinal cord injury. J
Neuroinflammation 9: 66. [Crossref]
23. Matsuda A, Yang WL,
Jacob A, Aziz M, Matsuo S et al. (2014) FK866, a visfatin inhibitor, protects
against acute lung injury after intestinal ischemia-reperfusion in mice via
NF-κB pathway. Ann Surg 259: 1007-1017. [Crossref]
24. Zhang XQ, Lu JT,
Jiang WX, Lu YB, Wu M et al. (2015) NAMPT inhibitor and metabolite protect
mouse brain from cryoinjury through distinct mechanisms. Neuroscience
291: 230-240. [Crossref]
25. Arranz LI, Rafecas
M, Alegre C (2014) Effects of obesity on function and quality of life in
chronic pain conditions. Curr Rheumatol Rep 16: 390. [Crossref]
26. Roane DS, Porter JR
(1986) Nociception and opioid-induced analgesia in lean (Fa/-) and obese
(fa/fa) Zucker rats. Physiol Behav 38: 215-218. [Crossref]
27. Croci T, Zarini E
(2007) Effect of the cannabinoid CB1 receptor antagonist rimonabant on
nociceptive responses and adjuvant-induced arthritis in obese and lean rats. Br
J Pharmacol 150: 559-566. [Crossref]
28. Iannitti T, Graham
A, Dolan S (2012) Increased central and peripheral inflammation and inflammatory
hyperalgesia in Zucker rat model of leptin receptor deficiency and genetic
obesity. Exp Physiol 97: 1236-1245. [Crossref]
29. Rossi HL, Luu AKS,
Kothari SD, Kuburas A, Neubert JK et al. (2013) Effects of diet-induced obesity
on motivation and pain behavior in an operant assay. Neuroscience 235:
87-95. [Crossref]
30. Tramullas M, Finger
BC, Dinan TG, Cryan JF (2016) Obesity takes its toll on visceral pain: High-fat
diet induces toll-like receptor 4-dependent visceral hypersensitivity. PLoS
One 11: e0155367. [Crossref]
31. Song Z, Xie W,
Strong JA, Berta T, Ulrich Lai YM et al. (2018) High-fat diet exacerbates
postoperative pain and inflammation in a sex-dependent manner. Pain 159:
1731-1741. [Crossref]
32. Blüher M, Fasshauer
M, Tönjes A, Kratzsch J, Schön MR et al. (2005) Association of interleukin-6,
C-reactive protein, interleukin-10 and adiponectin plasma concentrations with
measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin
Endocrinol Diabetes 113: 534-537. [Crossref]
33. Okifuji A, Bradshaw
DH, Olson C (2009) Evaluating obesity in fibromyalgia: neuroendocrine
biomarkers, symptoms, and functions. Clin Rheumatol 28: 475-478. [Crossref]
34. DeVon HA, Piano MR,
Rosenfeld AG, Hoppensteadt DA (2014) The association of pain with protein
inflammatory biomarkers: a review of the literature. Nurs Res 63: 51-62.
[Crossref]
35. Aslani MR,
Keyhanmanesh R, Alipour MR (2017) Increased Visfatin Expression Is Associated
with Nuclear Factor-κB in Obese Ovalbumin-Sensitized Male Wistar Rat Tracheae. Med
Princ Pract 26: 351-358. [Crossref]
36. Chang YH, Chang DM,
Lin KC, Shin SJ, Lee YJ (2011) Visfatin in overweight/obesity, type 2 diabetes
mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a
meta-analysis and systemic review. Diabetes Metab Res Rev 27: 515-527. [Crossref]
37. Moschen AR, Kaser
A, Enrich B, Mosheimer B, Theurl M et al. (2007) Visfatin, an adipocytokine
with proinflammatory and immunomodulating properties. J Immunol 178:
1748-1758. [Crossref]
38. Singh M, Benencia F
(2017) Inflammatory signals from fat: Visfatin promotes pro-inflammatory
activation and leukocyte interaction in endothelial cells. J Immunol
198: 206.24.
39. Darwish IES,
Dessouky IS (2015) Does Serum Visfatin Represent a Biochemical Marker to an
Experimental Peripheral Neuropathic Pain in Mice. Pharmacology 96:
248-252. [Crossref]
40. Ikeda H, Kiritoshi
T, Murase K (2012) Contribution of microglia and astrocytes to the central
sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol
Pain 8: 43. [Crossref]
41. Zhou LT, Wang KJ,
Li L, Li H, Geng M (2015) Pinocembrin inhibits lipopolysaccharide-induced
inflammatory mediators production in BV2 microglial cells through suppression
of PI3K/Akt/NF-κB pathway. Eur J Pharmacol 761: 211-216. [Crossref]
42. Wosikowski K,
Mattern K, Schemainda I, Hasmann M, Rattel B et al. (2002) WK175, a novel
antitumor agent, decreases the intracellular nicotinamide adenine dinucleotide
concentration and induces the apoptotic cascade in human leukemia cells. Cancer
Res 62: 1057-1062. [Crossref]
43. Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F et al. (2008)
Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin
enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS
One 3: e2267. [Crossref]
44. Evans L, Williams AS, Hayes AJ, Jones SA, Nowell M (2011) Suppression of leukocyte infiltration and cartilage degradation by selective inhibition of pre-B cell colony-enhancing factor/visfatin/nicotinamide phosphoribosyltransferase: Apo866-mediated therapy in human fibroblasts and murine collagen-induced arthritis. Arthritis Rheum 63: 1866-1877. [Crossref]
45. Lee YC, Lin CY, Chen YH, Chiu WC, Wang YY et al. (2019) Essential Role of Visfatin in Lipopolysaccharide and Colon Ascendens Stent Peritonitis-Induced Acute Lung Injury. Int J Mol Sci 20: 1678. [Crossref]
