Area Dose Response of Prevalent Childhood Thyroid Cancers after the Fukushima Nuclear Power Plant Accident
A B S T R A C T
Background and Methods: After the Fukushima Nuclear Power Plant accident, thyroid ultrasound screening was carried out on all residents of ages≤18 years at the accident. In order to find the cause for thyroid cancer prevalence, area dose dependence of childhood thyroid cancer proportion in the first and second round screening (E-I, E-II), and in 6 years as a whole (E-I+II) was analyzed for external dose estimated by Fukushima Health Management Survey and effective dose estimated by UNSCEAR. The results were analyzed by the regression analysis of Microsoft Excel.
Results: Thyroid cancer incidence within 4-6 years and prevalence in 6 years after the accident had tendency to increase with doses. In OM-model according to Ohira’s map, thyroid cancer prevalence in E-I+II after 6 years showed linear prevalence-effective dose response with positive coefficient 5.6 (CI=3.4-7.9) (cancer cases / 100,000 / mSv) in the 1.6–5 mSv range. In F-model according to official regional division, thyroid cancer proportion in E-II and E-I+II showed linear response to UNSCEAR effective dose with R2>0.86 and p<0.075. This correlation was higher than the correlation between external dose and effective dose with R2=0.71.
Conclusion: The observed linear area dose relation in 6 years after exposure suggests highly probable association between childhood thyroid cancer in Fukushima and radiation exposure. UNSCEAR effective dose was found to give the best fit to linear regression line for E-II and E-I+II, irrespective of models. Linear dose response for reasonable area division can be a direct evidence for radiation related thyroid cancer because linear dose response cannot be observed in mass-screening for sporadic thyroid cancers.
Keywords
Thyroid cancer, childhood, Fukushima, nuclear accident, ultrasound screening, prevalence–dose relation, incidence-dose response, external dose, effective dose
Introduction
After the radioactive fallout from Fukushima Daiichi Nuclear Power Plant (F1NPP) on March 11, 2011, Fukushima Prefecture initiated thyroid ultrasound screening for all residents aged ≤18 years at the time of the accident. The Fukushima Health Management Survey (FHMS) reported the results of the first round E-I (fiscal year FY2011-2013) and second round E-II (FY2014-2015) examinations, where 186 confirmed or suspected cancer cases were diagnosed [1]. Oversight Committee Meeting for FHMS evaluated that the prevalence thyroid cancer is several tens of times more than the one from thyroid cancer statistics, which may be because of diagnosing many cancers that will not be clinically diagnosed in the future [2]. The summary of FHMS was that thyroid cancer detected in E-I was unlikely to be associated with radiation exposure, and Suzuki et al. for the FHMS further concluded that the high prevalence of childhood thyroid cancer detected in the four-years study could be attributed to mass screening [3]. Yamamoto et al. reported that thyroid cancer detection rate and the radiation dose-rate in the 59 municipalities in the Fukushima prefecture showed statistically significant dose-response relationships [4]. They concluded that the radiation contamination due to the Fukushima nuclear power plant accidents is positively associated with the thyroid cancer detection rate in children and adolescents. Ohira et al. of Fukushima Medical University group (FMU) studied the associations between the incidence of childhood thyroid cancer and external radiation dose in the second-round screening E-II [5]. Their conclusions were 1st: individual external radiation dose was not associated with the incidence of thyroid cancer within 4 to 6 years after the nuclear power plant accident, 2nd: regional differences in external radiation dose were not associated with increased risk of thyroid cancer, and 3rd: obesity was positively associated with thyroid cancer incidence.
Contrary to their 1st conclusion, they showed positive correlation between thyroid cancer and individual external radiation dose in their analysis of the data of FHMS confined within the organization. They found the following: (a) The mean external radiation dose of participants with thyroid cancer in E-II, 1.1 mSv, was higher than the mean value 0.92 mSv of those without thyroid cancer (Table 1 in [5]). (b) The percentages with external radiation dose ≥ 1 mSv in participants with and without thyroid cancer were 58% and 42%, respectively. (c) Relative risk (RR, odds ratio) of thyroid cancer for participants with individual external radiation dose ≥ 2 mSv was twice as high, RR(CI)=2.09 (0.81-5.40) as compared with the one of participants with external dose <2 mSv. Higher radiation dose of detected thyroid cancer patients than the dose of participants without thyroid cancer (a, b) and higher risk of thyroid cancer in a group of high individual external radiation dose (c) suggested direct positive association between incidence of thyroid cancer and individual external radiation dose due to the accident. These discoveries seem to contradict with their conclusion of no association of individual external radiation dose with the incidence of thyroid cancer in E-II. FMU group concluded 2nd that regional differences in external radiation dose were not associated with increased risk of thyroid cancer in E-II. However, in our letter to the authors’ preceding paper, an analysis including the first and second-round examinations, E-I+II (FY2011-2015), revealed a linear relationship between thyroid cancer prevalence and external radiation dose after 6 years from the accident [6, 7]. The main purpose of this paper is to examine area dose response of thyroid cancer proportion on the basis of two models which divide Fukushima prefecture into 4 areas.
Methods
Subjects and Thyroid Ultrasound Screening
The results of thyroid ultrasound screening for all 367,649 residents aged ≤ 18 years on March 11, 2011, were reported for the first (E-I) and second (E-II) round examinations, where 150 confirmed and 36 suspected cancer cases (186 total) were detected [1]. In each examination, results of the primary examination are divided into four categories. A1: no nodule or cyst, A2: nodules <5.0 mm or cysts <20.0 mm, B: nodules >5.1 mm or cysts >20.1 mm, and C: immediate need for confirmatory examination [1]. Positives in confirmatory examination (B and C) underwent fine-needle aspiration cytology (FNAC) or medical follow-up. When cancer cells were detected by FNAC, the patient was followed and had surgery, where malignancy was finally confirmed.
Models dividing Fukushima Prefecture, area dose estimation, and statistical analysis
Area dose response of thyroid cancer proportion was analyzed on the basis of two models dividing Fukushima prefecture into 4 areas.
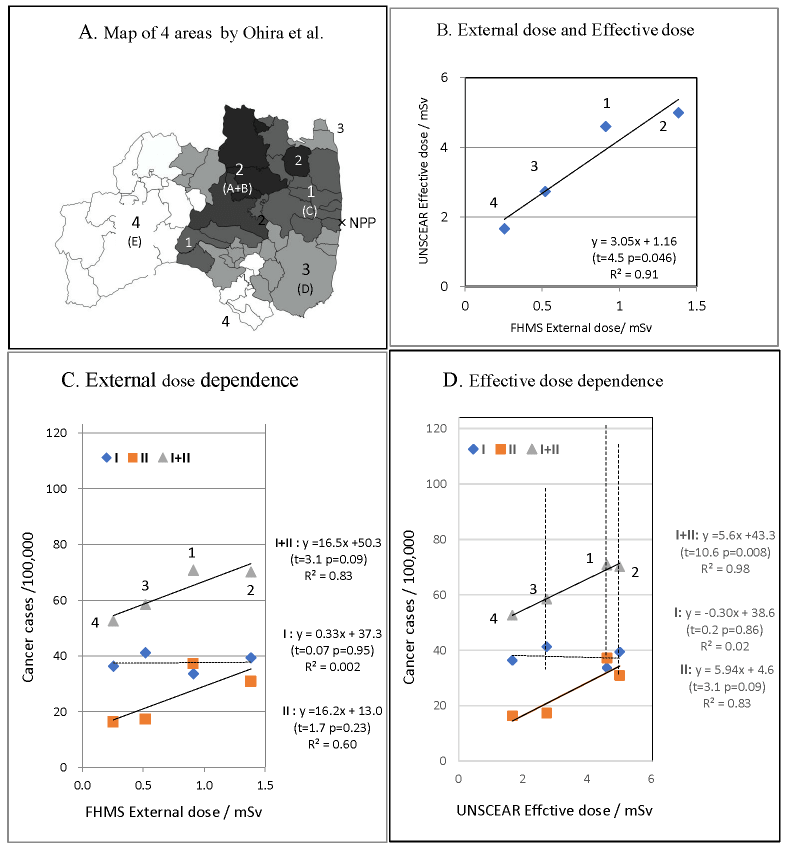
OM-model: Areas A and B were combined in the Ohira’s map of five areas A–E, which were classified according to the proportion of residents P’ (source of P’ was not clearly defined) with external exposure dose of ≥1 mSv, with borders of P’ = 66%, 55.4%, 5.7%, and 0.67%. Naming was changed; C to 1 the area including evacuation zone, A+B to 2 the central part Nakadori, D to 3 Iwaki and Soma area, and E to 4 the Aizu area. (Figure 1A) Four areas were characterized by P’(2) ≥ 55.4%>P’(1) ≥ 5.7%>P’(3) ≥ 0.67%>P’(4).
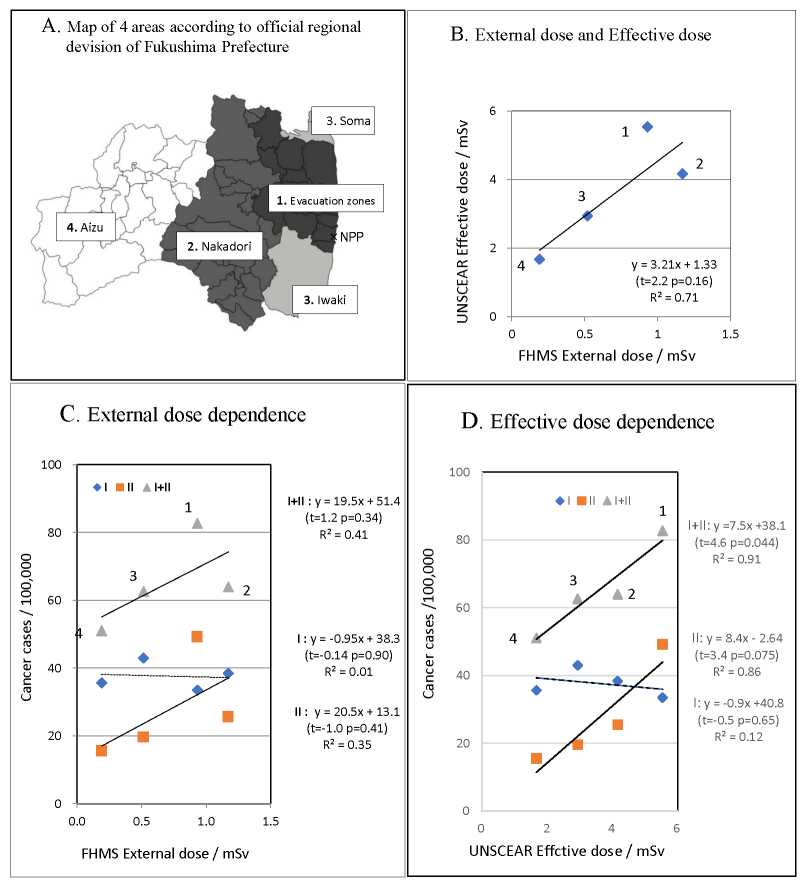
F-model: Official regional division adopted in FHMS reports of thyroid examination; 1. municipalities in evacuation zone, 2. Nakadori, 3. Iwaki and Soma, and 4. Aizu (Figure 2A) [1]. This model is important because FHMS canceled presentation of cancer cases by municipality from the third-round screening, and researchers outside FMU can only follow area dependence in the following rounds upon this model [1].
Enormous quantities of radionuclides were released into the environment following the F1NPP accident. Internal exposure of Iodine-131 (I-131) was found to be closely associated with thyroid cancer among children after the Chernobyl accident. Exposure information of I-131 from the Fukushima accident was uncertain because of the eight-day half-life of I -131 and few measurements of I-131 activity in the thyroid [8, 9]. While I-131 exposure is clearly important in thyroid cancer, external or effective radiation exposure can also be important. The study of CT scans among young people, where the exposure consisted entirely of external X-rays, showed that the increased risk of thyroid cancer was greater than the average increased risk for all solid cancers (Table 4 in ref. [10]). Dose response of thyroid cancer proportion was analyzed here for two independent dose estimations, external dose estimated in the Basic Survey by FHMS and effective dose, a sum of external, inhalation, and ingestion of 10-year-old children, estimated by the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) [1, 11, 12]. The external dose and effective dose of each area were estimated from the weighted average of dose of each municipality by the number of primary examinees in E-I, where the external dose for each municipality was an average of doses weighted by the number of the Basic Survey respondents. Dose responses of the four areas in two models were analyzed by simple regression analysis of Microsoft Excel. Odds ratio (OR) and 95% confidence interval (CI) of thyroid cancer proportion were estimated according to the formula of Rothman [13].
Results
Dose response of thyroid cancer in OM-model
External dose estimated from the FHMS basic survey, effective dose based on UNSCEAR report, number of primary examinees, confirmed or suspected thyroid cancer cases, thyroid cancer proportion per 100,000 people (CP), OR and CI in E-I, E-II, and E-I+II are listed in Table 1. Cancer proportion /100,000 in E-I+II is the sum of those for E-I and E-II. Because screening E-II was carried out for residents excluding confirmed or suspected cancer cases detected in E-I, cancer proportions CP-I and CP-I+II represent approximately the prevalence proportion after 4 years and 6 years of the nuclear accident, and CP-II represents approximately the incidence proportion of thyroid cancer detected in E-II after E-I. Dose response of cancer proportion CP-I, CP-II, and CP-I+II to FHMS external dose and UNSCEAR effective dose is shown in Figure 1 with regression line and statistics of regression analysis, R2, t- and p-values for slope parameters. Area dependence of CP-I was not observed because of the short elapsed-time from exposure to screening in high dose areas [4, 6]. Cancer proportions CP-II and CP-I+II showed tendency to increase with increasing FHMS external dose and UNSCEAR effective dose, except plot of CP-II versus external dose showed scatter from linearity (Figure 1C). Linear response to UNSCEAR effective dose was evident; CP-I+II increased linearly with positive coefficient 5.6 (CI=3.4-7.9) (cancer cases / 100,000 / mSv) (Figure 1D). Although CIs of cancer prevalence CP-I+II (broken lines) overlap each other, the plot shows perfect prevalence-effective dose response with p=0.01.
Table 1: External dose based on FHMS basic survey, effective dose based on UNSCESAR report, number of primary examinees, confirmed or suspected cancer cases, thyroid cancer proportion per 100,000 (CP), odds ratio (OR) and 95% confidence interval (CI) in E-I, E-II, and E-I+II in OM-model according to Ohira’s map.
|
Area† |
External |
Effective |
E-I (FY2011–2013) |
E-II (FY2014–2015) |
E-I+II (FY2011–2015) |
||||||||
|
mSv |
mSv |
Examinees |
Cases |
CP |
OR |
Examinees |
Cases |
CP |
OR (CI) |
Cases |
CP‡ |
OR (CI) |
|
|
1 (C) |
0.91 |
4.60 |
59,621 |
20 |
33.5 |
0.92 |
51,160 |
19 |
37.1 |
2.28(0.9-5.7) |
39 |
70.7 |
1.34(0.8-2.3) |
|
2 (A+B) |
1.38 |
4.99 |
119,648 |
47 |
39.3 |
1.08 |
107,107 |
33 |
30.8 |
1.89(0.8-4.5) |
80 |
70.1 |
1.33(0.8-2.2) |
|
3 (D) |
0.52 |
2.73 |
82,625 |
34 |
41.1 |
1.13 |
75,421 |
13 |
17.2 |
1.06(0.4-2.8) |
47 |
58.4 |
1.11(0.7-1.9) |
|
4 (E) |
0.25 |
1.66 |
38,579 |
14 |
36.3 |
1(Ref.) |
36,823 |
6 |
16.3 |
1 (Ref.) |
20 |
52.6 |
1 (Ref.) |
|
Ave. / Sum |
0.77 |
3.48 |
300,473 |
115 |
38.3 |
270,511 |
71 |
26.2 |
186 |
64.5 |
|||
† Area name in the parentheses is the name in Refs. [5,7]. ‡ CP in I + II is the sum of those for I and II.
† Area name in the parentheses is the name in Refs. [5,7]. ‡ CP in I + II is the sum of those for I and II.
Figure 1: Dose dependence of thyroid cancer proportion in OM-model
A). Map of four areas 1-4 in OM-model according to Ohira’s map. B). Correlation between FHMS external dose and UNSCEAR effective dose. C). Proportion of thyroid cancer per 100,000, CP-I, CP-II, and CP-I+II versus FHMS external dose with regression line. D). CP-I, CP-II, and CP-I+II versus UNSCEAR effective dose. 95%CIs of cancer prevalence CP-I+II are shown by broken lines.
Dose response of thyroid cancer in F-model
The results of analysis according to the official regional division (F-model) are listed in Table 2. Dose response of CP-I, CP-II, and CP-I+II to FHMS external dose and UNSCEAR effective dose is shown in Figure 2. Although only a weak relationship was expected between the official regional division and exposure doses in this model, ORs of thyroid cancer proportion CP-II and CP-I+II, using the least contaminated area Aizu (4) as reference, decreased in the order 1>2>3>4, which agreed with the order of decreasing UNSCEAR effective dose, and disagreed partially with the decreasing order of FHMS external dose (Table 2). Accordingly, cancer proportion CP-II and CP-I+II showed linear response to UNSCEAR effective dose with R2>0.86 and p<0.075 (Figure 2D), while CP-II and CP-I+II versus FHMS external dose showed scatter from linearity (Figure 2C). The correlation between FHMS external dose and UNSCEAR effective dose was not high with R2=0.71 (Figure 2B). External dose and effective dose are essentially the same quantity because ingestion was assumed to be constant for all municipalities and the dominant part of the effective dose was the external dose in the estimation by UNSCEAR [11, 12]. Low correlation between doses observed in some area divisions suggests considerable uncertainties in dose estimations, in FHMS external dose or in both, because exact estimation of doses should give nearly perfect correlation R2=1. Poor correlation patterns between effective dose and external dose and between cancer proportion and external dose are similar and may be the result of an inadequacy of external dose estimation by FHMS. If we consider that most evacuees presumably moved from evacuation area 1 to second highest dose area 2, Nakadori, the external dose of residents in evacuation area 1 is presumed to be higher than or comparable to the one of area 2 and external dose may decrease in the order 1>2>3>4, contrary to the present order 2>1>3>4. The present case is considered to be an example of how uncertainty in dose estimation violates incidence-dose relation.
Figure 2: Dose dependence of thyroid cancer proportion in F-model
A). Map of four areas 1-4 in F-model according to the official regional division B). Correlation between FHMS external dose and UNSCEAR effective dose. C). Proportion of thyroid cancer per 100,000, CP-I, CP-II, and CP-I+II versus FHMS external dose with regression line. D). CP-I, CP-II, and CP-I+II versus UNSCEAR effective dose.
Table 2: External dose based on FHMS basic survey, effective dose based on UNSCESAR report, number of primary examinees, confirmed or suspected cancer cases, thyroid cancer proportion per 100,000 (CP), odds ratio (OR) and 95% confidence interval (CI) in E-I, E-II, and E-I+II in F-model according to official regional division. 1. Evacuation zone, 2. Nakadori, 3. Iwaki Soma, 4. Aizu.
|
Area |
External |
Effective |
E-I (FY2011–2013) |
E-II (FY2014–2015) |
E-I+II (FY2011–2015) |
||||||||
|
mSv |
mSv |
Examinees |
Cases |
CP |
OR |
Examinees |
Cases |
CP |
OR (CI) |
Cases |
CP‡ |
OR (CI) |
|
|
1 |
0.93 |
5.54 |
41,810 |
14 |
33.5 |
1.03 |
34,557 |
17 |
49.2 |
3.17(1.2-8.6) |
31 |
82.7 |
1.62(0.9-2.9) |
|
2 |
1.17 |
4.17 |
169,155 |
65 |
38.4 |
1.08 |
152,693 |
39 |
25.5 |
1.65(0.7-4.2) |
104 |
64.0 |
1.25(0.8-2.1) |
|
3 |
0.52 |
2.94 |
55,788 |
24 |
43.0 |
1.05 |
51,053 |
10 |
19.6 |
1.26(0.4-3.7) |
34 |
62.6 |
1.22(0.7-2.2) |
|
4 |
0.19 |
1.67 |
33,720 |
12 |
35.6 |
1(Ref.) |
32,208 |
5 |
15.5 |
1 (Ref.) |
17 |
51.1 |
1 (Ref.) |
|
Ave. / Sum |
0.91 |
3.85 |
300,473 |
115 |
38.3 |
270,511 |
71 |
26.2 |
186 |
64.5 |
|||
‡ CP in E-I+II is the sum of those for E-I and E-II.
Discussion
Linear dose response of thyroid cancer proportions CP-II and CP-I+II
Thyroid cancer incidence proportion CP-II within 4-6 years and prevalence CP-I+II after 6 years from the nuclear accident had tendency to increase as effective dose increases in both models. In OM-model, although CIs of thyroid cancer prevalence CP-I+II, in the 6 years after exposure, overlap each other, the plot showed perfect linear prevalence-effective dose response with positive coefficient 5.6 (CI=3.4-7.9) (cancer cases / 100,000 / mSv) in the 1.6–5 mSv effective dose range (Figure 1D). The contamination due to diffusion of radioactive plumes changed continuously across areas, and CIs of the prevalence naturally spread and overlapped each other. It is important that linear prevalence-dose relation exists with high precision irrespective of uncertainties of the prevalence and dose estimations. These results of analyses suggest the possibility that childhood thyroid cancers in Fukushima are associated with radiation exposure due to the accident.
Absence of dose response in the first screening
Dose dependence of cancer proportion CP-I in the first screening was not observed for external dose and effective dose in both OM- and F-models. This agreed with Ohira et al.’s and Suzuki et al.’s conclusions that regional differences in the external dose in OM- and F-models, respectively, were not associated with thyroid cancer prevalence in the first 4 years after the accident [3, 7]. Area dependence of CP-I was not observed because of the short elapsed-time ~1.4 years from exposure in high dose evacuation area 1 compared to ~2.8 years for the lowest dose Aizu area 4 (elapsed time from [7]). Higher cancer prevalence expected in high-dose areas may have been counteracted by the short interval from exposure to screening [4, 6].
Dose response influenced by area division
Area dose response is affected by the selected area division. FMU concluded no association between regional radiation dose and risk of thyroid cancer within 4–6 years after exposure [5]. Conversely, linear dose response became apparent by combining A and B to form central part 2 in the present OM-model. In a stochastic process such as radiation induced thyroid cancer, randomness of cancer incidence of cases ~70 patients often violate dose response. It should be noted that the absence or presence of dose response for small number of cases is a function of selected border of areas, borders of the percentage of residents who received an external radiation exposure 1 mSv in the case of Ohira et al.’s model. Linear dose response for reasonable area division can be a direct evidence for radiation related thyroid cancer because linear dose response cannot be observed if cancer cases were not associated with radiation dose and were detected randomly by mass-screening. FMU concluded no association between radiation dose and the risk of thyroid cancer in E-II on the basis of division into 5 areas A-E. However, they inferred the increased risk of thyroid cancer RR=1.6-2.3 in areas A-C compared with the lowest dose area E. Cancer incidence of highly contaminated areas by radioactive plume A-C (evacuation area 1 + central part 2) was calculated to be 35.8/100,000 from Table 2 in [5], which was much higher than the incidence 15.8/100,000 of lowest contaminated areas E, with RR(CI) =2.27(0.9-5.7). The relative risk of areas A-D compared with lowest contaminate E was calculated RR(CI)=1.93(0.8-4.8). These facts derived from the data by Ohira et al. suggest a possibility that thyroid cancer cases in Fukushima were linked to radioactive fallout [5].
Dose response disappears for uncertain dose estimations
The present results of analyses by OM- and F-models show that UNSCEAR effective dose gives a clear explanation for dose response of childhood thyroid cancer in Fukushima irrespective of models. Low correlation between FHMS external dose and UNSCEAR effective dose observed in some area divisions suggested considerable uncertainties in dose estimations, because exact estimation of doses should give nearly perfect correlation R2=1. Dose response of cancer proportion may disappear if the analysis is based on an inadequate dose estimation. Estimations of thyroid dose in the present stage seem not adequate to be used for detecting dose response because it involves a lot of uncertainties and provides only representative values for the residents [9].
Changing purpose of FHMS
The authors of FMU consider that the purpose of FHMS is to detect the long-term effects of low-dose radiation exposure on thyroid cancer incidence and to reduce anxiety among residents in Fukushima [5]. There is a clear difference from the primary purpose of FHMS: to monitor the long-term health of residents, promote their future well-being, and confirm whether long-term low-dose radiation exposure has health effects [14]. If FMU is eager at reducing anxiety of residents by proving least probable health effect of radiation exposure, the analyses may have bias to radiation safety side, and the original purpose of confirming the health effects of long-term low-dose radiation exposure may not be achieved. FMU ascribed high RR of 1.6-2.3 of highly contaminated areas A-C as compared with the lowest dose area E to the high rate of FNAC (16.3, 14.7, 11.4% in areas A-C, and 7.0, 7.1 % in D and E areas), instead of recognizing the link between thyroid cancer and radiation exposure. It is formally advertised to the public that FNAC is carried out if the doctor determines its’ necessity. The idea of high RR of thyroid cancer coming from high rate of FNAC means 1st, a possibility of cancers becoming serious because of delayed discoveries by the low rate of FNAC, and 2nd the possibility of decreased cancer cases in FHMS report from low rate of FNAC, because cancer cases diagnosed during follow up period without FNAC in the confirmatory examination were found not included in the FHMS data [15].
After denying the associations of regional and individual external radiation doses with the incidence of thyroid cancer, the authors derived 3rd conclusion that obesity was positively associated with thyroid cancer incidence from the relative risk of thyroid cancer 1: 0.62: 2.20 for (No obesity nor overweight-): (Overweight-): (Obesity-) children. They defined overweight and obesity as age- and sex-specific BMI higher than the 85% (overweight) and 95% (obesity) percentiles of all Japanese in their age. It seems impossible to conclude that weight gain is a risk factor of thyroid cancer, because the data also showed negative risk (-38%) for overweight. Latency period of their ‘obesity associated thyroid cancer’ must be less than 2 years in concluding positive association of thyroid cancers detected in E-II and obesity measured in E-I or E-II, which is too short as compared with the latency period 4-5 years of radiation related thyroid cancer expected by Suzuki and FHMS [16].
Conclusion
Dose dependence of childhood thyroid cancer proportion in E-I, E-II and E-I+II in Fukushima was analyzed for external dose estimated by FHMS and effective dose estimated by UNSCEAR. Analyses were based on two model divisions of Fukushima prefecture, OM-model according to Ohira’s map in the order of decreasing external dose, and F-model according to the official regional division. We found the following:
1. Linear dose response of thyroid cancer incidence proportion CP-II during 4-6 years and prevalence CP-I+II after 6 years from the accident suggested a highly probable association between childhood thyroid cancer in Fukushima and radiation exposure due to the accident.
2. In OM-model according to Ohira’s map, thyroid cancer prevalence CP-I+II in the 6 years after exposure showed perfect linear prevalence-effective dose response with positive coefficient 5.6 (CI=3.4-7.9) (cancer cases / 100,000 / mSv) in the1.6–5 mSv effective dose range.
3. In F-model according to the official regional division, thyroid cancer proportion CP-II and CP-I+II decreased in the order of decreasing UNSCEAR effective dose 1>2>3>4 and showed linear response to UNSCEAR effective dose with R2>0.86 and p<0.075. This correlation between thyroid cancer proportion and effective dose was higher than the correlation between external dose and effective dose with R2=0.71.
4. Dose response is influenced by the adopted dose estimation. UNSCEAR effective dose was found to give best fit to linear regression line of the incidence and prevalence of childhood thyroid cancer in E-II and E-I+II, irrespective of models.
5. Area dose response is influenced by area division. In a stochastic process such as radiation induced thyroid cancer, randomness of cancer incidence in small number of cases often violates dose response. Linear dose response for reasonable area division can be a direct evidence for radiation related thyroid cancer because linear dose response cannot be observed in mass-screening for sporadic thyroid cancers.
6. Linear dose responses observed in the present study and in preceding studies as well suggested highly probable association between thyroid cancer in Fukushima and radiation exposure. [6, 17]
Funding
This research received no specific grant from any funding agency.
Conflicts of Interest
The author declares that there is no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 02, Dec 2019Accepted: Fri 20, Dec 2019
Published: Thu 26, Dec 2019
Copyright
© 2023 Toshiko Kato. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2019.06.16
Figures & Tables
Table 1: External dose based on FHMS basic survey, effective dose based on UNSCESAR report, number of primary examinees, confirmed or suspected cancer cases, thyroid cancer proportion per 100,000 (CP), odds ratio (OR) and 95% confidence interval (CI) in E-I, E-II, and E-I+II in OM-model according to Ohira’s map.
|
Area† |
External |
Effective |
E-I (FY2011–2013) |
E-II (FY2014–2015) |
E-I+II (FY2011–2015) |
||||||||
|
mSv |
mSv |
Examinees |
Cases |
CP |
OR |
Examinees |
Cases |
CP |
OR (CI) |
Cases |
CP‡ |
OR (CI) |
|
|
1 (C) |
0.91 |
4.60 |
59,621 |
20 |
33.5 |
0.92 |
51,160 |
19 |
37.1 |
2.28(0.9-5.7) |
39 |
70.7 |
1.34(0.8-2.3) |
|
2 (A+B) |
1.38 |
4.99 |
119,648 |
47 |
39.3 |
1.08 |
107,107 |
33 |
30.8 |
1.89(0.8-4.5) |
80 |
70.1 |
1.33(0.8-2.2) |
|
3 (D) |
0.52 |
2.73 |
82,625 |
34 |
41.1 |
1.13 |
75,421 |
13 |
17.2 |
1.06(0.4-2.8) |
47 |
58.4 |
1.11(0.7-1.9) |
|
4 (E) |
0.25 |
1.66 |
38,579 |
14 |
36.3 |
1(Ref.) |
36,823 |
6 |
16.3 |
1 (Ref.) |
20 |
52.6 |
1 (Ref.) |
|
Ave. / Sum |
0.77 |
3.48 |
300,473 |
115 |
38.3 |
270,511 |
71 |
26.2 |
186 |
64.5 |
|||
† Area name in the parentheses is the name in Refs. [5,7]. ‡ CP in I + II is the sum of those for I and II.
† Area name in the parentheses is the name in Refs. [5,7]. ‡ CP in I + II is the sum of those for I and II.
Table 2: External dose based on FHMS basic survey, effective dose based on UNSCESAR report, number of primary examinees, confirmed or suspected cancer cases, thyroid cancer proportion per 100,000 (CP), odds ratio (OR) and 95% confidence interval (CI) in E-I, E-II, and E-I+II in F-model according to official regional division. 1. Evacuation zone, 2. Nakadori, 3. Iwaki Soma, 4. Aizu.
|
Area |
External |
Effective |
E-I (FY2011–2013) |
E-II (FY2014–2015) |
E-I+II (FY2011–2015) |
||||||||
|
mSv |
mSv |
Examinees |
Cases |
CP |
OR |
Examinees |
Cases |
CP |
OR (CI) |
Cases |
CP‡ |
OR (CI) |
|
|
1 |
0.93 |
5.54 |
41,810 |
14 |
33.5 |
1.03 |
34,557 |
17 |
49.2 |
3.17(1.2-8.6) |
31 |
82.7 |
1.62(0.9-2.9) |
|
2 |
1.17 |
4.17 |
169,155 |
65 |
38.4 |
1.08 |
152,693 |
39 |
25.5 |
1.65(0.7-4.2) |
104 |
64.0 |
1.25(0.8-2.1) |
|
3 |
0.52 |
2.94 |
55,788 |
24 |
43.0 |
1.05 |
51,053 |
10 |
19.6 |
1.26(0.4-3.7) |
34 |
62.6 |
1.22(0.7-2.2) |
|
4 |
0.19 |
1.67 |
33,720 |
12 |
35.6 |
1(Ref.) |
32,208 |
5 |
15.5 |
1 (Ref.) |
17 |
51.1 |
1 (Ref.) |
|
Ave. / Sum |
0.91 |
3.85 |
300,473 |
115 |
38.3 |
270,511 |
71 |
26.2 |
186 |
64.5 |
|||
‡ CP in E-I+II is the sum of those for E-I and E-II.
A). Map of four areas 1-4 in OM-model according to Ohira’s map. B). Correlation between FHMS external dose and UNSCEAR effective dose. C). Proportion of thyroid cancer per 100,000, CP-I, CP-II, and CP-I+II versus FHMS external dose with regression line. D). CP-I, CP-II, and CP-I+II versus UNSCEAR effective dose. 95%CIs of cancer prevalence CP-I+II are shown by broken lines.
A). Map of four areas 1-4 in F-model according to the official regional division B). Correlation between FHMS external dose and UNSCEAR effective dose. C). Proportion of thyroid cancer per 100,000, CP-I, CP-II, and CP-I+II versus FHMS external dose with regression line. D). CP-I, CP-II, and CP-I+II versus UNSCEAR effective dose.
References
- (2017) The 27th Prefectural Oversight Committee Meeting for Fukushima Health Management Survey. Radiation Medical Science Center for the Fukushima Health Management Survey.
- (2016) Fukushima Prefectural Oversight Committee Meeting for FHMS March 2016, Interim report on prefectural health survey (in Japanese).
- Suzuki S, Suzuki S, Fukushima T, Midorikawa S, Shimura H et al. (2016) Comprehensive Survey Results of Childhood Thyroid Ultrasound Examinations in Fukushima in the First Four Years After the Fukushima Daiichi Nuclear Power Plant Accident. Thyroid 26: 843-851. [Crossref]
- Yamamoto H, Hayashi K, Scherb H (2019) Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine (Baltimore) 98: e17165. [Crossref]
- Ohira T, Ohtsuru A, Midorikawa S, Takahashi H, Yasumura S et al. (2019) External Radiation Dose, Obesity, and Risk of Childhood Thyroid Cancer after the Fukushima Daiichi Nuclear Power Plant Accident: The Fukushima Health Management Survey. Epidemiology 30: 853–860. [Crossref]
- Kato T (2019) Re: Associations Between Childhood Thyroid Cancer and External Radiation Dose After the Fukushima Daiichi Nuclear Power Plant Accident. Epidemiology 30: e9–e11. [Crossref]
- Ohira T, Takahashi H, Yasumura S, Ohtsuru A, Midorikawa S et al. (2018) Associations between childhood thyroid cancer and external radiation dose after the Fukushima Daiichi Nuclear Power Plant Accident. Epidemiology 29: e32-e34. [Crossref]
- Tokonami S, Hosoda M, Akiba S, Sorimachi A, Kashiwakura I et al. (2012) Thyroid doses for evacuees from the Fukushima nuclear accident. Sci Rep 2: 507. [Crossref]
- Kim E, Kurihara O, Kunishima N, Momose T, Ishikawa T et al. (2016) Internal thyroid doses to Fukushima residents-estimation and issues remaining. J Radiation Res 57 Suppl 1: i118–i126. [Crossref]
- Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK et al. (2013) Cancer risk in 680 000 people exposed to CT scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346: f2360. [Crossref]
- United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 2013 Report, Effective doses in Japan for the first year.
- United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 2013 Report, Estimated doses to evacuees in Japan for the first year.
- Rothman KJ (2012) Epidemiology: An introduction. 2nd edition. Oxford University Press, New York, USA. 2012: 148-163.
- Radiation Medical Science Center for the Fukushima Health Management Survey, Overview of the Fukushima Health Management Survey.
- Radiation Medical Science Center, Fukushima Medical University, Q&A for thyroid examination (in Japanese)
- Suzuki S (2016) Childhood and adolescent thyroid cancer in Fukushima after the Fukushima Daiichi nuclear power plant accident: 5 years on. Clin Oncol (R Coll Radiol) 28: 263–271. [Crossref]
- Kato T (2019) Dose dependence of pediatric thyroid cancer prevalence in the 6 years after the Fukushima nuclear power plant accident. Adv Pediatr Res.
