An Obscure Colon Dilatation and its Underlying Cause: Case Report and Literature Review
A B S T R A C T
Introduction: Congenital as well as acquired diseases may be responsible for the development of a megacolon. In adult patients, Clostridium difficileassociated infection as well as late-onset of Morbus Hirschsprung disease are known to cause a megacolon. In addition, malignant as well as benign colorectal strictures may lead to intestinal dilatation. In case of an idiopathic megacolon, the underlying cause remains unclear.
Case Presentation: We describe the case of a 44-year-old male patient suffering from a long history of chronic constipation. He presented himself with an obscurely dilated large intestine with bowel loops up to 17 centimeters in diameter. Radiological as well as endoscopic examination gave evidence of a spastic process in the sigmoid colon. The patient was treated with a subtotal colectomy and the intraoperative findings revealed a stenotic stricture in the sigmoid colon. Since the histological examination did not find a conclusive reason for the functional stenosis, an immunohistochemical staining was advised. This showed a decrease in interstitial cells of Cajal (ICC) in the stenotic part of the sigmoid colon.
Discussion: This case report describes a patient with an idiopathic megacolon, where the underlying cause remained unclear until an immunohistochemical staining of the stenotic colon showed a substantial decrease of ICCs. Various pathologies leading to a megacolon are reviewed and discussed.
Keywords
Megacolon, colon dilatation, interstitial cells of Cajal, mechanical obstruction of the colon
Introduction
The megacolon, with its variety of underlying causes, is a rare but potential life-threatening diagnosis in visceral surgery. Acquired as well as congenital forms are described in the literature. Moreover, idiopathic manifestations of the megacolon leave the causal condition unclear.
Acquired forms often originate from mechanical obstructions. In particular, stenotic processes due to malignant as well as benign strictures but also obstructions like sigma volvulus or functional stenosis without any morphologic reason can lead to a dilated colon [1]. Colonic dilatation caused by congenital factors frequently occur in patients suffering from Hirschsprung disease (HD) [2-4]. Late-onset forms of HD are described in the literature but are only rarely diagnosed [5]. If concomitant systemic toxicity is present, a toxic megacolon must be taken into consideration. Infectious colitis or an inflammatory bowel disease (IBD) may be part of the patient’s medical history. A dysfunction of ICCs, the intestinal pacemaker cells, seems to be involved in causing slow-transit-constipation and may be further associated with the development of a megacolon [6]. This idiopathic form comes along with a pathologically distended colon without organic reasons that might explain a dilatation and lengthening of the colon [7, 8].The management of patients with a megacolon can be challenging, especially if patients suffer from a slow-transit-constipation or a chronic constipation for a long period of time.
Here, we present a case of a patient suffering from a megacolon who finally underwent subtotal colectomy. However, the underlying cause for the functional stenosis of the colon remained unclear until a re-staining of the specimen was performed. Various pathologic conditions that may be associated with the development of a megacolon are reviewed in the discussion section.
Case Presentation
A 44-year-old male patient presented himself at our outpatient clinic with an obscurely dilated abdomen, increasing dyspnea and tensional pain. He reported sequences of constipation altering with sequences of diarrhea since his childhood. The first abnormal intestinal distensions occurred at an age of twenty. His past medical history describes a Hashimoto-thyroiditis, an evacuation of an epidural hematoma after traumatic head injury 30 years ago, and a laparoscopic removal of his appendix and his gallbladder several years ago.
Chronologically, his first visit at the Department of Internal Medicine was one year before the current visit due to abdominal pain especially in the right upper abdomen as well as nightly back pain. At that time, on physical examination the abdomen was clearly above the thoracic level, borborygmus was audible but the abdominal wall was soft, and the patient did not suffer from severe abdominal pain. He reported a weight loss of eleven kilograms within the last year. He suffered from constipation that aggravated as soon as his laxative medication was paused.
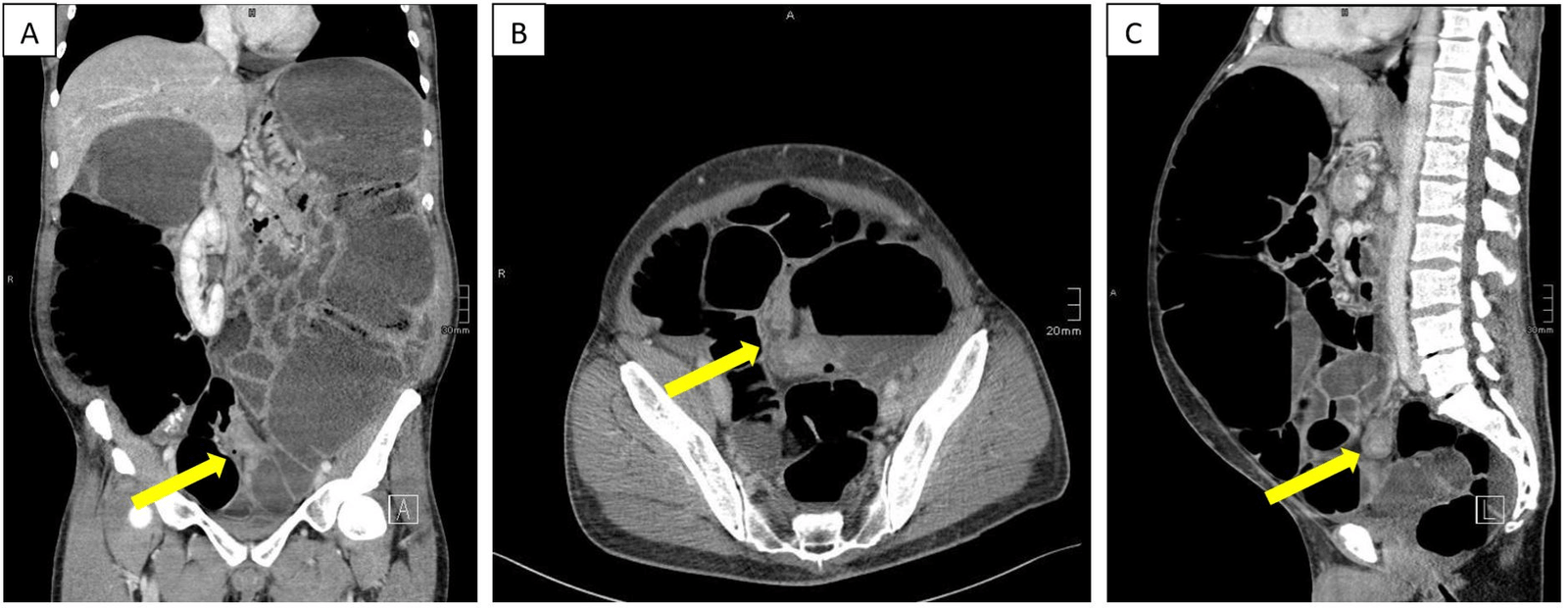
The attending gastroenterologist intensified the conservative treatment and a colonoscopy was performed. Due to an impassable obstruction in the proximal sigmoid colon, the examination had to be interrupted. Subsequently, an abdominal computer tomography was performed, which confirmed the colonic distension with segments dilated up to twelve centimeter in diameter. Additionally, a five-centimeter-long stenotic process in the sigmoid colon was described, which was considered responsible for the colonic distension (Figure 1). For further clarification, a second colonoscopy was performed in which a spastic but passable bowel segment in the sigmoid colon was described.
Figure 1: Abdominal computer tomography revealed a colonic distension up to twelve centimeters. There was evidence of a five-centimeter-long stenotic process in the sigmoid colon (yellow arrows).
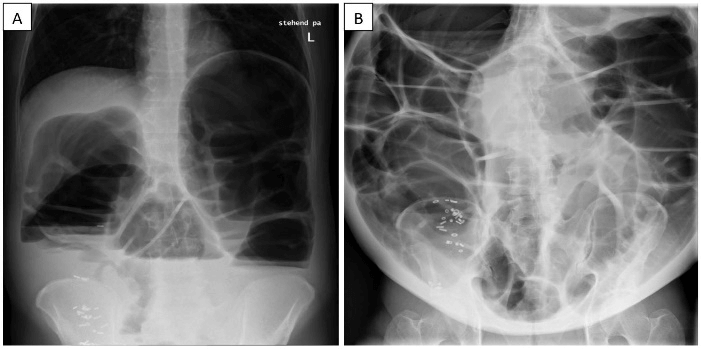
Figure 2: A) After orally applied radiopaque pellets, an abdominal X-ray was performed. It revealed a pathological colonic distension. About forty of the radiopaque pellets were projected onto the right colon. The small intestine appeared not distended. B) Nine months later, the initially applied radiopaque pellets were still detected in projection onto the right colon. The colon was distended up to 17 centimeters. This time, the small intestinal loops appeared dilated up to five centimeters as well.
In a further diagnostic step, the colonic transit time using radiopaque pellets was measured. The abdominal X-ray four days after application showed about forty pellets in the right colon (Figure 2). The patient was transferred to our abdominal surgery outpatient clinic. The results of all recently performed radiological examinations were discussed interdisciplinary, concluding that a tumor or an aganglionic segment in the sigmoid colon were the most probable cause for the megacolon. Considering all diagnostic results and medical circumstances, a subtotal colectomy was advised and discussed with the patient. At this moment, the patient didn’t consent to the recommended surgical treatment.
After nine months without any further recorded outpatient clinic visits, the patient presented himself again at our clinic with an obscurely dilated abdomen, increasing dyspnea and tensional pain. He reported on constipation altering with discharge of very small amount of liquid stool. In our outpatient clinic, a soft rectal tube was inserted, but neither rectal stool nor gas discharge was observed. In the abdominal X-ray (Figure 2), a colonic distension with bowel segments up to 17 centimeters in diameter was found. Additionally, nearly all of the nine-month-ago orally applied radiopaque pellets were still projected onto the right colon. Moreover, distended small intestine loops up to five centimeters in diameter were described as well. Blood tests revealed a leukocytosis of 12.95 G/L, a slight thrombocytosis (425 G/L), an increased fibrinogen (486 mg/dl) and a mild hyperkalemia (5.12 mmol/l). Neither viral antigens nor virus-specific antibodies for hepatitis-A, -B, -C or HIV were found. Furthermore, endomysial as well as anti-tissue transglutaminase antibodies were below detection level. The tumor marker alpha-fetoprotein was negative as well.
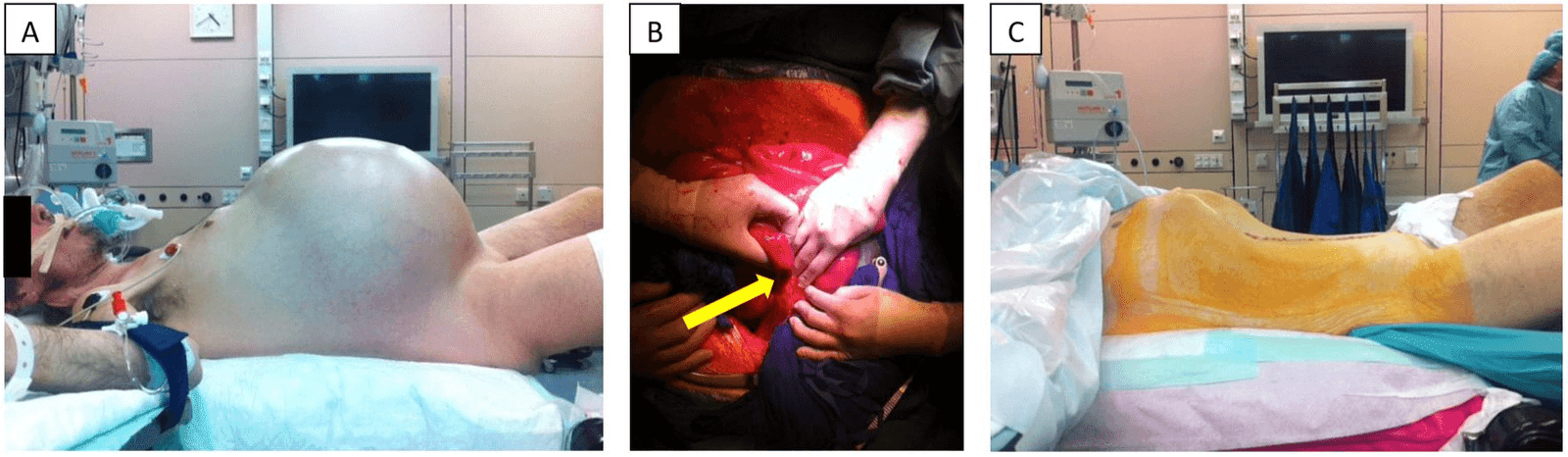
Now, given the substantial symptomatic burden, the patient gave his consent to the recommended operation. Subsequently, an explorative laparotomy was performed. As already radiographically assumed, intraoperatively, an obscurely dilated colon was found (Figure 3). A filiform stenotic process in the rectosigmoid junction, most likely triggered by inflammation, seemed to be causal. A subtotal colectomy with a side-to-side ileorectal anastomosis was performed.
Figure 3: The pictures show the patient before (A) and after (C) subtotal colectomy in patient’s profile. Picture A shows the extent of colonic distension as well as a slightly livid color of the abdominal skin. In picture (B), the yellow arrow points out the stenotic process in the sigmoid colon.
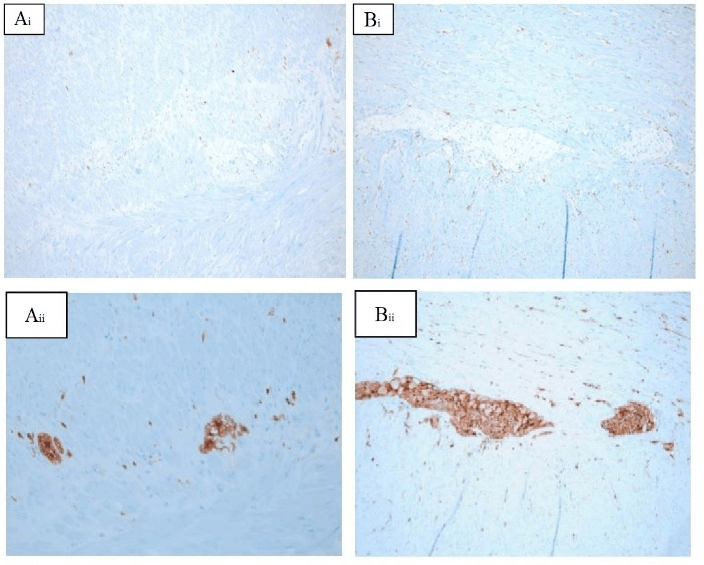
Figure 4: The figure shows A) immunohistochemical stainings of the stenosis in the sigmoid colon and B) an unaffected section of the sigmoid colon. i) c-Kit staining was used for interstitial cells of Cajal (ICC) and ii) S100 staining for glia cells of the nerval plexus. (Ai) and (Aii) show a decreased number of ICCs and glia cells in comparison to (Bi) and (Bii).
The histological work-up of the colon showed a non-inflamed diverticulosis in the sigmoid colon. Of particular interest was a section of the sigmoid colon, which showed a stenosis over a length of 13 centimeters. Neither a tumorous process nor any other underlying cause for the stenotic process was found. Therefore, an immunohistochemical staining of the stenotic part of the specimen was conducted with the main focus on the investigation of ICCs and structures of the nerval plexus. The staining of the ICCs was performed by using a c-Kit antibody whereas S100 was used as a marker for glia cells of the nerval plexus. A decrease of both, ICCs as well as glia cells of the nerval plexus, was observed in the stenotic part when compared to an unaffected part in the sigmoid colon (Figure 4).
During the postoperative course a stepwise progression to full diet was achieved and the patient was discharged 10 days after an uneventful postoperative period. During the following months, no further visits were documented. In a telephone contact, the patient reported a slightly increased defecation frequency up to 3 times a day but no other complaints.
Discussion
A variety of underlying diseases such as mechanical obstructions, infectious disease, inflammation as well as congenital disorders may lead to a megacolon. [4, 5, 9-15]. Besides benign and malign tumorous processes causing obstruction, a common cause of a mechanical bowel obstructions leading to a megacolon is the volvulus [9, 10]. It occurs when the intestine twists around a fixed point and may involve either the small or the large bowel [1]. The colonic volvulus mostly affects the sigmoid colon and the cecal region [10]. In the US, the sigmoid volvulus is the third most common cause for colonic obstruction after cancer and diverticulitis [11]. In this actual case, neither the preoperatively diagnostic workup nor the intraoperative findings gave evidence for colonic volvulus.
Non-mechanical causes may be an infectious colitis as well as an inflammatory bowel disease (IBD), potentially leading to a toxic megacolon [12, 13]. Severe colitis can be caused by Clostridium difficileinfection [12]. Typically, diffuse ulcerations, raised mucosal nodules and pseudomembranes are present at endoscopic examination [14]. Rarely, amoebic colitis, cytomegalovirus colitis as well as infections with Salmonella, Shigella and Campylobacter can also be associated with a toxic megacolon [14, 15]. A toxic megacolon is per definition accompanied by systemic toxicity [16]. Patients with IBD in their medical history are at an increased risk for developing a toxic megacolon. The underlying mechanism remains widely unclear but it is proposed that inflammation-triggered mucosal inflammatory mediators increase nitric oxide levels thereby inducing smooth muscle cells relaxation causing the development of the colon dilatation [16]. In the presented case, microbial diseases were ruled out as well as no IBD was found in the patient’s medical history. In addition, the patient denied any antibiotic intake that might have caused an antibiotic-associated colitis. Additionally, severe systemic toxicity signs were absent. The long period of colonic obstruction in this patient’s medical history further contradicts the hypothesis of a toxic megacolon.
In contrast to acquired pathogenetic factors, Hirschsprung disease (HD) is a congenital motor neuron disorder of the gut, caused by failure of the craniocaudal migration of neural crest cells from the neural crest to their destination in the distal end of the colon during the embryonic phase [15, 17-19]. The absence of neuroblasts severely impacts the nerval innervation in the distal colon [18]. Hirschsprung disease comes along with a functional obstruction in the respective colonic segment. In the majority of patients, only a short-segment of the colon, typically the rectosigmoid colon, is affected. In about every fifth patient, the proximal sigmoid colon is affected as well. In about two to fourteen percent of patients, the entire colon is affected by a total colonic aganglionosis [20]. The majority of patients with HD is diagnosed in the neonatal period; rarely, after the age of three [4]. If it is diagnosed in the adulthood, patients often present with colonic dilatation and a therapy-refractory constipation in their medical history [21].
In adults, HD sometimes occurs as an ultra-short segment disease, where an area of two to four centimeters proximal to the internal sphincter is affected [18]. In this case, a biopsy just proximal to the dentate line shows the absence of ganglion cells whereas biopsies from more proximal or distal regions show regular ganglion cells [22, 23]. In our patient, the initially suspected diagnosis of HD was not confirmed as the pathohistological work-up didn’t reveal any ganglionic disorder.
Neurological diseases may influence the nerval innervation of the intestines as well. For example, the characteristic finding of concentric hyaline inclusions (Lewy bodies) in neurons of Parkinson patients can also be found in ganglion cells of the myenteric plexus of the colon [24]. These patients suffer from a chronic constipation and a loss of muscle tone in the intestine. The impact of psychiatric medication on the development of a megacolon is described as well [25]. Anticholinergic side effects seem to explain the elevated risk of developing a megacolon in psychiatric patients [26]. In the presented case neither psychiatric nor neurological disorders were documented. Furthermore, the patient did not report on any intake of psychotropic drugs.
Two manifestations of a megacolon, where the causalities are not yet entirely clarified, are the Oglivie’s syndrome and the acquired megacolon (AMC). The Ogilvie’s syndrome describes an acute colonic pseudo-obstruction with massive dilatation of the respective colonic segment and occurs typically in hospitalized patients after surgery, particularly after cesarean section, or in debilitated patients suffering from acute critical illness [27]. The pathophysiology is poorly understood, as a lack or a restriction of movement in combination with an electrolyte imbalance as well as a disbalance of the autonomic nerval system is assumed to be causal [27, 28, 29]. In cases of Oglivie’s syndrome, no mechanical reasons for the intestinal obstruction can be found. The here presented patient unlikely suffered from an Ogilvie’s syndrome since no recent surgery took place and a long history of constipation was reported.
In the presented case the AMC may be the best fitting diagnosis. The term describes a condition of a pathologically distended colon without organic reasons, explaining the dilatation and lengthening of the colon [7, 8]. A review of Cuda et al. proposed following criteria for the diagnosis of AMC: (1) the exclusion of organic disease; (2) a sigmoid diameter of ~10 cm on abdominal X-ray or barium enema; (3) and symptoms including constipation, distension, abdominal pain and gas distress [8]. AMC still remains a diagnosis by exclusion but a dysfunction of ICCs was suggested to be the most profound histopathological finding [8]. These cells can undergo cyclic, spontaneous depolarisation leading to slow electric waves, which migrate towards the muscle cells [30]. Therefore, they play an important role as pacemaker cells, driving the activity of smooth muscle cells in the gastrointestinal tract [6, 31]. Lesions of these interstitial gut-pacemaker cells may cause a slow-transit constipation that further contributes to the development of a megacolon.
An immunohistochemical staining of the surgical specimen provided important insights: ICCs express c-kit, a receptor tyrosine kinase that is specific for ICCs. Hence, antibodies to the c-Kit protein label ICCs selectively. However, nerval or glia cells are not contributing to c-Kit positive cells [32, 33]. An antibody to S100 was used to stain glia cells. The immunohistochemical staining of the stenotic part of the colon revealed a lower number of ICCs in a reduced number of nerval plexus cells compared to an unaffected part of the sigmoid colon.
Taken together, the substantial decrease in ICCs seems to be the most reasonable pathophysiological mechanism, which led to the described megacolon.
Conflicts of Interest
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Tue 05, May 2020Accepted: Mon 22, Jun 2020
Published: Fri 10, Jul 2020
Copyright
© 2023 Anton Stift. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GSCR.2020.01.03
Author Info
Kerstin Wimmer Felix Harpain Katharina Wöran Thomas Mang Anton Stift
Corresponding Author
Anton StiftDepartment of Surgery, Division of General Surgery, Medical University Vienna, Austria
Figures & Tables
References
- Muneera R Kapadia (2017) Volvulus of the Small Bowel and Colon. Clin Colon Rectal Surg 30: 40-45. [Crossref]
- M A Parisi, R P Kapur (2000) Genetics of Hirschsprung disease. Curr Opin Pediatr 12: 610-617. [Crossref]
- Melissa A Parisi, Margaret P Adam, Holly H Ardinger, Roberta A Pagon, Stephanie E Wallace et al. (1993) Hirschsprung Disease Overview – archived chapter, for Historical reference only. GeneReviews. [Crossref]
- A Arshad, C Powell, P M Tighe (2013) Hirschsprung Disease. Praxis 102: 407-411. [Crossref]
- M J Wheatley, J R Wesley, A G Coran, T Z Polley Jr (1990) Hirschsprung's disease in adolescents and adults. Dis Colon Rectum 33:622-629. [Crossref]
- Othman A Al Shboul (2013) The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterol 19: 3-15. [Crossref]
- J Pereira, F D Horrigan (1987) Understanding adult acquired megacolon. Geriatr Nurs 8: 16-19. [Crossref]
- Tahleesa Cuda, Ronny Gunnarsson, Alan de Costa (2018) Symptoms and diagnostic criteria of acquired Megacolon - a systematic literature review. BMC Gastroenterol 18: 25. [Crossref]
- Carmen Mesas Burgos, Ulla Ullberg, Tomas Wester (2011) Gastric volvulus in children--rare but serious diagnosis. Prompt management is necessary to avoid severe complications. Lakartidningen 108: 1308-1310. [Crossref]
- P Ryan (1982) Sigmoid volvulus with and without megacolon. Dis Colon Rectum 25: 673-679. [Crossref]
- M S Rubin, L E Bodenstein, K C Kent (1995) Severe Clostridium difficile colitis. Dis Colon Rectum 38: 350-354. [Crossref]
- Priya D Farooq, Nathalie H Urrunaga, Derek M Tang, Erik C von Rosenvinge (2015) Pseudomembranous colitis. Dis Mon 61: 181-206. [Crossref]
- A P Wilson, G L Ridgway, M Sarner, P B Boulos, M G Broo et al. (1990) Toxic dilatation of the colon in shigellosis. BMJ 301: 1325-1326. [Crossref]
- V W Fazio (1980) Toxic megacolon in ulcerative colitis and Crohn's colitis. Clin Gastroenterol 9: 389-407. [Crossref]
- Jacob C Langer (2013) Hirschsprung disease. Curr Opin Pediatr 25: 368-374. [Crossref]
- S G Sheth, J T LaMont (1998) Toxic megacolon. Lancet 351: 509-513. [Crossref]
- J F Mayberry, M Atkinson (1986) Achalasia and other diseases associated with disorders of gastrointestinal motility. Hepatogastroenterology 33: 206-207. [Crossref]
- M Fu, P K H Tam, M H Sham, V C H Lui (2004) Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryology 208: 33-41. [Crossref]
- C G Fu, T Muto, T Masaki, H Nagawa (1996) Zonal adult Hirschsprung's disease. Gut 39: 765-767. [Crossref]
- Samuel W Moore, Monique Zaahl (2009) Clinical and genetic differences in total colonic aganglionosis in Hirschsprung's disease. J Pediatr Surg 44: 1899-1903. [Crossref]
- Tomas Wester, Anna Löf Granström (2017) Hirschsprung disease-Bowel function beyond childhood. Semin Pediatr Surg 26: 322-327. [Crossref]
- W Meier Ruge (1985) Ultrashort segment Hirschsprung disease. An objective picture of the disease substantiated by biopsy. Z Kinderchir 40: 146-150. [Crossref]
- I R Neilson, S Yazbeck (1990) Ultrashort Hirschsprung's disease: myth or reality. J Pediatr Surgery 25: 1135-1138. [Crossref]
- W J Kupsky, M M Grimes, J Sweeting, R Bertsch, L J Cote (1987) Parkinson's disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology 37: 1253-1255. [Crossref]
- Sheila Jorge Adad, Moisés Amâncio Souza, Gisele Barbosa E Silva, José do Carmo Junior, Charles Antônio Pires de Godoy et al. (2008) Acquired non-Chagas megacolon associated with the use of psychiatric medication: case report and differential diagnosis with Chagas megacolon. Rev Soc Bras Med Trop 41: 293-295. [Crossref]
- P Fehlow, F Walther, W Miosge (1995) [An increased incidence of megacolon in psychiatric and neurologic patients]. Nervenarzt 66: 57-59. [Crossref]
- P Pereira, F Djeudji, P Leduc, F Fanget, X Barth (2015) Ogilvie's syndrome-acute colonic pseudo-obstruction. J Visc Surg 152: 99-105. [Crossref]
- Muhammad Waqas Khan, Sanniya Khan Ghauri, Sara Shamim (2016) Ogilvie's Syndrome. J Coll Physicians Surg Pak 26: 989-991. [Crossref]
- Cameron I Wells, Gregory O'Grady, Ian P Bissett (2017) Acute colonic pseudo-obstruction: A systematic review of aetiology and mechanisms. World J Gastroenterol 23: 5634-5644. [Crossref]
- A Pasternak, M Szura, K Gil, A Matyja (2016) Interstitial cells of Cajal - systematic review. Folia Morphol 75: 281-286. [Crossref]
- Miyako Takaki (2003) Gut pacemaker cells: the interstitial cells of Cajal (ICC). J Smooth Muscle Res 39: 137-161. [Crossref]
- H M Young, D Ciampoli, B R Southwell, D F Newgreen DF (1996) Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol 180: 97-107. [Crossref]
- J D Huizinga, L Thuneberg, J M Vanderwinden, J J Rumessen (1997) Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci 18: 393-403. [Crossref]
