AI – New Avenue for Drug Discovery and Optimization
A B S T R A C T
The artificial intelligence (AI) used in drug treatment have to do with matching patients to their predicting drug-target or drug-drug interactions, optimal drug or combination of drugs, and optimizing treatment protocols. This review outlines some of the recently developed AI methods aiding the drug treatment and administration process. Selection of the suitable drug for a patient typically requires the patient data, such as genetics or proteomics, with drug data, like compound chemical descriptors, to score the therapeutic efficacy of drugs. The forecast of drug relations often relies on similarity metrics, pretentious that drugs with similar structures or targeted and similar behaviour or may interfere with each other. Deciding the dosage schedule for administration of drugs is performed using mathematical models to interpret pharmacokinetic and pharmacodynamics data.
Keywords
Artificial intelligence, machine learning, deep learning, combination therapy
Introduction
It is a science and engineering of making intelligent machines, mainly making excellent computer program. AI is branch of computer science which includes simulation of human intelligence by computer program. It involves the development of intelligence machines, thinking and working like human being, e.g., learning, planning, problem solving, recognition and speech. The AI research was constituted by John MC. Carthy in a conference held at Dartmouth College 1956. Allen Newell (Carnegie Mellon University), John Mc Carthy (Massachusetts Institute of Technology), Herbert Simon (Carnegie Mellon University) Marvit Minsky (Massachusetts Institute of Technology) and Arthur Samuel (IBM) were founder leaders of artificial intelligence. AI is a computer program able to perform task that need human intelligence. AI constitutes three types: human create algorithm, deep learning and machine learning [1].
i. Human created algorithm: It is evidence-based approaches programmed by researcher or clinician after installing known data into algorithm; computer can extract information and apply it to a given issue.
ii. Machine learning: It is the scientific study (Link1) of algorithms (Link2) and statistical models (Link3) that computer systems (Link4) use to perform a specific task without using explicit instructions, relying on patterns and inference (Link5) instead. Machine learning algorithms build a mathematical model (Link6) based on sample data, termed as ‘training data’ (Link7) in order to develop predictions without being explicitly programmed to perform the task.
iii. Deep learning: It is part of a broader family of machine learning (Link8) methods based on artificial neural networks (Link9) with representation learning (Link10). Learning can be supervised (Link11), semi-supervised (Link12) or unsupervised (Link13) Also known as deep neural learning or deep neural network.
AI play important role in health care, Banking industries, telecommunication, robotics, gaming, shipping, nuclear management, satellite control, Automotive etc. Health care related applications are in radiology, disease diagnosis, telehealth, drug interaction, electric health record etc. This review focuses on the role of AI in discovery and development of drugs.
Role of AI in Discovery of Anticancer Agents
The potent DDR1 kinase inhibitor they established deep generative model, generative tensorial reinforcement learning for de novo small molecule design [2]. Discoidin domain receptor kinase (DDR1 kinase) is Tran’s membrane receptor that belongs to the class of receptor tyrosine kinase. These molecules are involved in regulation of cell growth, differentiation and metabolism. Four discovered e-compounds were active in biochemical assay and two compounds were validated in cell-based assay. Developed new drug mechanism associated with drug response in disease beyond breast cancer with the help of this machine learning approved research work used MDA-MB-231 breast cancer single cell treated with antidiabetic drug Metformin [3]. The experiment on one of the gene, CDC42 confirmed that Metformin inhibited cancer cell migration and Proliferation.
A novel AI protocol for finding potential inhibitors of acute myeloid leukemia studied and give detail about [4]. They used 350 training results and nine artificial intelligence algorithm models were used to further verify the candidate’s potential. Molecular dynamics simulation evaluated the stability of the ligand-protein complex and achieved good results. AI models had unearthed the promising candidates for STAT3 inhibitors, and the good performance. Transforming cancer drug discovery with big data and AI by ADMET and clinical responses to drug treatment are clear areas where an insufficient amount of public data are currently available to get the most out of advanced AI algorithms [5]. They suggested that Big Data and AI methodology available, provision of training is essential to ensure that the next generation of drug discoverers are optimally enabled to take advantage of the powerful approaches and resources. They also said maximally exploit the benefits of Big Data and AI, these approaches must be adopted as an integral part of the whole drug discovery journey and continue into the clinical development and routine care.
CAD and AI for breast cancer-recent development and challenges discussed about by computer-aided diagnosis (CAD) machine learning methods and multidisciplinary knowledge and techniques are used to analyse the patient information and the results can be used to assist clinicians in their decision making process and CAD systems can be developed to provide decision support for many applications in the patient care processes, such as lesion detection, characterization, cancer staging, treatment planning and response assessment, recurrence and prognosis prediction. By deep learning (DL) and CAD, or artificial intelligence (AI), to medicine in general and to radiology in particular in breast imaging and solve problem for medical diagnosis field [6].
The CAD systems have been developed to help radiologists in order to increase diagnosis accuracy. A CAD system consists of four stages:
i. Pre-processing
ii. Segmentation of regions of interest
iii. Feature extraction and selection, and finally
iv. Classification [7].
They approached develop CAD systems on mammography and ultrasound images. AI in breast imaging worked on AI-CAD systems are focusing on breast diagnostic techniques such as ultrasound and magnetic resonance imaging (MRI) [8]. They fulfill gap in the market for contrast-enhanced spectral mammography AI-CAD tools. It is a cost-effectiveness assessment should be undertaken, with a large feasibility study carried out to ensure there are no unintended consequences.
Role of AI in Discovery of Anti-Diabetic Agents
Artificial intelligence (AI) is a quickly growing field, and its applications to diabetes research are growing even more rapidly. AI methods in combination with the latest technologies, including medical devices, mobile computing, and sensor technologies, have the potential to enable the creation and delivery of better management services to deals with diseases. The machine learning method of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was used to identify characteristic of patient at high cardiovascular risk from glycemic therapy for type-2 diabetes [9]. The participants were 40-79 years old with type 2 diabetes. The analysis involves four groups defined by age, BMI, and HGI (Haemoglobin glycosylation index) with varied risk for mortality under intensive glycemic therapy. The lowest risk group (HGI <0.44, BMI <30 kg/m2, age <61 years) had an absolute mortality risk decrease of 2.3% attributable to intensive therapy (95% CI 0.2 to 4.5, P = 0.038; number needed to treat: 43), whereas the highest risk group (HGI ≥0.44) had an absolute mortality risk increase of 3.7% attributable to intensive therapy (95% CI 1.5 to 6.0; P < 0.001; number needed to harm: 27). The overall results conclude that Age, BMI, and HGI may help individualize prediction of the benefit and harm from intensive glycemic therapy.
By AI techniques worked on a QSAR study on two diverse and enlarged α-amylase and α-glucosidase inhibitor databases collected from the ChEMBL [10]. They compared between different ML techniques for both datasets shown that k-NN (k-Nearest Neighbours) is the algorithm with the best fit. They also validated by two-way scheme based on ML-QSAR for the discrimination of active compounds from inactive chemicals in both α-amylase and α-glucosidase targets. AI for diabetes management and decision support is very important and by that we reduce the risk of death [11]. AI-powered tools for prediction and prevention of complications associated with diabetes. Our results indicate that AI methods are being progressively established as suitable for use in clinical daily practice, as well as for the self-management of diabetes. Consequently, these methods provide powerful tools for improving patients’ quality of life. They provide the General CRISP-DM model for the KDD process and how to that work for diabetes.
AI and machine learning in diabetes care they trying to optimize/automate therapy using machine learning algorithms at the patient level, on their routine visit scant at the global level and is nonexistent at the national level. Their work of area and data collection parameter was given prediction of diabetes, Glycemic control, prediction of glycemic events, and prediction of complications and Diagnosis of complications. Monitoring patients with diabetes using wearable sensors: predicting glycaemias using ECG and Respiration Rate by that they present a machine-learning based approach to predict and recognize anomalous blood glucose levels (hypo and hyper glycaemia) for patients with type I and II diabetes [12]. A general machine-learning approach was used to build classification models, based on attributes obtained from the ECG signals and respiration rate measurements. Two QSAR ways for anti-diabetic agents targeted using α-Amylase and α-Glucosidase Inhibitors [10]. In that they used model parameters settings in AI techniques. Two assay datasets that include α-amylase and α-glucosidase as enzyme targets are used. Optimized parameter values of the machine learning techniques by Workflow followed for the construction of the ML-QSAR models. They also checked parameter by ML-QSAR model for α-amylase inhibitory activity and α-glucosidase inhibitory activity. This paired virtual screening proposed could help to increase the successfully in the search of novel lead compounds for the inhibition of these hydrolytic enzymes and hence the management of type-2 DM.
AI proteomics improves cardiovascular risk assessment [13]. The integrin-binding structures promote leukocyte recruitment and stimulate expression of pro-inflammatory cytokines in the blood vessel wall, and by therefore represent important new biomarkers of CVD risk that could not be predicted based on earlier research. The study would therefore be able to use advanced proteomics methods to combine protein expression levels and PTM data with DPM profiles and analyses of EV cargo, thereby defining more effective bio signatures of clinical course in multi factorial disorders such as CVD. PEPPER was being developed using a patient-centric approach in order to improve patient self-efficacy and adherence to treatment. The software developed and adheres to international standards including those that apply to security and interoperability. The PEPPER system provides a portable personalized decision support system for insulin dosing that combines data from multiple sources such as body-worn sensors and manual inputs. The Case- Based Reasoning module is designed to provide a personalized insulin dose which adapts over time. A Model-Based Reasoning module is designed to maximize safety through prediction of adverse events and the detection of faults. The final system tested in silico before being clinically validated over a 6-month non-randomized open-label ambulatory trial [14]. Artificial Pancreas controller significantly improves all the evaluated glycemic outcomes in virtual type 1 diabetes on 11 adults, when compared against the Imperial College Artificial Pancreas without bolus adaptation over a three-month scenario with realistic inter-subject and intra-day variability. It is worth noting that the significant reduction in hyperglycemia was achieved without any increase in hypoglycemia. Trials have been planned to clinically validate the proposed technique [15].
Role of AI in Malaria Disease
Deep malaria AI driven discovery of potent antiplasmodials by given by AI, utilizing either structure-based or ligand-based approaches, has demonstrated highly accurate performances in the field of chemical property prediction [16]. Leveraging the existing data, AI would be a suitable alternative to blind-search HTS or fingerprint-based virtual Screening. The AI model would learn patterns within the data and help to search for hit compounds efficiently. In this work, we introduce Deep Malaria, a deep learning based process capable of predicting the anti-plasmodium falciparuminhibitory properties of compounds using their SMILES.
Investigate on cheminformatics based machine learning models for AMA1-RON2 abrogates for inhibiting plasmodium falciparumerythrocyte invasion [17]. This process was mediated by interaction between conserved Apical Membrane Antigen (AMA1) and Rhoptry Neck (RON2) protein, which is compulsory for successful invasion of erythrocyte by Plasmodium and manifestation of the disease Malaria. They used the physicochemical properties of the compounds available from a confirmatory high throughput screening. They tested for disruption capability of crucial molecular interaction; they trained supervised classifiers and validated their robustness by various statistical parameters. Integrative multi-kinase approach for the Identification of potent antiplasmodial hits and developed robust and predictive shape based and machine learning models, able to prioritize 10 promising hits as anti-malarial candidates [18]. Three compounds, LabMol-171, LabMol-172 and LabMol-181, reached activity in nano molar concentration against P. falciparumstrains, besides low cytotoxicity on mammalian cells (Figure 1). Moreover, these compounds did not show cross resistance with multidrug resistant strain, suggesting a different mechanism of action. Besides that, LabMol-171 and LabMol-181 also showed considerable inhibition of kinetic formation in P. Berghei standing out as powerful transmission blockers. Furthermore, a docking study shed some light into LabMol-171 interactions with CDPK1, CDPK4, and PK6 and suggests that could be a potential MKI, being able to bind with hinge and catalytic loop regions of proposed kinases.
Figure 1: Chemical structures of LabMol-171, LabMol-172 and LabMol-181.
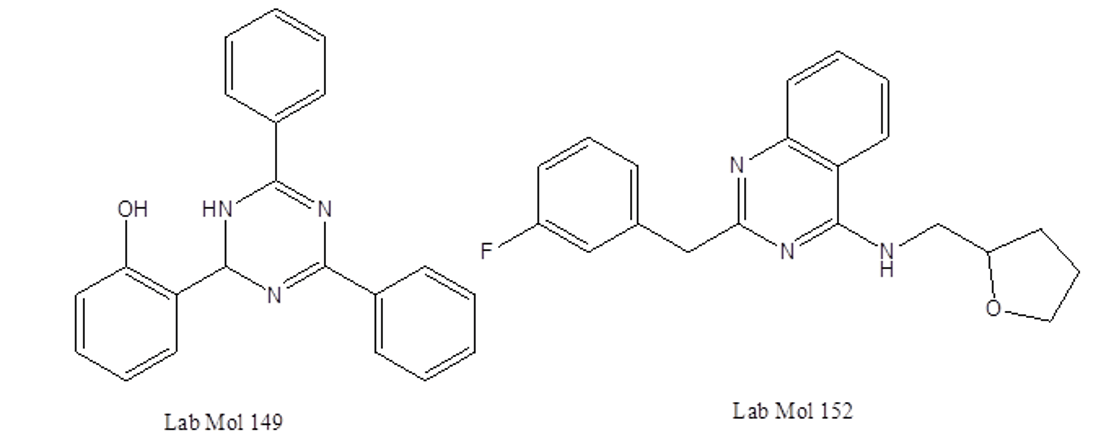
Deep learning-driven research for drug discovery in tackling malaria applied the best models for a virtual screening of a large database of chemical compounds [19]. The top computational predictions were evaluated experimentally against asexual blood stages of both sensitive and multi-drug-resistant plasmodium falciparumstrains. Among them, two compounds, LabMol-149 and LabMol-152, showed potent antiplasmodial activity at low nano molar concentrations (EC50 <500 nM) and low cytotoxicity in mammalian cells (Figure 2).
Figure 2: Chemical structures of LabMol-149 and LabMol-152.
Bayesian models trained with HTS data for predicting β-haematin inhibition and in vitro antimalarial activity studied both in vitro antimalarial activity and inhibitory data for β-haematin formation, largely obtained from publicly available sources, has been used to develop Bayesian models for inhibitors of β-haematin formation and in vitro antimalarial activity [20]. These models were used to screen two in-silico compound libraries. In the first, the 1510 U.S. Food and Drug Administration approved drugs available on PubChem were ranked from highest to lowest Bayesian score based on a training set of β-haematin inhibiting compounds active against P. falciparumthat did not include any of the clinical antimalarial or close analogues. The six known clinical antimalarial that inhibit β-haematin formation were ranked in the top 2.1% of compounds. Furthermore, the in vitro antimalarial hit-rate for this set of compounds was found to be 81% in the case of the subset where activity data are available in PubChem. In the second, a library of about 5,000 commercially available compounds (AldrichCPR) was virtually screened for ability to inhibit β-haematin formation and then for in vitro antimalarial activity. A selection of 34 compounds was purchased and tested, of which 24 were predicted to be β-haematin inhibitors. The hit rate for inhibition of β-haematin formation was found to be 25% and a third of these were active against P. falciparum, corresponding to enrichments estimated at about 25- and 140-fold relative to random screening, respectively [21].
Role of AI in Discovery of Anti-Microbial Agents
Machine learning-powered antibiotics phenotypic drug discovered and showed that a combined HCS and molecular information-based semi-automated phenotypic profiling platform, coupled to a ML-powered analysis pipeline, can effectively differentiate the MoA of novel antibacterial compound hits [22]. Hence the known antibacterial compound and target space of an existing pharmaceutical compound library is expanded. In addition, our approach enables SAR guidance and increases confidence that chemical modifications introduced during SAR, in an attempt to improve potency, retain desired compound MoA. They convinced that such a combined multipara metric HCS/genomic approach coupled to ML could be profitably applied in PDD across a broad range of biological problems.
Identification of novel antibacterial using machine learning techniques they develop an efficient in-silico model able to find compounds that have plenty of chances to exhibit antibacterial activity [23]. Based on a screening, they have accumulated a representative dataset of more than 140,000 molecules with antibacterial activity against Escherichia coli. They used a very large database of our proprietary HTS results to construct a highly discriminative and robust in-silico model able to score molecules by their antibacterial potency against E. coli. The main focus was placed on compounds with low similarity in structure to the reported antibacterial, as well as maximum diversity. Forty of the most reliable molecular descriptors were rationally selected from a whole pool of more than 1,700 calculated features. The final set of descriptors reflects several key aspects in privileged structures presented in antibacterial or non-antibacterial compounds and significant patterns hidden in the input chemical space.
The techniques of machine-learning for antibacterial drug discovery by Computer-Aided Drug Design (CADD), Ligand-Based Approaches and Receptor-Based Approaches because traditional drug-discovery paradigms have failed to keep up with the growing need for novel antibiotics [24]. Many pharmaceutical companies have abandoned antibiotic research entirely in search of more lucrative markets. Even those companies that have sought novel therapeutics have faced great challenges. Machine-learning methods have the potential and give the accuracy of high-throughput ligand- and receptor-based screening without sacrificing speed. They permit more nuanced binding estimates by freeing affinity prediction from predetermined formulaic or statistical forms. Rather, these techniques find patterns in observations of nature herself, independent of formulas or human theories.
Due to the rapid emergence of antibiotic-resistant bacteria, there was a growing need to discover new antibiotics. To address this challenge, we trained a deep neural network capable of predicting molecules with antibacterial activity for that A Deep Learning Approach to Antibiotic Discovery by Jonathan et al., Modern approaches to antibiotic discovery often includes screening large chemical libraries for those that elicit a phenotype of interest. Those screens, which are upper bound by hundreds of thousands to a few million molecules, were expensive, time consuming, and can fail to capture an expansive breadth of chemical space. In contrast, machine learning approaches afford the opportunity to rapidly and inexpensively explore vast chemical spaces in-silico. Our deep neural network model works by building a molecular representation based on a specific property, in our case the inhibition of the growth of E. coli, using a directed message passing approach. We first trained our neural network model using a collection of 2,335 diverse molecules for those that inhibited the growth of E. coli, augmenting the model with a set of molecular features, hyper parameter optimization, and assembling. Next, they applied the model to multiple chemical libraries, comprising >107 million molecules, to identify potential lead compounds with activity against E. coli. After ranking the candidates according to the model’s predicted score, they selected a list of promising candidates.
Worked on public health and epidemiology informatics: can AI help future global challenge they also emphasis on antimicrobial resistance and impact of climate change in disease epidemiology [25]. They give information of higher temperatures and increased precipitations are influencing the life cycle and the distribution of ticks, their spreading, development, and reproduction. As a consequence, tick-borne diseases are also spreading. Similarly, the West Nile virus, which was transmitted by mosquitos, was also expected to proliferate based on changes in mosquito populations. The increase in temperatures was allowing the overwintering of species and expanding the range of the disease-causing vectors. Chikungunya, a virus transmitted by the Aedes sp. mosquito, that caught a lot of attention from the media in the last years, was also affected by climate change: once again, the increase of the global temperature could lead to a proliferation of this mosquito in southern coastal regions. Many disease including dirofilariosis, tularemia, puumala virus, rabies, as well as airborne, food, and waterborne diseases caused.
AI-Driven Tools for Coronavirus
Epidemics of the COVID-19 discussed by K. C. Santosh, the importance of the AI-driven tools and their appropriate train and test models have been introduced and discussed [26]. AI-driven tools mostly employ RNA sequences. Besides, Electronic Health Record (EHRs), Computerized Tomography (CT) scans, Chest X-rays, and other data are considered and tested. Alibaba launched a new AI-based system to detect coronavirus infection via CT scans with an accuracy of up to 96% which was used to diagnose COVID-19 - can be complemented to the reverse-transcription polymerase chain reaction (RT-PCR) tests considering the spread rate of COVID-19 (across the globe), AI-driven tools are expected to work as cross population train/test models [27].
False-Negative Results of Real-Time Reverse-Transcriptase Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus-2 on Role of Deep-Learning-Based CT Diagnosis and Insights from Two Cases and confirm the result by rRT-PCR tests serve as the gold standard method to confirm the infection of SARS-CoV-2. They conclude their result by (Figure 3).
Figure 3: Chest CT scans for patient in Case 2. A) Thin-slice CT scan that shows glimpse of lesions. CT shows diffuse ground-glass opacities in dependent area of right lower lobe. B) Representative of DL-based segmentation of lesions in lower lobe of right lung that shows overview of automatically calculated ratios.
Artificial intelligence alarms suspected pneumonia based on relatively large proportion of abnormalities in lung. Detailed abnormality proportions in whole lungs, right upper lobe, right middle lobe, right lower lobe, left upper lobe, and left lower lobe were calculated and listed.
AI Distinguishes COVID-19 from Community Acquired Pneumonia on Chest CT a fully automatic framework to detect COVID-19 using chest CT and evaluate its performances [28]. Materials and Methods in this retrospective and multi-center study, a deep learning model, COVID-19 detection neural network (COVNet), was developed to extract visual features from volumetric chest CT exams for the detection of COVID-19. They also established deep learning model can accurately detect COVID-19 and differentiate it from community acquired pneumonia and other lung diseases. Identification of COVID-19 Can Be Quicker through AI Framework Using a Mobile Phone-Based Survey in the Populations When Cities/Towns Are Under Quarantine [29]. They used a mobile phone-based web survey. This reduced the spread in the susceptible populations and give very accurate data for COVID-19 disorder and spreading the community.
On the Coronavirus (COVID-19) outbreak and the smart city network on universal data sharing standards coupled with AI to benefit urban health monitoring and management the virus outbreak from an urban standpoint and advances how smart city networks should work towards enhancing standardization protocols for increased data sharing in the event of outbreaks or disasters, leading to better global understanding and management of the same [30]. They defeat coronvirus-19 in China by that technique and overcome that situation. Correlation of Chest CT and RT-PCR testing in COVID-19 in China on 1014 Cases in this study they conclude that cases had initial positive CT consistent with COVID-19 prior (or parallel) to the initial positive RT-PCR results [1].
Miscellaneous Role of Artificial Intelligence
Tumor necrosis factor (TNF) blocking agents are associated with lower risk for Alzheimer’s disease in patients with rheumatoid arthritis and psoriasis [31]. They analysed the value of a large, population-based database aggregating electronic health records from nearly 56 million adult patients for the rapid interrogation of the treatment benefit of prescription drugs. Patients diagnosed with a systemic inflammatory disease are at increased risk for developing Alzheimer’s disease, while TNF blocking agents were associated with decreased risk for comorbid Alzheimer’s disease in real-world patients diagnosed with rheumatoid arthritis or psoriasis.
Comparing multiple machine learning algorithms and metrics for estrogen receptor binding prediction in that work an exhaustive comparison of multiple machine learning algorithms, chemical spaces, and evaluation metrics for estrogen receptor binding was performed on numerous public datasets created using in-house chem [32]. informatics software, Assay Central. Chemical features were created with public tools, consisting of binary fingerprints and continuous molecular descriptors. Each feature set was subjected to either Classic Machine Learning algorithms or Deep Neural Networks of varying depth. Models were then evaluated using a variety of metrics, including five-fold cross validation which showed Deep Neural Networks had a clear advantage for prediction within the training set over Classic Machine Learning models.
The rom machine learning to deep learning: progress in machine intelligence for rational drug discovery and defined how machine intelligence, which is normally presented as artificial intelligence, refers to the intelligence exhibited by computers [33]. An artificial neural network integrated pipeline for biomarker discovery using Alzheimer's disease experiments they show greater understanding of the biology behind Alzheimer's disease, its progression and the mechanisms involved [34]. By expanding to other brain regions and datasets and focusing the questions on the most relevant genes, it is possible to identify new markers and drivers of the disease that can be used alongside the current ones to improve prognosis and provide more targets for therapy.
The precision psychiatry applications with pharmacogenomics, artificial intelligence and machine learning approaches application of AI in correlation with physical parameter works have applied [35]. AI and machine learning methods to predict diagnosis of certain psychiatric disorders such as Alzheimer’s disease, autism spectrum disorder, and schizophrenia. TopP–S: Persistent homology‐based multi‐task deep neural networks for simultaneous predictions of partition coefficient and aqueous solubility studied by [36, 37]. This work introduces an algebraic topology‐based method, called element‐specific persistent homology (ESPH), as a new representation of small molecules that is entirely different from conventional chemical and/or physical representations. ESPH describes molecular properties in terms of multi scale and multi component topological invariants. Such topological representation is systematically, comprehensive, and scalable with respect to molecular size and composition variations. This strategy leads to a more accurate prediction of relatively small datasets. A total of six datasets was considered in this work to validate the proposed topological and multitask deep learning approaches.
Machine learning models for lipophilicity and their domain of applicability. Unfavourable lipophilicity and water solubility cause many drug failures [38]. They used a modern Bayesian machine learning algorithm Gaussian process model this study constructs a log D7 model based on 14556 drug discovery compounds of Bayer Schering Pharma. Performance was compared with support vector machines, decision trees, ridge regression, and four commercial tools. The 81% were predicted correctly within 1 log unit, compared to only 44% achieved by commercial software by them.
Estimating the domain of applicability for machine learning QSAR models: a study on aqueous solubility of drug discovery molecules investigate error bars from a Bayesian model (Gaussian Process (GP), an ensemble based approach (Random Forest), and approaches based on the Mahalanobis distance to training data (Support Vector Machine and Ridge Regression models) [39]. They evaluate all approaches in terms of their prediction accuracy (in cross-validation, and on an external validation set of 536 molecules) and in how far the individual error bars can faithfully represent the actual prediction error.
Improved prediction of aqueous solubility of novel compounds by done deeper with deep learning [40]. Aqueous solubility was an important physicochemical property of compounds in anti-cancer drug discovery and development, impacting pharmacokinetic properties and formulations for that AI solubility prediction tools have been developed by employing regression and modelling, machine learning, and deep learning methods. Those compounds considered by medicinal chemistry experts as difficult for solubility estimations. To better explore the learning capability of deeper-net architectures, the molecular representations of the compounds may be selected for conforming to these architectures. Specifically, the superior local-feature learning capability of the CNN architectures may be better exploited by using the substructure-encoded molecular fingerprints for representing compounds. By novel approach may find broader applications in the development of high-performance deep learning models for the prediction of various pharmacodynamics, pharmacokinetic, and toxicological properties [41].
Deep architectures and deep learning in chemo informatics: The prediction of aqueous solubility for drug-like molecules that show that the UG-RNN approach can be used to build aqueous solubility predictors that match and sometimes outperform current state-of-the-art methods. One important difference between UG-RNN-based approaches with respect to other methods was the ability to automatically extract internal representations from the molecular graphs that are well suited for the specific tasks [42]. That aspect was an important advantage for a problem like aqueous solubility prediction, where the optimal feature set is not known and may even vary from one dataset to the other. It also saves time and avoids other costs and limitations associated with the use of human expertise to select features. Blood test for Alzheimer’s disease by candidate’ blood (and CSF) markers blood biomarker studies and clinical trials [43]. They perform RADAR-CNS (RADAR-CNS.org), a major goal of which is to develop a generalized real-time streaming platform that and enable active and passive remote monitoring, tracking phenotypes such as function and cognition. Gene expression has also been explored as a potential source of blood biomarkers for AD. Little consistency has been seen in the genes selected by these various studies, to suggest that greater concordance might be seen at the pathway level [44].
The in silico prediction inhibitory of constant of thrombin inhibitors by using machine learning techniques [45]. This work was carried out to predict Ki values of thrombin inhibitors based on a large data set by using machine learning methods. Total of 6554 descriptors for each compound were collected and four different methods including multiple linear regression (MLR), K Nearest Neighbours (KNN), Gradient Boosting Regression Tree (GBRT) and Support Vector Machine (SVM) were used to build prediction models with these selected descriptors. The SVM model was the best one among these methods with R2=0.84, MSE=0.55 for the training set and R2=0.83, MSE=0.56 for the test set.
Predict bioactivities of ligand molecules acting with G protein-coupled receptors by using weighted deep learning and random forest approach (WDL-RF) [37]. The algorithm consists of two consecutive stages:
i. Molecular fingerprint generation through a new weighted deep learning method
ii. Bioactivity calculations with a random forest model.
That research includes testing on a set of twenty-six non-redundant GPCRs that have a high number of active ligands (each with 200-4000 ligand associations). The overall result conclude that WDL-RF can generate bioactivity predictions with an average root-mean square error 1.33 and correlation coefficient (r2) 0.80 compared to the experimental measurements, which are significantly more accurate than the control predictors with different molecular fingerprints and descriptors. OCT-based deep learning algorithm for the evaluation and treatment indicated with anti-vascular endothelial growth factor medications [46]. The machine learning methods can offer the clinician support in the decision-making process. Care should be taken not to mistake neural network output as treatment recommendation and to ensure a final thorough evaluation by the treating physician.
Deep learning-based approached to predicted gene regulating effects of small molecules [47]. They successfully combined molecular fingerprint descriptors and gene descriptors to train deep neural networks that predict differential gene regulation endpoints collected in LINCS database. They achieved 10-fold cross-validation RAUC scores of and above 0.80, as well as enrichment factors of >5. The deep learning models can effectively synergize molecular and genomic descriptors and can be used to screen for novel drug candidates with the desired effect on gene expression. Mathematical deep learning for pose and binding affinity prediction and ranking in D3R grand challenges [48]. D3R grand challenge 2 focused on the pose prediction, binding affinity ranking and free energy prediction for Farnesoid X receptor ligands. Those models obtained the top place in absolute free energy prediction for free energy set 1 in stage 2. The latest competitions, D3R Grand Challenge 3 (GC3), were considered as the most difficult challenge so far. It has five sub challenges involving Cathepsin S and five other kinase targets, namely VEGFR2, JAK2, p38-α, TIE2, and ABL1. There were a total of 26 official competitive tasks for GC3. Our predictions were ranked 1st in 10 out of these 26 tasks. AI in drug discovery by while many of the new approaches have yet to bear fruit in terms of drugs being progressed to market, initial reports tend toward the belief that they became even more integral in the drug discovery process than has hitherto been seen [49]. New systems can design new chemical structures effectively, predicted for the desired molecular property profiles and even how to synthesize those compounds.
On MABAL: a novel deep-learning architecture for machine-assisted bone age labeling by that demonstrated the benefits of a customized neural network algorithm carefully calibrated to the evaluation of bone age utilizing a relatively large institutional dataset. In doing so, that study showed that advanced architectures can be successfully trained from scratch in the medical imaging domain and can generate results that outperform any existing proposed algorithm [50]. Deep learning based regression and multi-class models for acute oral toxicity prediction with automatic chemical feature extraction done prediction of human cytochrome P450 inhibition using a multi-task deep auto encoder neural network [51].
Deep learning in medical image analysis and applied to lesion detection or classification have reported superior performance compared to those by conventional techniques or even better than radiologists in some tasks [6]. The potential of applying deep-learning-based medical image analysis to computer-aided diagnosis (CAD), thus providing decision support to clinicians and improving the accuracy and efficiency of various diagnostic and treatment processes, has spurred new research and development efforts in CAD. Endoscopic diagnosis using AI, the CAD is expected to help endoscopists improve detection and characterization of polyp, cancer, and inflammation in all digestive area [52]. Some CAD systems showing ability better than endoscopists have been reported. Deep learning beyond cats and dogs: recent advances in diagnosing breast cancer with deep neural networks [53]. They evaluated the impact of deep learning based diagnostic systems that can help clinicians with screening and diagnosing breast cancer. AI in cardiology identified how cardiovascular medicine could incorporate artificial intelligence in the future [54]. They predicted modeling concepts relevant to cardiology such as feature selection and frequent pitfalls such as improper dichotomization. They describes the advent of deep learning and related methods collectively called unsupervised learning, provides contextual examples both in general medicine and in cardiovascular medicine, and then explains how these methods could be applied to enable precision cardiology and improve patient outcomes.
AI in medical imaging of the liver discussed that AI used and getting increasingly popular in the medical imaging of the liver, including radiology, ultrasound, and nuclear medicine [55]. It assisted physicians to make more accurate and reproductive imaging diagnosis and also reduce the physician’s workload. Artificially intelligent proteomics improves cardiovascular risk assessment by de novo integrin-binding structures promote leukocyte recruitment and stimulate expression of pro-inflammatory cytokines in the blood vessel wall and may therefore represent important new biomarkers of CVD risk that could not be predicted based on earlier research. He combines protein expression levels and PTM data with DPM profiles and analyses of EV cargo, thereby defining more effective bio-signatures of clinical course in multi factorial disorders such as CVD.
Conclusion
Traditional approaches of drug design were expensive and time-consuming. Over the past 25 years, medicinal chemistry has applied AI in various forms and with varying degrees of success to develop and design the compound and medicinal use. AI helps in the design of new inputs, i.e. drug discovery process from target selection, lead identification, and lead optimization to preclinical studies and clinical trials. From the above description, it is concluded AI can design new chemical structures effectively, predicted for the desired molecular property profiles, how to synthesize active compounds; identify the disease, physical parameter for the medical purpose.
Conflicts of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Article Info
Article Type
Review ArticlePublication history
Received: Tue 08, Dec 2020Accepted: Thu 21, Jan 2021
Published: Sat 30, Jan 2021
Copyright
© 2023 Rupesh Dudhe. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2021.01.02
Author Info
Anshu Chaudhary Dudhe Rupesh Dudhe Suhas N. Sakarkar Omji Porwal
Corresponding Author
Rupesh DudheSchool of Pharmacy, G H Raisoni University, Saikheda, Saunsar, Chhindwara, Madhya Pradesh, India
Figures & Tables


References
- Ai T, Yang Z, Hou H, Zhan C, Chen C (2020) Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 296: E32-E40. [Crossref]
- Ivanenkov YA, Zhavoronkov A, Yamidanov RS, Osterman IA, Sergiev PV et al. (2019) Identification of Novel Antibacterials Using Machine Learning Techniques. Front Pharmacol 10: 913. [Crossref]
- Athreya AP, Gaglio AJ, Cairns J, Kalari KR, Weinshilboum RM et al. (2018) Machine Learning helps Identify New Drug Mechanisms in Triple Negative Breast Cancer. IEEE Trans Nanobioscience 17: 251-259. [Crossref]
- Chen X, Chen HY, Chen ZD, Gong JN, Chen CY (2020) A Novel Artificial Intelligence Protocol for Finding Potential Inhibitors of Acute Myeloid Leukemia. J Mater Chem B 8: 2063-2081. [Crossref]
- Workman P, Antolin AA, Lazikani BA (2019) Transforming cancer drug discovery with Big Data and AI. Expert Opin Drug Discov 14: 1089-1095. [Crossref]
- Chan HP, Samala RK, Hadjiiski LM (2020) CAD and AI for Breast Cancer-Recent Development and Challenges. Br J Radiol 93: 20190580. [Crossref]
- Jalalian A, Mashohor SBT, Mahmud HR, Saripan MIB, Ramli ARB et al. (2013) Computer-aided Detection/Diagnosis of Breast Cancer in Mammography and Ultrasound: a Review. Clin Imaging 37: 420-426. [Crossref]
- Le EPV, Wang Y, Huang Y, Hickman S, Gilbert FJ (2019) Artificial intelligence in breast imaging 74: 357-366. [Crossref]
- Basu S, Vellakkal S, Agrawal S, Stuckler D, Popkin B et al. (2014) Averting obesity and Type 2 Diabetes in India through Suga-Sweetened Beverage Taxation: An Economic Epidemiologic Modeling Study. PLoS Med 11: e1001582. [Crossref]
- Santana KD, Oscar M, Borroto R, Puris A, Le Thi Thu H et al. (2017) A Two QSAR Way for Antidiabetic Agents Targeting Using α-Amylase and α-Glucosidase Inhibitors: Model Parameters Settings in Artificial Intelligence Techniques. Lett Drug Design Discov 14: 1-7.
- Contreras I, Vehi J (2018) Artificial Intelligence for Diabetes Management and Decision Support: Literature Review. J Med Internet Res 20: 5e1075. [Crossref]
- Bozidara C, Urska P, Anton G, Mitja L (2016) Monitoring Patients with Diabetes Using Wearable Sensors: Predicting Glycaemias Using ECG and Respiration Rate. 22nd Eur Confe AI 18-21.
- Sze SK (2019) Artificially intelligent proteomics improves cardiovascular risk assessment. EBioMedicine 40: 23-24. [Crossref]
- Herrero P, ´Opez BL, Martin C (2016) PEPPER: Patient Empowerment Through Predictive Personalised Decision Support. Artificial Intelligence for Diabetes. 22nd Eur Confere AI 8-10.
- Herrero P, Bondia J, Pesl P, Oliver N, Georgiou P (2016) Enhancing an Artificial Pancreas with an Adaptive Bolus Calculator based on Case-Based Reasoning. AI for Diabetes. 22nd Eur Confere AI 10-12.
- Arshadi AK, Salem M, Collins J, Yuan JS, Chakrabarti D (2020) Deep Malaria: Artificial Intelligence Driven Discovery of Potent Antiplasmodials. Fro Pharmacol 10: 1526. [Crossref]
- Maindola P, Jamal S, Grover A (2015) Cheminformatics Based Machine Learning Models for AMA1-RON2 Abrogators for Inhibiting Plasmodium Falciparum Erythrocyte Invasion. Mol Inform 34: 655-664. [Crossref]
- Lima MNN, Cassiano GC, Tomaz KC, Silva AC, Sousa BKP et al. (2019) Integrative Multi-Kinase Approach for the Identification of Potent Antiplasmodial Hits. Front Chem 7: 773. [Crossref]
- Neves BJ, Braga RC, Alves VM, Lima MNN, Cassiano GC et al. (2020) Deep Learning-driven research for drug discovery: Tackling Malaria. PLoS Comput Biol 16: e1007025. [Crossref]
- Wicht KJ, Combrinck JM, Smith PJ, Egan TJ (2015) Bayesian models trained with HTS data for predicting β-haematin inhibition and in vitro antimalarial activity. Bioorg Med Chem 23: 5210-5217. [Crossref]
- Romm EL, Tsigelny IF (2020) Artificial Intelligence in Drug Treatment. Annu Rev Pharmacol Toxicol 60: 353-369. [Crossref]
- Zoffmann S, Vercruysse M, Benmansour F, Maunz A, Wolf L et al. (2019) Machine learning-powered antibiotics phenotypic drug discovery. Sci Rep 9: 5013. [Crossref]
- Zhavoronkov A , Ivanenkov YA, Aliper A, Veselov MS, Aladinskiy VA et al. (2019) Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat Biotechnol 37: 1038-1040. [Crossref]
- Durrant JD, Amaro RE (2015) Machine-Learning Techniques Applied to Antibacterial Drug Discovery. Chem Biol Drug 85: 14-21. [Crossref]
- González AR, Zanin M, Ruiz EM (2019) Public Health and Epidemiology Informatics: Can Artificial Intelligence Help Future Global Challenges? An Overview of Antimicrobial Resistance and Impact of Climate Change in Disease Epidemiology. Yearb Med Inform 28: 224-232. [Crossref]
- Santosh KC (2020) AI-Driven Tools for Coronavirus Outbreak: Need of Active Learning and Cross-Population Train/Test Models on Multitudinal/Multimodal Data. J Med Syst 44: 93. [Crossref]
- Dasheng Li, Wang D, Dong J, Wang N, Huang H et al. (2020) False-Negative Results of Real-Time Reverse-Transcriptase Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus 2: Role of Deep-Learning-Based CT Diagnosis and Insights from Two Cases. Korean J Radiol 21: 505-508. [Crossref]
- Li L, Qin L, Xu Z, Yin Y, Wang X et al. (2020) Artificial Intelligence Distinguishes COVID-19 From Community Acquired Pneumonia on Chest CT. Radiology 19: 200905. [Crossref]
- Rao ASR, Vazquez JA (2020) Identification of COVID-19 Can Be Quicker Through Artificial Intelligence Framework Using a Mobile Phone-Based Survey in the Populations When Cities/Towns Are Under Quarantine. Infect Control Hosp Epidemiol 41: 826-830. [Crossref]
- Allam Z, Jones DS (2020) On the Corona virus (COVID-19) Outbreak and the Smart City Network: Universal Data Sharing Standards Coupled With Artificial Intelligence (AI) to Benefit Urban Health Monitoring and Management. Healthcare (Basel) 8: 46. [Crossref]
- Zhou M, Xu R, Kaelber DC, Gurney ME (2020) Tumor Necrosis Factor (TNF) blocking agents is associated with lower risk for Alzheimer’s disease in patients with rheumatoid arthritis and psoriasis. PLoS One 15: e0229819. [Crossref]
- Russo DP, Zorn KM, Clark AM, Zhu H, Ekins S (2018) Comparing Multiple Machine Learning Algorithms and Metrics for Estrogen Receptor Binding Prediction. Mol Pharm 15: 4361-4370. [Crossref]
- Zhang L, Tan J, Han D, Zhu H (2017) From machine learning to deep learning: progress in machine intelligence for rational drug discovery. Drug Discov Today 22: 1680-1685. [Crossref]
- Zafeiris D, Rutella S, Ball GR (2018) An Artificial Neural Network Integrated Pipeline for Biomarker Discovery Using Alzheimer's Disease as a Case Study. Comput Struct Biotechnol J 16: 77-87. [Crossref]
- Lin E, Lin CH, Lane HY (2020) Precision Psychiatry Applications with Pharmacogenomics: Artificial Intelligence and Machine Learning Approaches. Int J Mol Sci 21: 969. [Crossref]
- Wu K, Zhao Z, Wang R, Wei GW (2018) TopP-S: Persistent homology‐based multi‐task deep neural networks for simultaneous predictions of partition coefficient and aqueous solubility. J Computat Chem 39: 1444-1454. [Crossref]
- Wu J, Zhang Q, Wu W, Pang T, Hu H et al. (2018) WDL-RF: Predicting Bioactivities of Ligand Molecules Acting With G Protein-Coupled Receptors by Combining Weighted Deep Learning and Random Forest. Bioinformatics 34: 2271-2282. [Crossref]
- Schroeter TS, Schwaighofer A, Mika S, Laak AT, Suelzle D et al. (2007) Machine learning models for lipophilicity and their domain of applicability. Mol Pharm 4: 524-538. [Crossref]
- Schroeter TS, Schwaighofer A, Mika S, Laak AT, Suelzle D et al. (2007) Machine learning models for lipophilicity and their domain of applicability. Mol Pharm 4: 524-538. [Crossref]
- Cui Q, Lu S, Ni B, Zeng X, Tan Y et al. (2020) Improved Prediction of Aqueous Solubility of Novel Compounds by Going Deeper With Deep Learning. Front Oncol 10: 121. [Crossref]
- Stokes JM, Yang K, Swanson K, Jin W, Cubillos Ruiz A et al. (2020) A Deep Learning Approach to Antibiotic Discovery. Cell 180: 688-702. [Crossref]
- Lusci A, Pollastri G, Baldi P (2013) Deep architectures and deep learning in chemoinformatics: the prediction of aqueous solubility for drug-like molecules. Chem Inf Model 53: 1563-1575. [Crossref]
- Kiddlea SJ, Voylea N, Dobson RJ (2018) A Blood Test for Alzheimer's Disease: Progress, Challenges, and Recommendations. J Alzheimers Dis 64: 1. [Crossref]
- Han G, Wang J, Zeng F, Feng X, Yu J et al. (2013) Characteristic transformation of blood transcriptome in Alzheimer's disease. J Alzheimers Dis 35: 373-386. [Crossref]
- Zhao J, Zhu L, Zhou W, Yin L, Wang Y et al. (2018) In Silico Prediction of Inhibitory Constant of Thrombin Inhibitors Using Machine Learning. Comb Chem High Throughput Screen 21: 662-669. [Crossref]
- Prahs P, Raeck V, Mayer C, Cvetkov Y, Cvetkova N et al (2018) OCT-based deep learning algorithm for the evaluation of treatment indication with anti-vascular endothelial growth factor medications. Graefes Arch Clin Exp Ophthalmol 256: 91-98. [Crossref]
- Woo G, Fernandez M, Hsing M, Lack NA, Cavga AD et al. (2020) Deep COP: deep learning-based approach to predict gene regulating effects of small molecules. Bioinformatics 36: 813-818. [Crossref]
- Nguyen DD, Cang Z, Wu K, Wang M, Cao Y et al. (2019) Mathematical deep learning for pose and binding affinity prediction and ranking in D3R Grand Challenges. J Comp Aided Mol Des 33: 71-82. [Crossref]
- Sellwood MA, Ahmed M, Segler MH, Brown N (2018) Artificial intelligence in drug discovery. Future Med Chem 10: 2025-2028. [Crossref]
- Mutasa S, Chang PD, Shapiro CR, Ayyala R (2018) MABAL: a Novel Deep-Learning Architecture for Machine-Assisted Bone Age Labeling. J Digital Imaging 31: 513-519. [Crossref]
- Sushko I, Salmina E, Potemkin VA, Poda G, Tetko IV (2012) ToxAlerts: A Web Server of Structural Alerts for Toxic Chemicals and Compounds with Potential Adverse. Reactions. J Chem Inf Model 52: 2310-2316. [Crossref]
- Hirasawa T, Ikenoyama Y, Horie Y, Ishioka M, Tamashiro A et al. (2019) Endoscopic Diagnosis Using Artificial Intelligence. Gan To Kagaku Ryoho 46: 412-417. [Crossref]
- Burt JR, Torosdagli N, Khosravan N, RaviPrakash H, Mortazi A (2018) Deep learning beyond cats and dogs: recent advances in diagnosing breast cancer with deep neural networks. Br J Radiol 91: 20170545. [Crossref]
- Johnson KW, Soto JT, Glicksberg BS, Shameer K, Miotto R et al. (2018) Artificial Intelligence in Cardiology. J Am Coll Cardiol 71: 2668-2679. [Crossref]
- Zhou LQ, Wang JY, Yu SY, Wu GG, Wei Q et al. (2019) Artificial intelligence in medical imaging of the liver. World J Gastroenterol 25: 672-682. [Crossref]

