Journals
Adiposity and depressive symptoms in women with and without HIV infection. The Women?s Interagency HIV Study
A B S T R A C T
Depression is a common neuropsychiatric disorder in women, particularly among women with HIV infection. The association of adiposity with depressive symptoms in adult women is unclear. We evaluated the cross-sectional association of depressive symptoms measured using the Centre for Epidemiological Studies Depression scale (CES-D) score, with anthropometric (body mass index, waist-to-hip ratio and waist circumference) and metabolic (adipokines: leptin, total adiponectin, and high molecular weight adiponectin) adiposity measures. This was accomplished in HIV-infected or at-risk HIV-uninfected participants at the Brooklyn, New York site of the Women’s Interagency HIV Study. Participants (250 HIV+, 107 HIV-; average age 38.9 years), with measured levels of leptin and adiponectins were included. Adiposity measures were considered as continuous and categorical variables. A clinically relevant depressive symptom burden was defined as CES-D > 16. Spearman correlations, T-tests, multivariable linear and logistic regression models, and Receiver Operating Characteristic tests were used. Neither anthropometric nor metabolic adiposity measures were associated with depressive symptoms in this sample. To our knowledge, this is the first report on the association between depressive symptoms and anthropometric and metabolic adiposity measures in HIV-infected or HIV-uninfected women. Despite null findings, these results contribute to our understanding of adiposity-associated risk related to neuropsychiatric outcomes in at risk women.
K E Y W O R D S
Depressive symptoms, adipokine, leptin, adiponectin, HIV, women, overweight, obesity, neuropsychiatry
I N T R O D U C T I O N
Chronic Human Immunodeficiency Virus (HIV) infection is prevalent worldwide. With the advent of antiretroviral therapies (ART), adults with HIV infection are living longer, primarily due to a decrease in deaths secondary to AIDS (Acquired Immune Deficiency Syndrome) related conditions [1]. In addition, the wasting syndrome that characterized HIV infection and AIDS prior to the availability of ART has almost disappeared since weight gain is often a side effect of adherence to ART [2]. Thus, adults living with HIV infection because of adherence to their medication regimens, also tend to be overweight or obese and at risk for chronic diseases associated with overweight and obesity [3, 4].
Overweight and obesity are leading causes of disability and death in the United States, and around the world [4, 5]. Overweight and obesity have been associated with elevated risk of developing a number of peripheral diseases, including Type 2 diabetes, certain cancers, and immune-mediated disorders [6-8]. Overweight and obesity are also associated with neuropsychiatric disorders, such as depression and cognitive disorders including dementias [9]. Interestingly, overweight and obesity associations are often inconsistent for neuropsychiatric disorders. For example, obesity has been positively associated with depression, increasing the risk of major depression by 37% [10-12]. However, overweight and obesity have also been associated with less depression, a theory originating in part from the Jolly Fat hypothesis first published in 1976. The Jolly Fat hypothesis was based on clinical study observations that obese people (men and women who were above 20% and 40% of standard weight, respectively) were less anxious, and that men were less depressed [13].
Inconsistent associations observed between overweight and obesity and depressive symptoms have led to the hypothesis that adipokines may be better indicators of underlying pathophysiologic adipose tissue mechanisms related to depression and other disorders [14]. Adipose tissue functions as the largest endocrine organ by secreting approximately 800 adipokines (hormones, peptides, and cytokines) [15, 16]. Two adipokines, leptin and adiponectin, influence processes in the peripheral and central nervous systems and may be dysregulated in HIV+ adults, on or off ART [17, 18].
Leptin is a 16 kDa protein hormone, primarily secreted by adipose tissue, that decreases food intake and energy expenditure [19]. Leptin is positively correlated, r>0.70, with anthropometric measures including body mass index (BMI) and waist circumference in adults, including among women enrolled in the Brooklyn Women’s Interagency HIV Study (WIHS), who, on average, are overweight and obese [20, 21]. Leptin receptors are expressed in limbic areas such as the hippocampus and amygdala that are responsible for mood regulation. In animal models, leptin has shown to improve memory and have behavioral effects consistent with anti-depressant action [21].
Data on leptin and depressive symptoms in humans are inconsistent. Cross-sectionally, higher levels of blood leptin and waist circumference have been positively associated with Center for Epidemiological Studies Depression scale (CES-D) score, particularly among those having higher levels of both [22]. In contrast, higher leptin levels have been independently associated with decreased depressive and anxiety symptoms in women; but not in patients with metabolic disorders [23, 24].
Adiponectin is a protein secreted by adipocytes and is involved in glucose and lipid homeostasis. It is also anti-atherogenic, anti-inflammatory, and cardioprotective. An inverse correlation has been found between BMI and circulating adiponectin (r = -0.30- -0.40); and serum levels are decreased in adults with visceral obesity and insulin resistance [25-27]. Adiponectin circulates in several multimeric forms. It is suspected that one form, a high molecular weight complex, is the active form [28, 29]. Adiponectin has also been shown to protect against apoptosis and hyperglycemia-induced oxidative stress of endothelial cells [30, 31]. Since depression is also associated with cardiovascular disease, adiponectin may be a critical link among overweight and obesity, depression and cardiovascular disease [32]. Adiponectin has also been linked to mood states [33-36].
To our knowledge, there are no publications on leptin or adiponectin levels in association with depressive symptoms among women with or at risk for HIV-infection. Based on previously published reports from the WIHS, higher levels anthropometric measures and lower levels of leptin were associated with better cognition [25, 37]. Since depression and cognitive disorders tend to co-exist, we utilized the rich Brooklyn WIHS data base to analyze whether these adiposity measures were similarly associated with depressive symptoms, and evidence of the Jolly Fat hypothesis.
Methods
The WIHS is an ongoing prospective study of women with or at-risk for HIV infection [38]. The WIHS began in 1994 and enrolled 3766 women across six sites, San Francisco, Los Angeles, Chicago, Washington DC, Brooklyn and the Bronx. Participants were evaluated every six months with an extensive interview that included history of interval illnesses and interval substance abuse, current medications and medication adherence, physical exam, and blood and gynecological specimen collection. Participants were also asked to report their current smoking status, and use of marijuana, ‘crack’, cocaine, and heroin. At-risk HIV- women were matched on the demographic and risk profile of HIV-infected women.
The Brooklyn WIHS site has participated since the WIHS’ inception. In 2005, 347 participants (243 HIV+, 104 HIV-) enrolled in Brooklyn WIHS had CES-D scores, anthropometric measures, and leptin and adiponectin levels available. Therefore, only Brooklyn WIHS participants form the effective sample for these analyses. The Brooklyn WIHS protocol was approved by the State University of New York Downstate Medical Centre (SUNY DMC) Institutional Review Board (IRB). All participants provided written informed consent. All methods were performed in accordance with SUNY DMC IRB guidelines and regulations.
I Demographic measures
All demographic measures were self-reported. Race was reported as white, Hispanic, African-American, or ‘other’ (self-reported as Native American/Alaskan, Asian/Pacific Islander or other).
II Clinical measures
Anthropometric measures were conducted according to the National Health and Nutrition Examination Survey III protocol and included body weight (pounds), body height (inches), waist and hip circumferences (cm). Anthropometric measurements were conducted with participants wearing light clothing. Body weight was recorded to the nearest 1.0 pound, and body height was measured to the nearest 1.0 inch. After conversion of body weight and height to metric units, BMI was calculated as kilograms per meter squared (kg/m2). Categories of BMI used to estimate total body adiposity are: underweight, <18.5 kg/m2; healthy, 18.5-24.9 kg/m2 overweight, > 25.0-29.9 kg/m2, and obesity, >30 kg/m2. Waist and hip circumferences were measured to the nearest 0.50cm. waist-to-hip ratio was calculated as the ratio of waist circumference (cm) to hip circumference (cm). Central obesity was defined as waist-to-hip ratio >0.85.
III Adipokine measures
Eight hour fasted blood samples were collected. Plasma samples for leptin and adiponectin analyses were drawn within one visit of testing for depressive symptoms in patients. Standards and controls were tested in duplicate using a leptin ELISA measured by Luminex multiplex assays (Millipore, Billerica, MA). For leptin, undiluted samples were tested, and plates were prepared according to protocol. The 7-point standard curve ranges from 0.5 – 100 ng/ml. Plates were read using a Molecular Devices Plate reader and Softmax Pro data analysis software (Molecular Devices, Sunnyvale, CA). A 4-point logistic curve fit was used. For adiponectin, diluted samples were assayed, and plates were prepared according to protocol. The 7-point standard curve range was 1.56 – 100 ng/mL and tested at a final dilution of 1:500.
IV HIV-related variables
Methods for determining HIV status, AIDS diagnosis, CD4 count, viral load, and duration of ART use have been described previously in other studies [38-40].
V Depressive symptoms
The CES-D is a 20-item self-report scale, scores ranging from 0-60 points, that assesses the frequency of depressive symptoms according to the Diagnostic and Statistical Manual of Mental Disorders 5 criteria. A cut point of > 16 indicates a clinically relevant depressive symptom burden and association with major depressive disorder [41]. The CES-D is administered to all English-speaking WIHS participants. These analyses used a CES-D score obtained one time during visits 21 to 24 (October 2004 to September 2006).
Table 1: Characteristics of all Brooklyn WIHS participants and by HIV infection status
|
Characteristic |
N |
All (n=365) mean (SD) |
N |
HIV + (n=257) mean (SD) |
N |
HIV - (n=108) mean (SD) |
P* |
|
Age |
363 |
38.9 (9.1) |
255 |
39.9 (8.5) |
108 |
36.5 (9.9) |
0.001 |
|
CD4 count |
|
|
252 |
517.0 (323.0) |
|
|
|
|
Viral load |
|
|
252 |
28175.1 (139978.6) |
|
|
|
|
CES-D scores |
355 |
12.1 (11.2) |
250 |
12.47 (11.7) |
105 |
11.45 (10.1) |
0.429 |
|
≥16 |
108 |
25.9 (9.5) |
74 |
27.0 (10.3) |
34 |
23.7 (7.1) |
|
|
<16 |
247 |
6.13 (4.6) |
176 |
6.36 (4.7) |
71 |
5.6 (4.4) |
|
|
Adipokines |
|
|
|
|
|||
|
Leptin (ng/ml) |
357 |
30.7 (29.6) |
250 |
28.9 (28.5) |
107 |
34.8 (31.7) |
0.091 |
|
Total Adiponectin (ng/ml) |
358 |
6985.8 (3863.7) |
251 |
7017.6 (4114.4) |
107 |
6193.0 (3238.1) |
0.799 |
|
HMW Adiponectin (ng/ml) |
216 |
3968.5 (5127.1) |
160 |
4120.3 (5646.3) |
56 |
3534.9 (3215.0) |
0.449 |
|
BMI (kg/m2) |
357 |
29.2 (8.0) |
252 |
28.7 (7.3) |
105 |
30.3 (9.1) |
0.088 |
|
<18.5 |
7 |
17.0 (1.0) |
6 |
17.1 (1.0) |
1 |
15.8 |
|
|
18.5-24.9 |
115 |
22.5 (1.6) |
83 |
22.6 (1.6) |
32 |
22.3 (1.8) |
|
|
25.0-29.9 |
107 |
27.2 (1.4) |
78 |
27.3 (1.5) |
29 |
27.2 (1.3) |
|
|
≥30.0 |
128 |
37.6 (6.9) |
85 |
37.0 (6.1) |
43 |
38.7 (8.1) |
|
|
Waist-to-hip Ratio |
270 |
0.90 (0.1) |
185 |
0.9 (.1) |
85 |
0.8 (.1) |
0.000 |
|
Waist Circumference (cm) |
272 |
91.1 (16.2) |
186 |
91.0 (15.4) |
86 |
91.5 (17.8) |
0.807 |
* P-values were calculated using independent samples two tailed T-Test, comparing HIV+ and HIV- participants with equal variances assumed. The significant level is p<0.05. Abbreviations: HMW, high molecular weight; BMI, body mass index.
VI Statistical analysis
Anthropometric factors (BMI, waist-to-hip ratio, and waist circumference) were considered as continuous, quintiled and categorical variables according to commonly defined cut points. The adipokines (leptin and total and high molecular weight adiponectin) were considered as continuous and quintiled variables. Mean levels of anthropometric measures and adipokines were compared by dichotomous CES-D category (< vs > 16), and a t-test was used to estimate significance of the differences. BMI, waist-to-hip ratio, and waist circumference were quintiled to understand potential nonlinear relationships with CES-D score. Commonly used overweight and obesity categories were also used. Spearman Correlation coefficients were calculated to assess the ranked associations among CES-D scores, adipokine levels, and anthropometric measures. Analysis of variance was used to compare mean CES-D score by anthropometric risk category. The Receiver Operating Characteristic curve was used to assess the sensitivity and specificity of leptin and adiponectin in relation to CES-D score.
Multivariate logistic regression analyses allowed estimation of the odds (Odds Ratio, 95% Confidence Interval) of clinically relevant depressive symptom burden, CES-D > 16 versus CES-D < 16. Primary predictors included anthropometric and adipokine measures. Several covariates were considered, including: age, HIV status, viral load, CD4 count, self-reported diabetes, current smoking status, number of years smoked, use of marijuana/ hash since last visit, use of crack/ freebase cocaine since last visit, heroin use, injection drug use, race, and number of cigarettes currently smoked per day. Regression models were run separately for women with versus without HIV infection. Due to the skewness of the continuous CES-D score, multivariate linear regression analyses were not attempted. Results were considered statistically significant at p < 0.05 (two-sided). SPSS version 21 was used for all statistical analyses.
Table 2: Spearman correlations of CES-D score by adipokine and anthropometric measures in the entire sample and by HIV infection status: Brooklyn Women’s Interagency HIV Study.
|
All |
|
|
HIV+ |
|
|
HIV- |
|
||
|
N |
r |
P |
N |
r |
P |
N |
r |
P |
|
|
Leptin |
347 |
0.018 |
0.738 |
243 |
0.004 |
0.953 |
104 |
0.041 |
0.683 |
|
Total Adiponectin |
348 |
0.048 |
0.367 |
244 |
0.107 |
0.094 |
104 |
0.087 |
0.380 |
|
HMW Adiponectin |
211 |
0.076 |
0.270 |
155 |
0.062 |
0.446 |
56 |
0.118 |
0.388 |
|
BMI |
349 |
0.066 |
0.220 |
247 |
0.049 |
0.422 |
102 |
0.104 |
0.299 |
|
Waist-to-hip Ratio |
262 |
0.006 |
0.917 |
180 |
0.038 |
0.615 |
82 |
0.029 |
0.794 |
|
Waist Circumference |
264 |
-0.050 |
0.417 |
181 |
0.052 |
0.488 |
83 |
0.041 |
0.712 |
Abbreviations: P, P-value; r, Spearman correlation coefficient; HMW, high molecular weight; BMI, body mass index
Table 3: Odds of a clinically relevant depressive symptom burden (CES-D > 16) by adipokine and anthropometric measures in the entire sample and by HIV infection status.
|
All |
|
HIV+ |
HIV- |
|||||||||
|
n |
P* |
OR |
95%CI |
n |
P* |
OR |
95%CI |
n |
P* |
OR |
95%CI |
|
|
Leptin |
347 |
0.21 |
0.21 |
0.99-1.04 |
243 |
0.48 |
1.00 |
0.99-1.01 |
104 |
0.99 |
1.00 |
0.99-1.01 |
|
Adiponectin |
348 |
0.80 |
1.00 |
1.00-1.00 |
244 |
0.30 |
1.00 |
1.00-1.00 |
104 |
0.80 |
1.00 |
1.00-1.00 |
|
HMW Adiponectin |
211 |
0.58 |
1.00 |
1.00-1.00 |
155 |
0.91 |
1.00 |
1.00-1.00 |
56 |
0.70 |
1.00 |
1.00-1.00 |
|
BMI |
349 |
0.97 |
0.97 |
0.84-1.20 |
247 |
0.94 |
1.00 |
0.96-1.04 |
102 |
0.19 |
0.97 |
0.92-1.02 |
|
Waist-to-hip Ratio |
262 |
0.89 |
0.58 |
0.00-1912.50 |
180 |
0.89 |
0.74 |
0.01- 49.74 |
82 |
0.41 |
0.04 |
0.00-71.65 |
|
Waist Circumference |
264 |
0.76 |
0.99 |
0.90-1.08 |
181 |
0.42 |
0.99 |
0.97-1.01 |
83 |
0.70 |
0.99 |
0.97-1.02 |
* Abbreviations: P, P-value; OR, Odds Ratio; 95% CI, 95% Confidence Interval; HMW, high molecular weight; BMI, body mass index.
Results
Demographic, anthropometric, and health characteristics of WIHS participants are presented in Table 1. Thirty percent (n=108, 69% HIV+, 31% HIV-) presented with a clinically relevant depressive symptom burden (CES-D score ≥16). The majority of women (66%) were overweight or obese (BMI ≥ 25.0 kg/m2) and the prevalence of central obesity was 59.1% (waist-to-hip ratio>0.85).
There was no correlation between CES-D score and adipokine or anthropometric measures (Table 2). There were no differences in CES-D score across traditional BMI categories (p=0.155). Age-adjusted logistic regression models predicting the presence of a clinically relevant depressive symptom burden by anthropometric or adipokine measures yielded no association in HIV+ or HIV- women (Table 3).
As previously reported, given the observed plateau of leptin levels with BMI > 40 kg/m2, in a post-hoc analysis, we stratified the sample using that cut point [25]. The BMI-leptin correlation was r=0.2 for BMI > 40 vs r=0.7 for BMI < 40 kg/m2. Further analyses by BMI strata using this cut point were uninformative.
The Receiver Operating Characteristic test for leptin and total adiponectin and CES-D scores demonstrated that blood adipokine levels in HIV+ and HIV- women were not predictive of CES-D scores (Figure 1). Similar results were observed for high molecular weight adiponectin (data not shown).
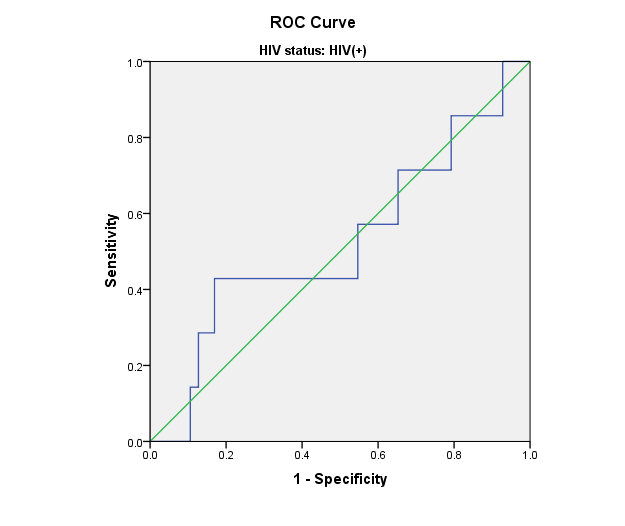
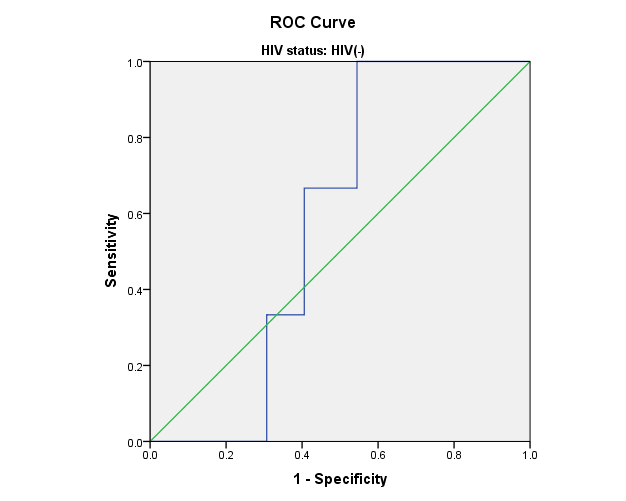
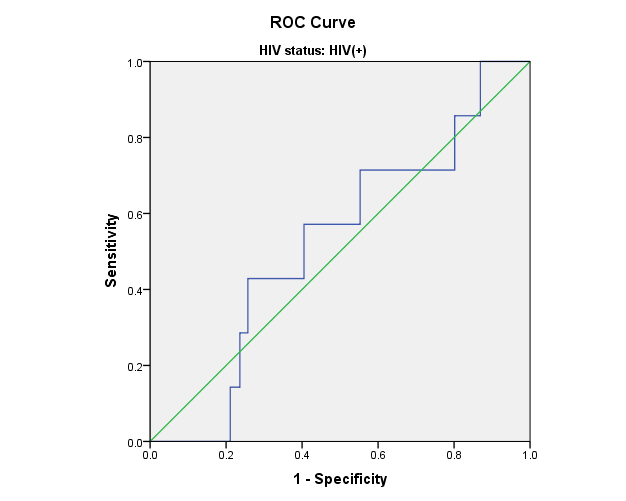
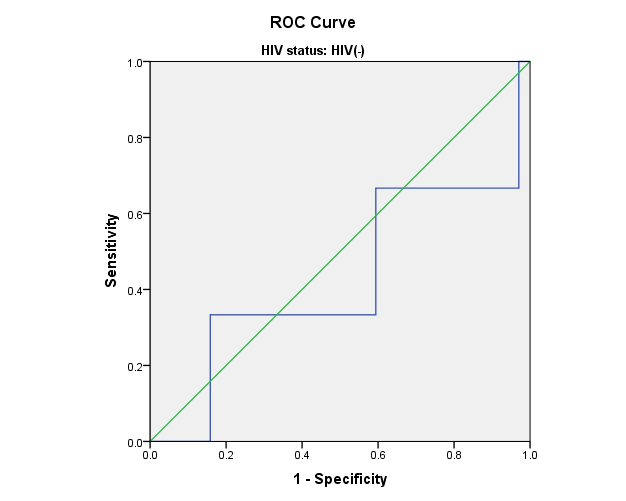
Figure 1: Receiver Operating Characteristic (ROC) curves for leptin and adiponectin in relation to CES-D score. The Women’s Interagency HIV Study.
a) HIV+ women: CES-D scores and Leptin levels
Area=0.525; p= 0.120
b) HIV- women: CES-D scores and Leptin levels
Area= 0.581; p=0.072
c) HIV+ women: CES-D scores and Total Adiponectin levels
Area=0.524; p=0.098
d) HIV- women: CES-D scores and Total Adiponectin levels
Area=0.426; p=0.194
Receiver Operating Characteristic (ROC) curves were used to assess the sensitivity and specificity of leptin and adiponectin in relation to CES-D score. ROC curves for leptin and total adiponectin and CES-D scores show that blood adipokine levels in HIV+ and HIV- women were not predictive of CES-D scores. Similar results were observed for high molecular weight adiponectin (data not shown).
Discussion
To our knowledge, this is the first report on the association between depressive symptoms, and serum adipokine and anthropometric measures in a sample of women with or at risk for HIV infection. With rigorous analyses we found that among women participating in the Brooklyn WIHS, multiple adiposity indicators were not associated with depressive symptoms, despite associations observed with cognition in the same sample. While these findings support some published literature from uninfected populations we cannot disambiguate a true null finding from a type II error given our small sample size [33, 42]. At the same time, exploratory studies such as this one are vital for uncovering the variability in adiposity measures across human samples and contribute to a foundation of knowledge for further investigation.
The association between depressive symptoms and adipose-derived adipokines is sparsely studied, and directions of associations remain unclear. In a case-control study of patients clinically diagnosed with major depression, adiponectin levels were lower compared to their healthy counterparts; and adiponectin was significantly correlated with severity of depression [43]. However, another study reported no association between depression and adiponectin levels in 90 healthy adolescents [42].
Consensus on leptin’s associations with neuropsychiatric health has not been reached, despite the presence of leptin receptors in the brain and in areas involved in emotion. One preclinical study demonstrated that direct microinjection of leptin into the hippocampus of rats elicited antidepressant-like behavior [44]. Streptozotocin-induced diabetic mice exhibited low leptin levels and depression-like behavior during a tail suspension test. Treatment with leptin reversed this behavior [45]. In women, low leptin levels were associated with higher depressive symptoms across the body weight spectrum, independent of body fat [23]. In another study, patients with depression evidenced elevated nocturnal profiles of serum leptin [46].
HIV+ women who take ART and gain weight, may experience an increase in central and decrease in peripheral obesity [17]. It is unknown whether HIV+ adults also experience significant changes in levels of adiponectin, leptin, and other adipose tissue hormones. Lipodystrophy had no effect on quality of life and depression in HIV-infected men or women who are taking ART therapy [47].
Leptin resistance in the overweight and obese may explain the differential association between depressive symptoms and leptin levels. Administration of leptin in people with healthy body weight results in a reduction in adipose tissue and weight loss. However, administration of leptin in obese people does not inhibit food intake [48]. Leptin resistance occurs because of a defect in the leptin signaling pathway including impairment in transport of leptin across the blood brain barrier and reduced function of the leptin receptors [49]. The remaining question is whether leptin resistance is associated with depressive symptoms [50]. If that were the case, it would create an opportunity for investigating many therapeutic options that target the leptin signaling pathway to treat leptin resistance.
Investigators have suggested that adiponectin plays a role in the association between obesity and psychopathology, with hypoadiponectinemia related to the two conditions [36]. However, reports are variable.
An earlier diagnosis of depression is crucial since depression can take a serious toll on physical health and exacerbate existing health problems. Among HIV+ women, this can be debilitating and even life threatening, due to the high number of depressive symptom correlates often observed in HIV+ women, including stigma, disparities related to socioeconomics, access to health care, and multi-ethnoracial backgrounds, and poor ART adherence [11, 51]. Co-morbid depressive symptoms among HIV+ women have other downstream consequences. Based on a systematic review, the American Heart Association declared that depression should be considered a risk factor for poor prognosis among patients with acute coronary syndrome [52]. Recent studies exploring health and major depression have found that adults with major depression and recovering from strokes or heart attacks have more difficulty following their healthcare provider’s instructions and coping with the challenges of their conditions [53-57].
The main strength of this study is that we have explored adiposity-related endocrine and anthropometric measures that may be associated with the neuropsychiatric health of women coping with HIV infection, and those at risk. We accomplished this in the longest running US HIV cohort of women. These women, with diverse ethnoracial backgrounds and health disparities, are underrepresented in research studies.
Certain limitations should be considered. First, depressive symptoms were measured via self-report using the CES-D, which while a validated method for assessing depressive symptoms, is not as robust as a clinically diagnosed depression using the Diagnostic and Statistical Manual of Mental Disorders 5 criteria. However, the sensitivity of CES-D to detect major depression is high [58, 59]. Second, depression is episodic and a cross-sectional analysis not ideal. Third, we are not able to determine potential causal relationships between adipokine levels and depressive symptoms. Finally, other overwhelming factors may associate with depressive symptom burden stronger than measures of adiposity in this group of vulnerable women. Not surprisingly, of the one-third (n=110) of participants with a CES-D score ≥16, representative of a clinically depressive symptom burden, 67% were HIV+ while 33% were HIV-. HIV+ participants also had a higher average CES-D score, 27.0, versus 23.7 in HIV- participants. Despite these issues, this is the first cross-sectional study of leptin and adiponectins in association with depressive symptoms among HIV+ and HIV- women, thereby providing a novel perspective on endocrine function and neuropsychiatric health in this population.
Depressive symptoms were not associated with anthropometric or metabolic adiposity indicators, among the HIV+ and HIV- women in the Brooklyn WIHS. Further studies are needed to replicate or refute these findings given the inconsistencies in the literature and given our small sample size. Despite null findings, these results contribute to our understanding of adiposity-associated risk in the neuropsychiatric health of HIV+ and at-risk HIV- women and the large variability in these measures.
Acknowledgement
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), and P30-AI-050410 (UNC CFAR). In addition, Dr. Gustafson received support from NIH/NIAID ARRA Supplement No. 54492, Swedish Research Council Diarienummer: 523-2005-8460, and the SUNY Research Foundation. In addition, we wish to thank John Heitman, BS, for performing the leptin and adiponectin assays.
Competing Financial Interests
The authors declare no financial or non-financial competing interests.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
We thank the women participating in WIHS for their time, cooperation, and support.
Author Contributions
Anna Y. Groysman, MD was involved in hypothesis generation, conducting the data analyses, and drafted the manuscript. Sheila Keating, PhD oversees the laboratory where leptin and adiponectin were analyzed, reviewed the hypothesis, and edited and approved the manuscript. Susan Holman, RN, MS is the Project Director for the Brooklyn WIHS site and oversees clinical operations, reviewed the hypothesis, and edited and approved the manuscript. Jeremy Weedon, PhD guided the statistical analysis, reviewed the hypothesis and edited and approved the manuscript. Howard Minkoff, MD is Co-Principal Investigator of the Brooklyn WIHS, reviewed the hypothesis, and edited and approved the manuscript. Deborah R. Gustafson, MS, PhD is Co-Principal Investigator of the Brooklyn WIHS, and supervised Dr.Groysman, reviewed the hypothesis, assisted in gaining WIHS approval to obtain the data, guided the statistical analyses, and assisted in drafting, finalizing and submitting the manuscript.
Availability of materials and data
All materials, data and associated protocols are promptly available to readers without undue qualifications in material transfer agreements. There are no restrictions. Requests for these materials and data can be made to the Corresponding Author, Dr. Deborah Gustafson, via email at Deborah.gustafson@downstate.edu
Article Info
Article Type
Research ArticlePublication history
Received: Sat 15, Sep 2018Accepted: Thu 04, Oct 2018
Published: Fri 19, Oct 2018
Copyright
© 2023 Deborah Gustafson. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2018.02.002
Author Info
Anna Y. Groysman Deborah Gustafson Howard Minkoff Jeremy Weedon Sheila Keating Susan Holman
Corresponding Author
Deborah GustafsonDepartment of Neurology, State University of New York Downstate Medical Center, Brooklyn, NY
Figures & Tables
References
1.The Antiretroviral Therapy Cohort (2010) Causes of Death in HIV-1–Infected Patients Treated with Antiretroviral Therapy, 1996–2006: Collaborative Analysis of 13 HIV Cohort Studies. Clin Infect Dis 50: 1387-1396. [Crossref]
2. Palella FJ Jr, Phair JP (2011) Cardiovascular Disease in HIV Infection. Curr Opin HIV AIDS 6: 266-271. [Crossref]
3. Nancy Crum-Cianflone, Raechel Tejidor, Sheila Medina, Irma Barahona, Anuradha Ganesan (2008) Obesity among HIV Patients: The Latest Epidemic. AIDS patient care and STDs 22: 925-930. [Crossref]
4. Abdelaal M, le Roux CW, Docherty NG (2017) Morbidity and mortality associated with obesity. Ann Transl Med 5: 161. [Crossref]
5. Ogden CL, Carroll MD, Flegal KM (2014) Prevalence of obesity in the United States. JAMA 312: 189-190. [Crossref]
6. Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4: 579-591. [Crossref]
7. Mannino DM, Mott J, Ferdinands JM, Camargo CA, Friedman M, et al. (2006) Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY). Int J Obes (Lond) 30: 6-13. [Crossref]
8. Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115: 1111-1119. [Crossref]
9. Emmerzaal TL, Kiliaan AJ, Gustafson DR (2015) 2003-2013: a decade of body mass index, Alzheimer's disease, and dementia. J Alzheimers Dis 43: 739-755. [Crossref]
10. Stunkard AJ, Faith MS, Allison KC (2003) Depression and obesity. Biol Psychiatry 54: 330-337. [Crossref]
11. Faith MS, Matz PE, Jorge MA (2002) Obesity-depression associations in the population. J Psychosom Res 53: 935-942. [Crossref]
12. K M Carpenter, D S Hasin, D B Allison, M S Faith (2000) Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health 90: 251-257. [Crossref]
13. A H Crisp, B McGuiness (1976) Jolly fat: relation between obesity and psychoneurosis in general population. Br Med J 1: 7-9. [Crossref]
14. Kiliaan AJ, Arnoldussen IA, Gustafson DR (2014) Adipokines: a link between obesity and dementia? Lancet Neurol 13: 913-923. [Crossref]
15. Lehr S, Hartwig S, Sell H (2012) Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl 6: 91-101. [Crossref]
16. Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9: 191-200. [Crossref]
17. Mallewa JE, Wilkins E, Vilar J, Mallewa M, Doran D, et al. (2008) HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J Antimicrob Chemother 62: 648-660. [Crossref]
18. Sweeney L L, Brennan A M, Mantzoros C S (2007) The role of adipokines in relation to HIV lipodystrophy. AIDS (London, England) 21: 895-904.
19. Jequier E (2002) Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 967: 379-388. [Crossref]
20. Gustafson DR, Backman K, Joas E, Waern M, Ostling S, et al. (2012) 37 years of body mass index and dementia: observations from the prospective population study of women in Gothenburg, Sweden. J Alzheimers Dis 28: 163-171. [Crossref]
21. Milaneschi Y, Sutin AR, Terracciano A, Canepa M, Gravenstein KS, et al. (2014) The association between leptin and depressive symptoms is modulated by abdominal adiposity. Psychoneuroendocrinology 42: 1-10. [Crossref]
22. Milaneschi Y, Sutin AR, Terracciano A, Canepa M, Gravenstein KS, et al. (2014) The association between leptin and depressive symptoms is modulated by abdominal adiposity. Psychoneuroendocrinology 42: 1-10. [Crossref]
23 Lawson EA, Miller KK, Blum JI, Meenaghan E, Misra M, et al. (2012) Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf) 76: 520-525. [Crossref]
24. Chirinos DA, Goldberg R, Gellman M, Mendez AJ, Gutt M, et al. (2013) Leptin and its association with Somatic Depressive Symptoms in Patients with the Metabolic Syndrome. Ann Behav Med 46: 31-39. [Crossref]
25. Gustafson DR, Mielke MM, Keating SA, Holman S, Minkoff H, et al. (2015) Leptin, Adiponectin and Cognition in Middle-aged HIV-infected and Uninfected Women. The Brooklyn Women’s Interagency HIV Study. J Gerontol Geriatr Res 4: 240. [Crossref]
26. Freitas P, Carvalho D, Santos AC, Madureira AJ, Martinez E, et al. (2014) Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis 14: 347. [Crossref]
27. Samaras K, Gan SK, Peake PW, Carr A, Campbell LV (2009) Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 17: 53-59. [Crossref]
28. Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, et al. (2006) Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes 55: 1954-1960. [Crossref]
29. Wang Y, Lam KS, Yau MH, Xu A (2008) Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J 409: 623-633. [Crossref]
30. Cotter BR (2006) Endothelial dysfunction in HIV infection. Curr HIV/AIDS Rep 3: 126-131. [Crossref]
31. Narita K, Murata T, Takahashi T, Kosaka H, Omata N, et al. (2006) Plasma levels of adiponectin and tumor necrosis factor-alpha in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry 30: 1159-1162. [Crossref]
32. Alexopoulos G S, Bruce M L, Silbersweig D, Kalayam B, Stern E (1999) Vascular depression: a new view of late-onset depression. Dialogues Clin Neurosci 1: 68-80. [Crossref]
33. Pan A, Ye X, Franco OH, Li H, Yu Z, et al. (2008) The Association of Depressive Symptoms with Inflammatory Factors and Adipokines in Middle-Aged and Older Chinese. PLoS ONE 3: 1392. [Crossref]
34. Kershaw EE, Flier JS (2002) Adipose tissue as an endocrine organ. Best practice & research. J Clin Endocrinol Metab 16: 639-651. [Crossref]
35. Taylor VH, Macqueen GM (2010) The Role of Adipokines in Understanding the Associations between Obesity and Depression. J Obes 10: 1155. [Crossref]
36. Yilmaz Y (2008) Psychopathology in the context of obesity: the adiponectin hypothesis. Med Hypotheses 70: 902-903. [Crossref]
37. Deborah R Gustafson, Michelle M Mielke, C Tien, Victor Valcour, Mardge Cohen, et al. (2013) Anthropometric measures and cognition in middle-aged HIV-infected and uninfected women. The Women's Interagency HIV Study. J Neurovirol 19: 574-585. [Crossref]
38. Bacon MC, von Wy V, Alden C, Sharp G, Robison E, et al. (2005) The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 12: 1013-1019. [Crossref]
39. Robert C Kaplan, Lawrence A Kingsley, Stephen J Gange, Lorie Benning, Lisa P Jacobson, et al. (2008) Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 22: 1615-1624. [Crossref]
40. Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, et al. (1998) The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 9: 117-125. [Crossref]
41. Radloff L S (1977) The CES-D Scale. Applied Psychological Measurement 1: 385-401.
42. Mamalakis G, Kiriakakis M, Tsibinos G, Hatzis C, Flouri S, et al. (2006) Depression and serum adiponectin and adipose omega-3 and omega-6 fatty acids in adolescents. Pharmacol Biochem Behav 85: 474-479. [Crossref]
43. Leo R, Di Lorenzo G, Tesauro M, Cola C, Fortuna E, et al. (2006) Decreased plasma adiponectin concentration in major depression. Neurosci Lett 407: 211-213. [Crossref]
44. Lu XY, Kim CS, Frazer A, Zhang W (2006) Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A 103: 1593-1598. [Crossref]
45. Hirano S, Miyata , Kamei J (2007) Antidepressant-like effect of leptin in streptozotocin-induced diabetic mice. Pharmacol Biochem Behav 86: 27-31. [Crossref]
46. Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, et al. (1998) Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res 32: 403-410. [Crossref]
47. Steel JL, Landsittel D, Calhoun B, Wieand S, Kingsley LA (2006) Effects of lipodystrophy on quality of life and depression in HIV-infected men on HAART. AIDS Patient Care STDS 20: 565-575. [Crossref]
48. Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, et al. (1999) Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. Jama 282: 1568-1575. [Crossref]
49. Munzberg H, Myers MG Jr (2005) Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8: 566-570. [Crossref]
50. Lu X Y (2007) The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 7: 648-652. [Crossref]
51. Mardge H COHEN, Anna L HOTTON, Ronald C HERSHOW, Alexandra LEVINE, Peter BACCHETTI, et al. (2015) Gender-Related Risk Factors Improve Mortality Predictive Ability of VACS Index Among HIV-Infected Women. J Acquir Immune Defic Syndr 70: 538-544. [Crossref]
52. Lichtman JH, Bigger JT Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, et al. (2008) Depression and Coronary Heart Disease. Circulation 118: 1768-1775. [Crossref]
53. DiMatteo MR, Lepper HS, Croghan TW (2000) Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 160: 2101-2107. [Crossref]
54. Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, et al. (2000) Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 160: 1818-1823. [Crossref]
55. Ciechanowski PS, Katon WJ, Russo JE (2000) Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 160: 3278-3285. [Crossref]
56. Bautista LE, Vera-Cala LM, Colombo C, Smith P (2012) Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens 25: 505-511. [Crossref]
57. Jeffrey S Gonzalez, Abigail W Batchelder, Christina Psaros, Steven A Safren (2011) Depression and HIV/AIDS Treatment Nonadherence: A Review and Meta-analysis. J Acquir Immune Defic Syndr 58: 1097. [Crossref]
58. Cary L Gay, Anders Kottorp, Anners Lerdal, Kathryn A Lee (2016) Psychometric Limitations of the Center for Epidemiologic Studies-Depression Scale for Assessing Depressive Symptoms among Adults with HIV/AIDS: A Rasch Analysis. Depress Res Treat 2016. [Crossref]
59. Truc Thanh Thai, Mairwen K Jones, Lynne M Harris, Robert C Heard (2016) Screening value of the Center for epidemiologic studies – depression scale among people living with HIV/AIDS in Ho Chi Minh City, Vietnam: a validation study. BMC Psychiatry 16: 145. [Crossref]
